Abstract
The production of H2O2, which is essential to thyroid hormone synthesis, involves two NADPH oxidases: dual oxidases 1 and 2 (DuOx1 and DuOx2). A functional study with human DuOx genes and their 5′-flanking regions showed that DuOx1 and −2 promoters are different from thyroid-specific gene promoters. Furthermore, their transcriptional activities are not restricted to thyroid cells. While regulation of Tg (thyroglobulin) and TPO (thyroperoxidase) expression have been extensively studied, DuOx2 promoter regulation by hormones and transcriptional factors need to be more explored. Herein we investigated the role of TSH, insulin and insulin-like growth factor 1 (IGF-1), as well as the cAMP effect on DuOx2 promoter (ptx41) activity in transfected rat thyroid cell lines (PCCL3). We also assessed DuOx2 promoter activity in the presence of transcriptional factors crucial to thyroid development such as TTF-1 (thyroid transcription factor 1), PAX8, CREB, DREAM, Nkx2.5 and the coactivator TAZ in HeLa and HEK 293T-transfected cells. Our results show that TSH and forskolin, which increase cAMP in thyroid cells, stimulated DuOx2 promoter activity. IGF-1 led to pronounced stimulation, while insulin induction was not statistically different from DuOx2 promoter basal activity. All transcriptional factors selected for this work and coactivator TAZ, except DREAM, stimulated DuOx2 promoter activity. Moreover, Nkx2.5 and TAZ synergistically increased DuOx2 promoter activity. In conclusion, we show that DuOx2 expression is regulated by hormones and transcription factors involved in thyroid organogenesis and carcinogenesis, reinforcing the importance of the control of H2O2 generation in the thyroid.
Key Words: DuOx2 promoter, Thyroid, Nkx2.5, TAZ
Introduction
The production of H2O2, which is essential for thyroid hormone synthesis, involves NADPH oxidase enzymes, namely dual oxidases 1 and 2 (DuOx1 and DuOx2) [1]. They are first detected at embryonic day 15.5 in mice thyroid when iodide organification sets up [2] and share 83% gene similarity. Molecular studies have revealed that both DuOx genes lie on chromosome 15q, arranged in a head-to-head configuration, and are separated by a 16-kb-long region [1]. In human thyroid, DuOx2 mRNA is 1.5-5 times more abundant than DuOx1 [3]. Biochemical studies have revealed that DuOx activity is stimulated by calcium [4] and TSH through the cAMP cascade [5]. On the other hand, thyroid H2O2 generation is inhibited by iodide in vivo, the Wolff-Chaikoff effect [6,7] and in cell culture [8]. Moreover, impaired Ca2+/NADPH-dependent H2O2-generating activity has been associated with goiter and hypothyroidism [9]. Indeed, mutations in the DuOx2 gene leading to a truncated protein with impaired H2O2 generation activity have been found in hypothyroid patients with a thyroid iodide organification defect [10,11]. A functional study with human DuOx genes and their 5′-flanking regions showed that DuOx1 and −2 promoters are different from thyroid-specific gene promoters and that their transcriptional activities are not restricted to thyroid cells [3]. Besides, the expression of both genes are positively controlled by cAMP in dog thyrocytes [12], although cAMP failed to stimulated DuOx1/2 human promoter transcriptional activity in a rat thyroid cell line [3].
Despite its importance in thyroid hormone biosynthesis, no conclusive data about DuOx promoter regulation have been proposed. Thus, the current work explores DuOx regulation by TSH, cAMP and CREB (cAMP response element-binding protein), as these factors also regulate TPO (thyroperoxidase) and Tg (thyroglobulin) expression [13]. Furthermore, as insulin and insulin-like growth factor 1 (IGF-1) have permissive effect on the mitogenic action of TSH [12,13], we also evaluated their contribution to the transcriptional activity of the DuOx2 promoter (ptx41), the most relevant isoform in humans [10]. We focused on the role of TTF-1 (thyroid transcription factor 1/Nkx2-1) and PAX8 (paired box gene 8) on DuOx2 promoter activity regulation, as they are simultaneously expressed only in the thyroid gland and are required for proper thyroid development [14,15,16,17]. We also investigated the role of TAZ (transcriptional coactivator with PDZ-binding motif) which is overexpressed in papillary thyroid carcinomas [18,19], Nkx2.5 which is expressed during thyroid organogenesis [20,21], and DREAM (downstream regulatory element antagonist modulator) [22] which is responsible for thyroid enlargement and nodular development [23,24] on DuOx2 promoter regulation.
Given that DuOx2 produces H2O2, which is essential to thyroid hormone synthesis, it is of great importance to understand the mechanisms involved in the regulation of this gene. Thus, the present study describes the effect of the main thyroid regulators and the role of transcriptional factors (TF) involved in thyroid dysgenesis on human thyroid DuOx2 promoter activity.
Material and Methods
Cell Culture
PCCL3 cells, an immortalized rat thyroid cell line, was grown in Coon's modified Ham's F-12 medium (HiMedia Laboratories, Mumbai, India) supplemented with 5% donor calf serum and a 6-hormone mixture (complete medium): 1 nM TSH, 10 µg/ml insulin, 10 ng/ml somatostatin, 5 µg/ml transferrin, 10 µg/ml hydrocortisone and 10 ng/ml glycyl-L-histidyl-L-lysine acetate. HeLa (human cervical cancer cell line) and C (human embryonic kidney cell line), heterologous system, were grown in Dulbecco's modified Eagle's medium (HiMedia Laboratories) supplemented with 10% fetal calf serum, 1% glutamine and 1% pyruvate. To evaluate DuOx2 promoter activity modulation by TSH and insulin/IGF-1, cells were cultured in a ‘starvation’ medium for 3 and 5 days in the absence of TSH or insulin/IGF-1, respectively, and before DuOx2 promoter activity assays were performed.
Plasmids and Expression Constructs
Four luciferase reporter plasmids, ptx 41 (0.6 kpb), ptx 43 (0.5 kpb), ptx 46 (4.1 kpb) and ptx 52 (6.1 kpb), containing the proximal 5′-flanking region of the DuOx gene were cloned in the pGL3 vector according to Pachucki et al. [3] and were kindly provided by Dr. Françoise Miot (Faculté de Médecine, Université Libre de Bruxelles, Brussels, Belgium). Human luciferase reporter plasmid sequences were described by Pachucki et al. [3]. Tg promoter was also cloned in pGL3 vector. Human cDNA for transcription factor TTF-1 [25], PAX8 [26], CREB and DREAM [22] were cloned into pcDNA3 vector. Mouse cDNA for Nkx2.5, mutated Nkx2.5 I183P [27] and the coactivator TAZ [19] were also cloned into pcDNA3 vector. To correct transfection efficiency, pRL-CMV (Promega, Madison, Wisc., USA) containing renilla luciferase cDNA was used.
Transfection and Luciferase Assays
PCCL3 (6 × 105 cells/well) and HeLa (4 × 105 cells/well) cells were cultured in the presence of 0.2% fetal bovine serum (starvation medium) for 72 h. Cells were seeded in 6-well tissue culture plates for 48 h and transiently transfected with 3 µg of tested plasmid (DuOx1 and −2 promoters) and 100 ng of pRL-CMV renilla by calcium phosphate coprecipitation, as previously described [28]. Hormones were added to the culture medium at the following final concentrations: 10 µM forskolin, 1 or 2 nM TSH, 10 µM insulin (Sigma-Aldrich, St. Louis, Mo., USA), and 100 ng/ml IGF-1, (PeproTech, Rocky Hill, N.J., USA).
To assess DuOx2 promoter activity regulation by TF, pTX41 (3 µg) was transiently transfected alone or in combination with 500 ng of the mentioned TF in PCCL3 and HeLa cells, by calcium phosphate coprecipitation or in HEK 293T cells (2 × 105 cells/well), using lipofectamine (Invitrogen, Carlsbad, Calif., USA). Tg promoter (1 µg) was cotransfected in the presence of TTF-1 or PAX8 in HeLa cells, in the same conditions as DuOx2 promoter.
After 24 h, the cells were harvested and collected for firefly and renilla luciferase activity by the Dual-Luciferase Reporter Assay System (Promega). Luminescence was measured in a Victor X4 Multilabel Plate Reader (PerkinElmer, Norwalk, Conn., USA). In all cotransfection experiments, the amount of DNA was normalized using the corresponding insertless expression vector. Results were expressed as relative activity compared to the control in each experiment. The data are expressed as means ± SEM of at least three independent experiments in triplicate.
Statistical Analysis
All transfections were performed in triplicate, at least twice. Statistical analysis was performed using a nonparametric Kruskal-Wallis test followed by Dunn's multiple comparison tests. Statistical analyses were conducted using the software GraphPad Prism (version 5; GraphPad Software Inc., San Diego, Calif., USA), and the level of significance was established at p < 0.05.
Results
DuOx1 and −2 Promoter Activities Are Differentially Regulated in PCCL3 and HeLa Cells
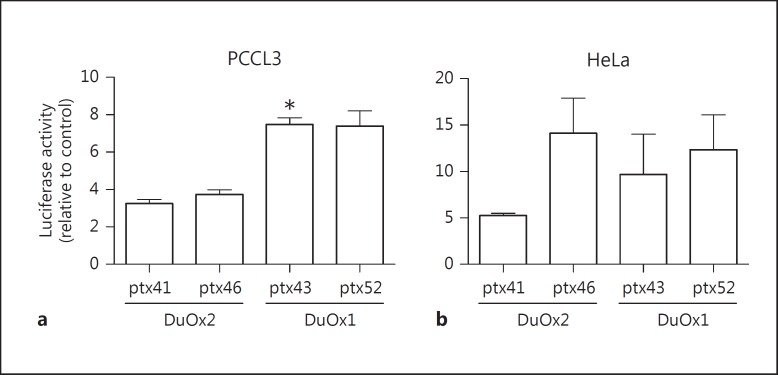
We evaluated DuOx1 and −2 promoter transcription activity using two different promoter constructs: DuOx1: ptx43 (0.5 kpb – short promoter) and ptx52 (6.1 kpb – long promoter), and DuOx2: ptx41 (0.6 kpb – short promoter) and ptx46 (4.1 kpb – long promoter) in a rat thyroid cell line (PCCL3) and in a nonthyroid cell line (HeLa), as shown in figure 1a, b, respectively. In PCCL3 cells, short promoter luciferase activity of both DuOx1 and −2 was similar to their long promoter activity (fig. 1a), although DuOx2 promoters exhibited lower luciferase activity than DuOx1 (fig. 1a). On the other hand, in HeLa cells, DuOx2 promoter ptx46 exhibited higher activity among promoters and DuOx2 short and long promoter activities were similar (fig. 1b). Because mutations in DuOx2 are related to hypothyroidism [10], we focused our attention on the study of the DuOx2 short promoter regulation since the ptx41 sequence has binding domains for TF.
Fig. 1.
Transcriptional activity of DuOx1 and −2 promoters in a rat thyroid cell line, PCCL3 (a), and in a nonthyroid cell line, HeLa (b). DuOx1 and −2 promoter transcription activity was measured in two different promoter constructs: DuOx1 (ptx43, 0.5 kpb, and ptx52, 6.1 kpb) and DuOx2 (ptx41, 0.6 kpb, and ptx46, 4.1 kpb). 3 µg of each DuOx promoter sequence cloned in pGL3 luciferase reporter vector coding for firefly luciferase was cotransfected with 0.02 µg of pRL renilla luciferase reporter vector to normalize luciferase activity in PCCL3 or HeLa cells. * p < 0.05 vs. ptx41. All transfections were done in triplicate, at least twice.
DuOx2 Promoter Activity Is Modulated by TSH, Insulin and IGF-1
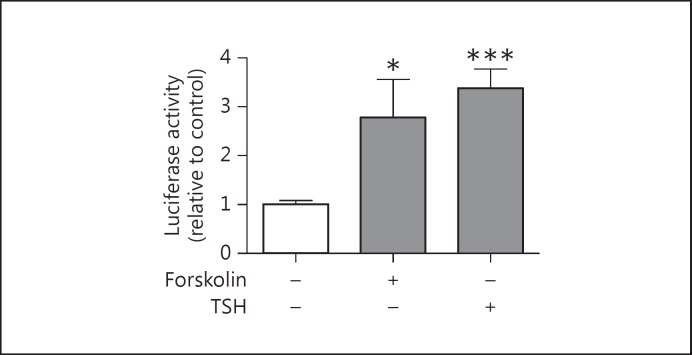
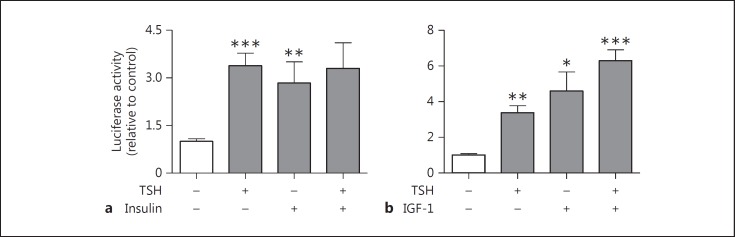
Since TSH activity through the cAMP pathway is involved in the control of Tg and TPO gene transcription [2] and also stimulates H2O2 production [13], we measured DuOx2 short promoter transcription activity in the presence of TSH and forskolin, which increases cAMP in thyroid cells (PCCL3 cells). TSH and forskolin enhanced DuOx2 promoter transcription activity (fig. 2), suggesting that TSH stimulates DuOx2 promoter activity via activation of adenylyl cyclase. Because insulin and IGF-1 have permissive effects on the mitogenic action of TSH on thyroid cells [12], we investigated the effect of both hormones on DuOx2 promoter activity in the presence of TSH. Indeed, we found a significant DuOx2 promoter activity induction in the presence of TSH and insulin (fig. 3a) and IGF-1 (fig. 3b). We also observed an additional transcriptional activity increase in the presence of TSH and IGF-1 (fig. 3b), but not for TSH and insulin (fig. 3a).
Fig. 2.
DuOx2 promoter activity regulation by forskolin or TSH in PCCL3 cells. PCCL3 cells were cultivated in the presence or absence of forskolin (10 µM) or TSH (1 nM) and transfected with 3 µg of DuOx2 short promoter (ptx41, 0.6 kpb) sequence cloned in pGL3 luciferase reporter vector coding for firefly luciferase. DuOx2 promoter was cotransfected with 0.02 µg of pRL renilla luciferase reporter vector to normalize luciferase activity. * p < 0.05 vs. ptx41 and *** p < 0.001 vs. ptx41. All transfections were done in triplicate at least twice.
Fig. 3.
DuOx2 promoter activity regulation by insulin (a) and IGF-1 (b) in the presence or absence of TSH in PCCL3 cells. a PCCL3 cells were transfected with 3 µg of DuOx2 short promoter (ptx41, 0.6 kpb) sequence cloned in pGL3 luciferase reporter vector coding for firefly luciferase. DuOx2 promoter was cotransfected with 0.02 µg of pRL renilla luciferase reporter vector to normalize luciferase activity. Cells were cultivated in the presence or absence of TSH (1 nM) and/or insulin (10 µM) (a) and in the presence or absence of TSH (1 nM) and/or IGF-1 (100 ng/ml) (b). * p < 0.05 vs. ptx41; ** p < 0.01 vs. ptx41; *** p < 0.001 vs. ptx41. Mann-Whitney tests were applied in the luciferase activity in cells treated with insulin. All transfections were done in triplicate at least twice.
DuOx2 Promoter Activity Is Modulated by Transcription Factors
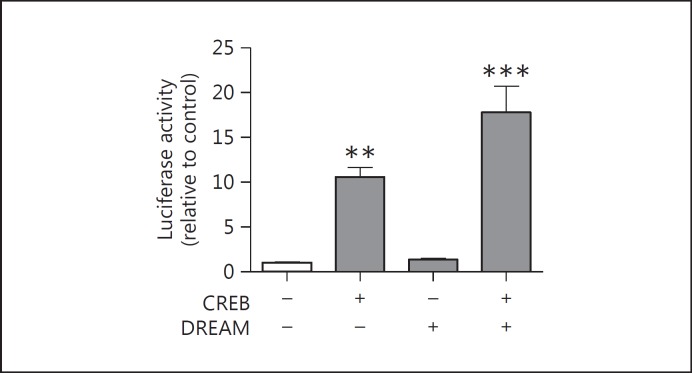
The maintenance of thyroid differentiation phenotype depends on the expression of specific TF that regulate the expression of the main thyroid differentiation markers. Studies concerning the role of thyroid TF on DuOx2 promoter activity regulation are rare. We have assessed DuOx2 short promoter in the presence of CREB, DREAM, TTF-1 and PAX8 in HeLa cells and Nkx2.5 and the coactivator TAZ in HEK 293T cells as a heterologous system in order to study the role of each TF on DuOx2 promoter activity without the interference of other thyroid TF. Since CREB binds to specific DNA sequences in response to cAMP increase and regulates thyroid metabolism, growth and differentiation [29], we also investigated CREB effects on DuOx2 promoter activity, cotransfecting it in HeLa cells. We found a significant increase in DuOx2 promoter activity in the presence of CREB (fig. 4). As DREAM is a transcriptional repressor that impairs Tg promoter transactivation by TTF-1 [24] and DREAM/CREB interaction displaces CREB from CRE sites and prevents the recruitment of CBP to phospho-CREB impairing the transcription [30], we expected a decrease in DuOx2 promoter activity in the presence of CREB and DREAM. Indeed, DuOx2 promoter activity in the presence of DREAM alone remained unchanged compared to the promoter basal levels, but the cotransfection of CREB and DREAM resulted in a higher DuOx2 promoter activity (fig. 4). It is likely that additional factors might be involved in the mechanism of cooperation of CREB and DREAM on DuOx2 promoter activity.
Fig. 4.
DuOx 2 promoter activity regulation by CREB and DREAM in HeLa cells. HeLa cells were transfected with 3 µg of DuOx2 promoter (ptx41) sequence and cotransfected with 0.5 µg of CREB or DREAM, or both TF together. CREB and DREAM sequences were cloned in pcDNA3 vector, while the DuOx2 sequence was cloned in pGL3 luciferase reporter vector coding for firefly luciferase, and were cotransfected with 0.02 µg of pRL renilla luciferase reporter vector to normalize luciferase activity in HeLa cells. ** p < 0.01 vs. ptx41; *** p < 0.001 vs. ptx41. All transfections were done in triplicate, at least twice.
TTF-1 and PAX8 are critically involved in the transcriptional control of thyroid differentiation markers expression. In thyroid cells, PAX8 interacts with TTF-1 and activates Tg [15] and TPO expression [16,17]; however, there is no convincing evidence that PAX8 and TTF-1 are able to regulate DuOx1 and −2 promoter activity. In fact, a study using fusion proteins composed of the DNA-binding domain of either TTF-1 or PAX8 fused to the repressive domain of the drosophila-engrailed protein to competitively counteract the activity of endogenous TTF-1 or PAX8 failed to activate DuOx2 promoter activity [31]. In order to clarify this point, we cotransfected PAX8 and TTF-1 in HeLa cells with the DuOx2 short promoter and Tg promoter (online suppl. fig. S1; see www.karger.com/doi/10.1159/000379749). Our results revealed that PAX8 and TTF-1 increased DuOx2 promoter transcriptional activity and they had a synergistic action on this promoter, producing a 35-fold promoter activity increase compared to the promoter basal levels (fig. 5). This result suggests not only TPO and Tg promoter activities but also the DuOx2 promoter are stimulated by these two TF. The discrepancy found between our results and the one described before [31] might be related to the experimental model used.
Fig. 5.
Effect of the transcriptional factors TTF-1 and PAX8 on DuOx2 short promoter activity in HeLa cells transfected with ptx41. HeLa cells were transfected with 3 µg of DuOx2 short promoter (ptx41, 0.6 kpb) and cotransfected with 0.5 µg of TTF-1 or PAX8, or both TF. The DuOx2 sequence was cloned in pGL3 luciferase reporter vector coding for firefly luciferase, while TTF-1 and PAX8 sequences were cloned in pcDNA3 vector. DuOx2 promoter was cotransfected with 0.02 µg of pRL renilla luciferase reporter vector to normalize luciferase activity. ** p < 0.01 vs. ptx41. All transfections were done in triplicate at least twice.
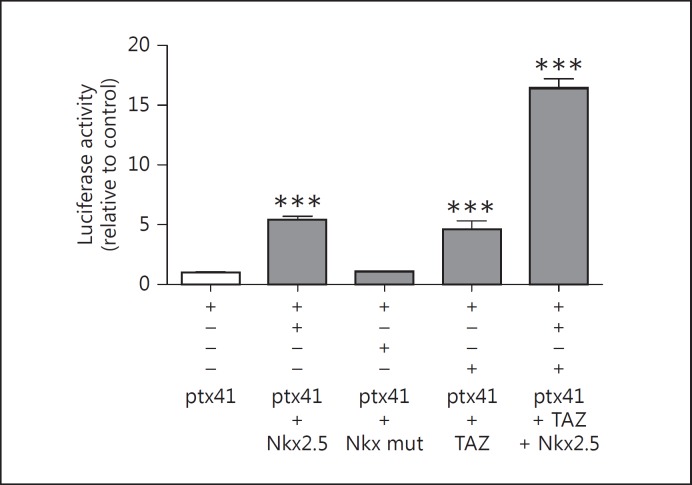
It was shown that Nkx2.5 increases type 2 deiodinase, Tg, TPO and sodium-iodide symporter promoter activities [32], but its role on DuOx2 promoter activity has not been evaluated so far. To investigate the role of Nkx2.5 in DuOx2 promoter regulation, we cotransfected Nkx2.5 in HEK 293T cells once the efficiency of the transfection was very low in HeLa cells. In this sense, our results have shown that wild-type Nkx2.5 induced DuOx2 promoter activity while mutated Nkx2.5 did not change the basal activity of the promoter in HEK 293T cells (fig. 6).
Fig. 6.
Effect of Nkx2.5 and TAZ on DuOx2 short promoter activity in HEK 293T cells. Wild-type Nkx2.5 (Nk2 transcription factor related, locus 5), mutated Nkx2.5 (Ile183 to Pro in the homeodomain of Nkx2.5 – Nkx mut), TAZ (transcriptional coactivator with PDZ-binding motif), and both TAZ and wild-type Nkx2.5 were transfected in HEK 293T cells and the activity of ptx41 (DuOx2 short promoter) was evaluated. 3 µg of DuOx2 promoter (ptx41) was cotransfected alone or together with 0.5 µg of wild-type Nkx2.5, mutated Nkx2.5, TAZ, or TAZ and wild-type Nkx2.5. The DuOx2 promoter sequence was cloned in pGL3 luciferase reporter vector coding for firefly luciferase, while TAZ, wild-type and mutated Nkx2.5 were cloned in pcDNA3 vector. The DuOx2 promoter was cotransfected with 0.02 µg of pRL renilla luciferase reporter vector to normalize luciferase activity in HEK 293T cells. *** p < 0.01 vs. ptx41. All transfections were done in triplicate at least twice.
We also investigated the role of the general coactivator TAZ on DuOx2 promoter since it cooperates with TTF-1 and PAX8, stimulating the Tg promoter [29]. DuOx2 promoter activity in the presence of TTF-1, PAX8 and TAZ was not different from the promoter activity evaluated in the presence of TTF-1 and PAX8 only [unpubl. data]. Interestingly, we found that TAZ significantly increased DuOx2 promoter transcriptional activity and the cotransfection of Nkx2.5 and TAZ produced a 15-fold increase in DuOx2 promoter activity when compared to basal transcriptional activity (fig. 6), thus suggesting that Nkx2.5 and TAZ act synergistically to increase DuOx2 promoter activity. Moreover, the cotransfection of TAZ and mutated Nkx2.5 showed the same effect as TAZ alone on DuOx2 promoter transcriptional activity (data not shown).
Discussion
In the present study, we explored basal functional activity of DuOx1 and −2 promoters and the regulation of DuOx2 promoters by hormones and TF in thyroid cells (PCCL3 cells) and in a heterologous system (HeLa and HEK 293T cells). Our results have shown that DuOx1 promoters exhibited higher luciferase activity than DuOx2 promoters, at least in PCCL3 cells. Indeed, DuOx1 mRNA is more abundant than DuOx2 mRNA in this cell type [33]. In HeLa cells, DuOx2 long promoter (ptx46) activity was higher than the short promoter (ptx41) activity, while in PCCL3 cells the short and long promoter activities were similar. It is likely that the TF found in PCCL3 cells might have interacted within the DuOx2 long promoter sequence and decreased its activity. We also demonstrated that human DuOx2 promoter activity is induced by TSH and forskolin in PCCL3. In fact, TSH, through the cAMP cascade, regulates H2O2 production, which is essential for thyroid hormone biosynthesis [5], in accordance with our data. A previous study using forskolin 10-5M suggested that human DuOx promoter is not positively controlled by cAMP in thyroid cells [3]. We assessed human DuOx2 promoter activity in the presence of 10 and 20 μM (data not shown) of forskolin. While the dose of 10 μM forskolin increased the promoter activity, the dose of 20 μM decreased it (data not shown). Therefore, it seems that modulation of DuOx2 promoter activity by cAMP depends on the dose of forskolin in PCCL3 cells. Previous reports have demonstrated that insulin and IGF-1 are able to stimulate Tg transcription [34] as well as TPO promoters [35]. Our results revealed that DuOx2 promoter activity was stimulated by insulin and IGF-1 and that there was synergism between TSH and IGF-1, which is interesting since it has been shown that both hormones stimulate thyroid function [34,35].
DREAM is expressed in the brain [22] and thyroid [23], where it is important to nociception and thyroid enlargement, and probably nodular development [23], respectively. In thyroid FRTL5 cells, DREAM seems to impair TTF-1 binding to the Tg promoter, blocking promoter transactivation [22] and raising the possibility of DREAM modulating other thyroid promoters regulated by TTF-1 such as DuOx2. Concerning the role of TF involved in the maintenance of thyroid differentiation phenotype, we have shown that CREB-increased DuOx2 promoter activity and, surprisingly, its cotransfection with the transcriptional repressor DREAM elicited a higher activation of DuOx2 promoter activity in HeLa cells. Thus, a possible interaction between CREB and DREAM in regulating the DuOx2 promoter cannot be discarded. As DuOx2 promoter activity was stimulated in the presence of both CREB and DREAM, the cAMP pathway might contribute to this induction. In fact, elevated levels of DREAM in human multinodular goiters have been shown [23], and it has been postulated that an imbalance between H2O2 production during thyroid hormone synthesis might be related to the high frequencies of mutagenesis in the thyroid gland, probably due to oxidative stress [36]. For this reason, we could speculate that the stimulatory effect of CREB in the presence of DREAM would increase DuOx2 expression and H2O2 generation, predisposing the gland to mutagenesis and multinodular goiter. Additional experiments, however, are needed to support this idea. PAX8 and TTF-1 are the most important TF involved in thyroid organogenesis [37] because they regulate thyroid genes such as Tg [15] and TPO [16]. Moreover, it has been shown that PAX8 induces DuOx2 promoter transcriptional activity [38]. Accordingly to our results, DuOx2 promoter is a possible target for PAX8 and TTF-1, as the cotransfection of both TF synergistically increased DuOx2 promoter activity. As Nkx2.5 stimulates Tg, TPO and NIS promoter activities [21,32], we were also interested in a possible activation of this TF on the DuOx2 promoter. Indeed, we have shown that Nkx2.5 induced DuOx2 activity, probably through its homeodomain since Nkx2.5 carrying a single missense mutation Ile to 183 Pro in the homeodomain, which preserves homodimerization function but totally abolishes DNA binding, did not change DuOx2 promoter basal activity. It is also possible that the activation of DuOx2 promoter by Nkx2.5 occurs through the same binding site of TTF-1 since Nkx2.5 share high homology to TTF-1, which also contains a conserved 23-amino-acid NK2-specific domain [21].
It has been shown that a combination of PAX8, TTF-1 with Foxe1 [16,39] or TAZ [19,29] stimulates Tg and TPO promoters. We have shown that the coactivator TAZ induced DuOx2 promoter activity almost in the same magnitude as Nkx2.5, and we have found an important synergism between TAZ and Nkx2.5, suggesting that TAZ could be a relevant partner to Nkx2.5 on DuOx2 regulation. We could raise a possible mechanism of control of DuOx2 expression in the thyroid by TAZ and Nkx2.5, which can be related to thyroid tumorigenesis as TAZ is overexpressed in thyroid papillary carcinoma and is involved in epithelial-mesenchymal transition, suggesting an important role in papillary thyroid carcinomas development [18]. Moreover, Nkx2.5 mRNA and TAZ protein are detectable in the TPC-1 (thyroid papillary carcinoma) cell line (unpubl. data) and Nkx2.5 is overexpressed in the anaplastic thyroid carcinoma cell line (unpubl. data), suggesting that they could also play a role in regulating DuOx2 expression in pathological conditions such as cancer.
To sum up, we have shown that the DuOx2 promoter is regulated by hormones important for normal thyroid function and growth [40], and by transcription factors involved in thyroid organogenesis, underlining the importance of the fine control of H2O2 generation for thyroid homeostasis.
Disclosure Statement
The authors have nothing to disclose
Supplementary Material
Supplementary data
Ackowledgements
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do estado do Rio de Janeiro (FAPERJ), Dirección General de Proyectos de Investigación (Grant SAF2013-44709-R) and TIRONET from the Comunidad de Madrid (Spain) (Grant S2011/BMD-2328).
References
- 1.De Deken X, Dantong W, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 2.Milenkovic M, De Deken X, Jin L, De Felice M, Di Lauro R, Dumont JE, Corvilain B, Miot F. DuOx expression and related H2O2 measurement in mouse thyroid: onset in embryonic development and regulation by TSH in adult. J Endocrinol. 2007;192:615–626. doi: 10.1677/JOE-06-0003. [DOI] [PubMed] [Google Scholar]
- 3.Pachucki J, Wang D, Christophe D, Miot F. Structural and functional characterization of the two human ThOX/Duox genes and their 5´-flanking regions. Mol Cell Endocrinol. 2004;214:53–62. doi: 10.1016/j.mce.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Dupuy C, Dème D, Kaniewski J, Pommier J, Virion A. Ca2+ regulation of thyroid NADPH-dependent H2O2 generation. FEBS Lett. 1988;233:74–78. doi: 10.1016/0014-5793(88)81358-9. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho DP, Dupuy C, Gorin Y, Legue O, Pommier J, Haye B, Virion A. The Ca2+ and reduced nicotidamide adenine dinucleotide phosphate-dependent H2O2 generation system is induced by thyrotropin in porcine thyroid cells. Endocrinology. 1996;137:1007–1012. doi: 10.1210/endo.137.3.8603567. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso LC, Martins DCL, Figueiredo MDL, Domingos MG, Vaisman M, Carvalho DP. Ca2+/nicotinamide adenine dinucleotide phosphate-dependent H2O2 generation is inhibited by iodide in human thyroids. J Clin Endocrinol Metab. 2001;86:4339–4343. doi: 10.1210/jcem.86.9.7823. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso LC, Martins DCL, Campos DVB, Santos LM, Costa VMC, Rosenthal D, Vaisman M, Violante AHD, Carvalho DP. Effect of iodide or iopanoic acid on thyroid Ca2+/NADPH-dependent H2O2 generating activity and thyroperoxidase in toxic diffuse goiters. Eur J Endocrinol. 2002;147:293–298. doi: 10.1530/eje.0.1470293. [DOI] [PubMed] [Google Scholar]
- 8.Morand S, Chaaraoui M, Kaniewski J, Dème D, Ohayon R, Noel-Hudson MS, Virion A, Dos Santos OF, Dupuy C. Identification of a truncated dual oxidase 2 (DUOX2) messenger ribonucleic acid (mRNA) in two rat thyroid cell lines. Insulin and forskolin regulation of DUOX2 mRNA levels in FRTL-5 cells and porcine thyrocytes. Endocrinology. 2003;144:567–574. doi: 10.1210/en.2002-220824. [DOI] [PubMed] [Google Scholar]
- 9.Figueiredo MDL, Cardoso LC, Ferreira ACF, Campos DVB, Domingos MG, Corbo R, Nasciutti LE, Vaisman M, Carvalho DP. Goiter and hyporthyroidism in two siblings due to impaired Ca2+/NADPH-dependent H2O2 generating activity. J Clin Endocrinol Metab. 2001;86:4843–4848. doi: 10.1210/jcem.86.10.7934. [DOI] [PubMed] [Google Scholar]
- 10.Moreno JC, Bikker H, Kempers MJ, van Trostsenburg AS, Bass F, de Vijlder JJ, Vulsma T, Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (Thox 2) and congenital hypothyroidism. N Engl J Med. 2002;347:95–102. doi: 10.1056/NEJMoa012752. [DOI] [PubMed] [Google Scholar]
- 11.Moreno JC, Visser TJ. New phenotypes in thyroid dyshormonogenesis: hypothyroidism due to DUOX2 mutations. Endocr Dev. 2007;10:99–117. doi: 10.1159/000106822. [DOI] [PubMed] [Google Scholar]
- 12.Deleu S, Pirson I, Coulonval K, Drouin A, Taton M, Clermont F, Roger PP, Nakamura T, Dumont JE, Maenhaut C. IGF-1 or insulin, and the TSH cyclic AMP cascade separately control dog and human thyroid cell growth and DNA synthesis, and complement each other in inducing mitogenesis. Mol Cell Endocrinol. 1999;149:41–51. doi: 10.1016/s0303-7207(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 13.Dumont JE, Lamy F, Roger P, Maenhaut C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev. 2002;72:667–697. doi: 10.1152/physrev.1992.72.3.667. [DOI] [PubMed] [Google Scholar]
- 14.Pasca Di Magliano M, Di Lauro R, Zannini M. Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci USA. 2000;97:13144–13149. doi: 10.1073/pnas.240336397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinoza CR, Schmitt TL, Loos U. Thyroid transcription factor 1 and Pax8 synergistically activate the promoter of the human thyroglobulin gene. J Mol Endocrinol. 2001;27:59–67. doi: 10.1677/jme.0.0270059. [DOI] [PubMed] [Google Scholar]
- 16.Di Palma T, Nitsch R, Mascia A, Nitsch L, Di Lauro R, Zannini M. The paired domain-containing factor Pax8 and the homeodomain-containing factor TTF-1 directly interact and synergistically activate transcription. J Biol Chem. 2003;278:3395–3402. doi: 10.1074/jbc.M205977200. [DOI] [PubMed] [Google Scholar]
- 17.Damante G, Tell G, Di Lauro R. A unique combination of transcription factors controls differentiation of thyroid cells. Prog Nucleic Acid Res Mol Biol. 2001;66:307–356. doi: 10.1016/s0079-6603(00)66033-6. [DOI] [PubMed] [Google Scholar]
- 18.de Cristofaro T, Di Palma T, Ferraro A, Corrado A, Lucci V, Franco R, Fusco A, Zannini M. TAZ/WWTR1 is overexpressed in papillary thyroid carcinoma. Eur J Cancer. 2011;7:926–933. doi: 10.1016/j.ejca.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CY, Schwartz RJ. Identification of novel DNA binding targets and regulatory domains of a murine tinman homeodomain factor, Nkx-2.5. J Biol Chem. 1995;270:15628–15633. doi: 10.1074/jbc.270.26.15628. [DOI] [PubMed] [Google Scholar]
- 21.Dentice M, Cordeddu V, Rosica A, Ferrara AM, Santarpia L, Salvatore D, Chiovato L, et al. Missense mutation in the transcription factor NKX2-5: a novel molecular event in the pathogenesis of thyroid dysgenesis. J Clin Endocrinol Metab. 2006;91:1428–1433. doi: 10.1210/jc.2005-1350. [DOI] [PubMed] [Google Scholar]
- 22.Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- 23.Rivas M, Mellström B, Torres B, Cali G, Ferrara AM, Terracciano D, Zannini M, Morreale de Escobar G, Naranjo JR. The DREAM protein is associated with thyroid enlargement and nodular development. Mol Endocrinol. 2009;23:862–870. doi: 10.1210/me.2008-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivas M, Mellström B, Naranjo JR, Santisteban P. Transcriptional repressor DREAM interacts with thyroid transcription factor-1 and regulates thyroglobulin gene expression. J Biol Chem. 2004;279:33114–33122. doi: 10.1074/jbc.M403526200. [DOI] [PubMed] [Google Scholar]
- 25.Pohlenz J, Dumitrescu A, Zundel D, Martiné U, Schönberger W, Koo E, Weiss RE, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109:469–73. doi: 10.1172/JCI14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilain C, Rydlewski C, Duprez L, Heinrichs C, Abramowicz M, Malvaux P, Renneboog B, et al. Autosomal dominant transmission of congenital thyroid hypoplasia due to loss-of-function mutation of PAX8. J Clin Endocrinol Metab. 2001;86:234–238. doi: 10.1210/jcem.86.1.7140. [DOI] [PubMed] [Google Scholar]
- 27.Kasahara H, Usheva A, Ueyama T, Aoki H, Horikoshi N, Izumo S. Characterization of homo- and heterodimerization of cardiac Csx/Nkx2.5 homeoprotein. J Biol Chem. 2001;276:4570–4580. doi: 10.1074/jbc.M004995200. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6:632–639. [PubMed] [Google Scholar]
- 29.Di Palma T, D'Andrea B, Liguorib GL, Liguorob A, De Cristofaroa T, Del Pretea D, Pappalardoa A, Masciaa A, Zannini M. TAZ is a coactivator for Pax8 and TTF-1, two transcription factors involved in thyroid differentiation. Exp Cell Res. 2009;315:162–175. doi: 10.1016/j.yexcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Ledo F, Kremer L, Mellström B, Naranjo JR. Ca2+-dependent block of CREB-CBP transcription by repressor DREAM. EMBO J. 2002;21:4583–4592. doi: 10.1093/emboj/cdf440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christophe-Hobertus C, Christophe D. Human thyroid oxidases genes promoter activity in thyrocytes does not appear to be functionally dependent on thyroid transcription factor-1 or Pax8. Mol Cell Endocrinol. 2007;264:157–163. doi: 10.1016/j.mce.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Dentice M, Luongo C, Elefante A, Romino R, Ambrosio R, Vitale M, Rossi G, Fenzi G, Salvatore D. Transcription factor Nkx-2.5 induces sodium/iodide symporter gene expression and participates in retinoic acid- and lactation-induced transcription in mammary cells. Mol Cell Biol. 2004;24:7863–7877. doi: 10.1128/MCB.24.18.7863-7877.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rigutto S, Hoste C, Dumont JE, Corvilain B, Miot F, De Deken X. DuOx1 is the main source of hydrogen peroxide in the rat thyroid cell line PCCl3. Exp Cell Res. 2007;313:3892–3901. doi: 10.1016/j.yexcr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Santisteban P, Acebrón A, Polycarpou-Schwarz M, Di Lauro R. Insulin and insulin-like growth factor I regulate a thyroid-specific nuclear protein that binds to the thyroglobulin promoter. Mol Endocrinol. 1992;6:1310–1317. doi: 10.1210/mend.6.8.1406708. [DOI] [PubMed] [Google Scholar]
- 35.Aza-Blanc P, Di Lauro R, Santisteban P. Identification of a cis-regulatory element and a thyroid-specific nuclear factor mediating the hormonal regulation of rat thyroid peroxidase promoter activity. Mol Endocrinol. 1993;7:1297–1306. doi: 10.1210/mend.7.10.8264661. [DOI] [PubMed] [Google Scholar]
- 36.Krohn K, Maier J, Paschke R. Mechanisms of disease: hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors. Nat Clin Pract Endocrinol Metab. 2007;3:713–720. doi: 10.1038/ncpendmet0621. [DOI] [PubMed] [Google Scholar]
- 37.De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev. 2004;25:722–746. doi: 10.1210/er.2003-0028. [DOI] [PubMed] [Google Scholar]
- 38.D'Andrea B, Iacone R, Di Palma T, Nitsch R, Baratta MG, Nitsch L, Di Lauro R, Zannini M. Functional inactivation of the transcription factor Pax8 through oligomerization chain reaction. Mol Endocrinol. 2006;20:1810–1824. doi: 10.1210/me.2005-0463. [DOI] [PubMed] [Google Scholar]
- 39.Fernández LP, López-Márquez A, Martínez AM, Gómez-López G, Santisteban P. New insights into FoxE1 functions: identification of direct FoxE1 targets in thyroid cells. PLoS One. 2013;8:e62849. doi: 10.1371/journal.pone.0062849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev. 2011;22:631–656. doi: 10.1210/edrv.22.5.0444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data