Abstract
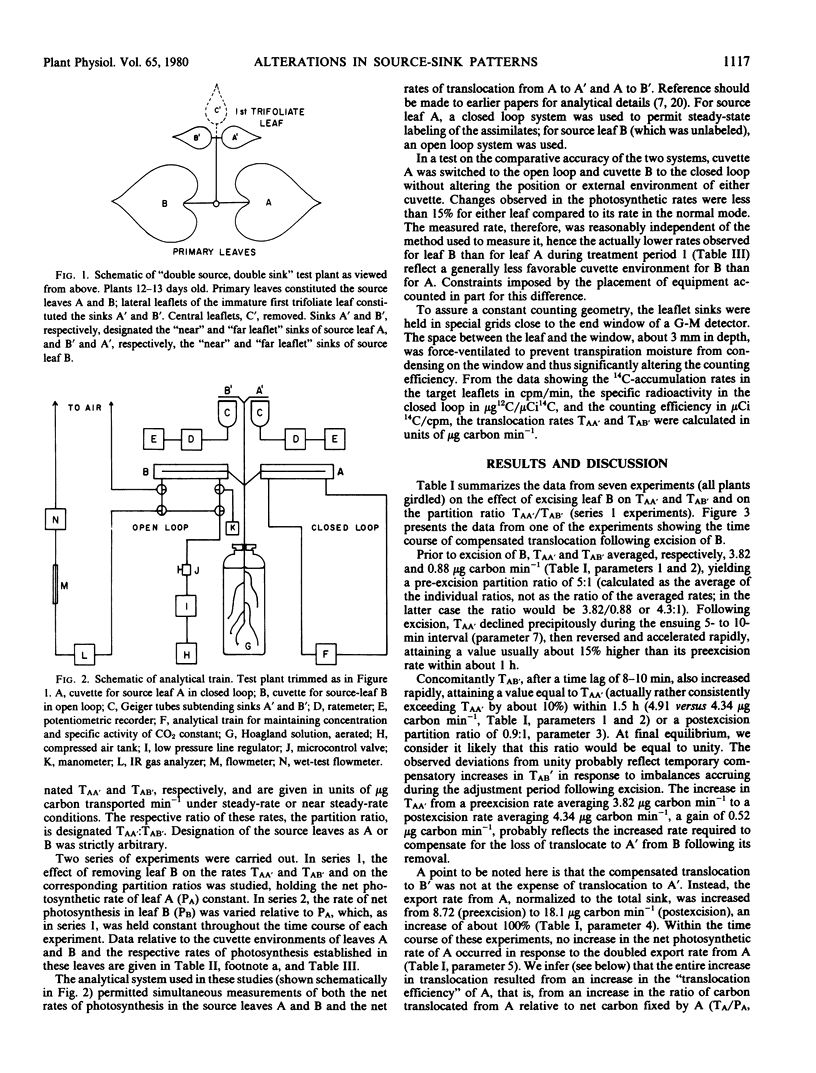
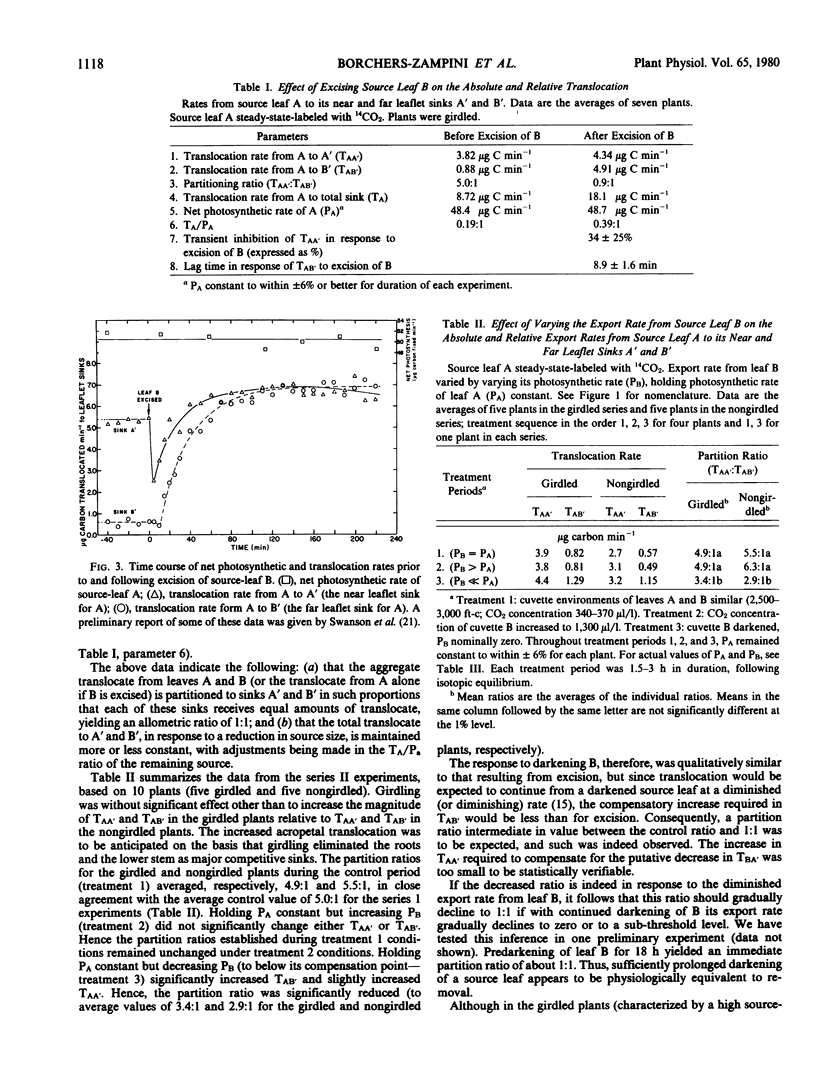
Bean plants, trimmed to a simplified “double source, double sink” translocation system (the paired primary leaves serving as the double source and the paired lateral leaflets of the immature first trifoliate leaf as the double sink) were used to study the magnitude and short-term time course of change in the allocation ratio (partition ratio) of assimilates translocated from the labeled primary leaf to its respective “near” and “far leaflet” sinks in response to an increase or decrease in the source strength of the opposite primary leaf (the “control” leaf). If the rates of net photosynthesis in the two primary leaves were similar, assimilates from the labeled source leaf partitioned to the leaflet sinks in the ratio of 5:1 or higher, the dominant sink being the leaflet “nearer” to the labeled source leaf. If the rate of net photosynthesis in the control leaf was increased substantially above that of the labeled source leaf, the rate of translocation from the labeled source to either the near leaflet sink or far leaflet sink remained unaffected, despite, presumably, a higher translocation rate from the control leaf, and hence a higher phloem pressure gradient (or increased cross-sectional area) in the transport pathway from the control leaf to the leaflet sinks. If the control leaf was excised, thus reducing the source leaf area by about a half, the translocation rate from the remaining source leaf rapidly doubled, the partition ratio becoming equal to unity. If the control leaf was darkened, the partition ratio adjusted to an intermediate value. Although export rates from the labeled source leaf were increased either by excising or darkening the control leaf, the rate of net photosynthesis in the labeled leaf remained constant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biddulph O., Cory R. Translocation of C Metabolites in the Phloem of the Bean Plant. Plant Physiol. 1965 Jan;40(1):119–129. doi: 10.1104/pp.40.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson C. L., Christy A. L., Cataldo D. A., Swanson C. A. Carbohydrate translocation in sugar beet petioles in relation to petiolar respiration and adenosine 5'-triphosphate. Plant Physiol. 1972 Jun;49(6):919–923. doi: 10.1104/pp.49.6.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Swanson C. A. Evaluation of Selected Parameters in a Sugar Beet Translocation System. Plant Physiol. 1965 Sep;40(5):942–947. doi: 10.1104/pp.40.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. E. TRANSLOCATION OF CARBOHYDRATES IN MAIZE. Science. 1945 Apr 20;101(2625):398–400. doi: 10.1126/science.101.2625.398. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., King R. W. Indirect Action of Benzyladenine and Other Chemicals on Flowering of Pharbitis nil Chois: Action by Interference with Assimilate Translocation from Induced Cotyledons. Plant Physiol. 1979 Apr;63(4):643–649. doi: 10.1104/pp.63.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C., Geiger D. R. Effects of light intensity and oxygen on photosynthesis and translocation in sugar beet. Plant Physiol. 1974 Oct;54(4):575–578. doi: 10.1104/pp.54.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson C. A., Hoddinott J. Effect of light and ontogenetic stage on sink strength in bean leaves. Plant Physiol. 1978 Sep;62(3):454–457. doi: 10.1104/pp.62.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]


