Summary
Background
Hematopoietic stem and progenitor cell (HPC) motility is essential for HPC transplantation. The chemokine CXCL12 is key for HPC motility. Further regulators are of interest to improve HPC transplantation and regenerative medicine. Here the impact of the human chemokine CCL15 on HPC motility was investigated.
Methods
CCL15 plasma concentrations were determined during HPC mobilization in humans. Activity of CCL15 on HPCs was investigated in murine assays, including chemotaxis, adhesion, and CFU-A assays, and competitive repopulation assays.
Results
During HPC mobilization with granulocyte colony-stimulating factor, blood plasma contains increased concentrations (1.1 ± 0.1 ng/ml) of activated CCL15(27–92) versus 0.4 ± 0.1 ng/ml in controls (p = 0.02). CCL15(27–92) significantly enhanced CXCL12-induced transwell migration of Lin-/Sca1+ HPCs and strengthened shear stress-dependent adhesion to vascular cell adhesion molecule-1 (VCAM-1). CCL15(27–92) dose-dependently reduced the colony size in CFU-A assays performed with murine bone marrow and Lin-/Sca1+ HPCs. CCL15(27–92) did not show a direct impact on cell cycle status of HPCs. In murine repopulation assays, pretreatment of bone marrow with CCL15(27–92) significantly increased competitive repopulation.
Conclusion
Our results point to a regulation of HPCs by CCL15 by modulating migratory and adhesive properties of HPCs with the potency to improve HPC short-term engraftment in stem cell transplantation.
Keywords: Hematopoietic progenitor cells, Cell cycle, Cell migration, Cell adhesion, Chemokines
Introduction
Today, hematopoietic stem and progenitor cells (HPCs) are used for transplantation of malignant and non-malignant disorders, in regenerative medicine, and in gene therapy. For the success of these therapies, the motility of HPCs is essential determining their migration to the bone marrow or to other tissues of interest. Thus, deciphering mechanisms that regulate HPC motility may be of interest to improve HPC transplantation and regenerative medicine [1, 2, 3].
It is well established that conditioning chemotherapy and repeated administration of granulocyte colony-stimulating factor (G-CSF) induce HPC mobilization. One important regulatory mechanism inducing this HPC mobilization is the activation of neutrophils and increased release of neutrophil proteases. These proteases were shown to shed and release membrane-bound stem cell factor (SCF), activate and degrade adhesion molecules such as VLA-4, P-, and E-selectins, and induce inactivation of the chemokine CXCL12 (stromal cell-derived factor-1; SDF-1) [4]. Cycles of activation, inactivation, and degradation of these bone marrow components by proteolytic enzymes, also including CD26 and various matrix metalloproteinases (MMPs), induce a clinically relevant HPC mobilization [5]. Inversely, homing and engraftment of HPCs to the bone marrow after transplantation is dependent on the residence of VLA-4, P-, and E-selectins on the cell surface and an intact CXCL12–CXCR4 signaling, which regulate adhesion of HPCs to endothelial cells and subsequent transendothelial migration [6]. Clearly CXCL12 plays a dominant role in the induction of migration, adhesion, and mobilization from bone marrow and engraftment of HPCs after transplantation [7, 8]. However, only few other chemokines, e.g. CXCL8 (IL-8) and CXCL2 (GRO-β) have so far been shown to impact HPC adhesion and migration, or mobilization from bone marrow into the blood [9]. It has been suggested that they may act indirectly by first stimulating mature cells in the bone marrow, which further interact to mobilize HPCs from bone marrow to blood [9].
We have previously described the isolation of the chemokine CCL15 (HCC-2, MIP-5, Lkn1) from human plasma, which is N-terminally processed and activated by the neutrophil proteases cathepsin G and elastase. This low-molecular-weight(LMW)-CCL15 form primarily acts on monocytes [10]. However, CCL15 and its murine orthologue CCL9 were recently found to be of significance for recruitment of CD34+ Gr-1-immature myeloid cells into colon cancer metastases, indicating that CCL15 also acts on HPCs [11, 12, 13, 14].
We here show that LMW-CCL15 plasma concentrations are increased in healthy stem cell donors and diseased patients treated with G-CSF. The impact of CCL15 was investigated in murine HPCs, building the basis for further investigation in different stem cell transplantation and regenerative medicine models with non-immunocompromised mice [15, 16, 17]. The N-terminally truncated form of CCL15 acts on HPCs by amplifying CXCL12-induced transwell migration and directly inducing integrin-mediated adhesion. Furthermore, we show that truncated but not full-length CCL15 modulates CFU-A colony formation and improves competitive repopulation of HPCs. Thus, CCL15 provides means to modulate adhesion/migration of HPCs with the potency to improve HPC regeneration in stem cell transplantation or regenerative medicine.
Material and Methods
Determination of Chromatographic Retention Time for CCL15 Immunoreactivity in Blood Plasma
The chromatographic retention time (tR) for CCL15 immunoreactivity (CCL15-IR) was determined as recently described [10]. Shortly, tR of CCL15-IR material was determined using a standard chromatography method. Chromatography was performed on a preparative reverse phase (RP) HPLC, 250 mm × 10 mm inner diameter (Source RPC15; Pharmacia, Stockholm, Sweden). The separations were performed at a flow rate of 2.5 ml/min using a linear binary gradient of 100% solvent A (0.1% v/v trifluoroacetic acid (TFA)) to 60% solvent B (100% acetonitrile and 0.1% v/v TFA) in 60 min and from 60% solvent B to 100% solvent B in 5 min. One fraction was collected each minute, starting at minute 20 of the chromatography. The fractions obtained were analyzed by CCL15 radioimmunoassay (RIA). CCL15-IR in human plasma was identified after chromatography of 1.5 ml of plasma. The lower detection limit of the CCL15 RIA was 5 pg/tube.
Peptide Synthesis
CCL15(27–92) was prepared by F-moc solid-phase peptide synthesis as described previously [18]. The purified products were characterized by HPLC, capillary zone electrophoresis, electrospray mass spectrometry (ESMS), and sequence analysis. The net peptide content was determined by amino acid analysis.
Migration and Adhesion Assays
For transmigration experiments, migration medium (Iscove's Modified Dulbecco's Medium (IMDM; Life Technologies) supplemented with 0.2% BSA, penicillin/streptomycin and variable concentrations of CXCL12) was added to the lower wells of 96-well chambers (NeuroProbe, Gaithersburg, MD, USA). Filters with a pore size of 5 μm were mounted, and the upper wells were filled with 20,000 Lin–/Sca1+ cells in migration medium in the presence or absence of CCL15. After 6 h in a humidified incubator at 37 ° C, assays were stopped by removing the medium from the upper wells and carefully removing the filters. Cells in the lower well were counted by determining the DNA amount using the CyQuant cell count assay (Molecular Probes; Life Technologies, Carlsbad, CA, USA). FDCP-mix HPCs were maintained in IMDM/20% horse serum substituted with 20 ng/ml rmIL-3 (R&D Systems, Wiesbaden, Germany). For adhesion assays, 105 FDCP-mix or freshly isolated bone marrow Lin– cells were allowed to rest for 3 min on a laminar flow chamber slide (μ-slide, ibiTreat; IBIDI Systems, Munich, Germany) mounted on an inverted microscope. Slides were pre-coated with 2 μg/ml rm Ig-VCAM-1 fusion protein (R&D Systems). Subsequently, HBSS/0.1% BSA (pre-warmed to 37 ° C) was flushed through the chambers at the indicated calculated shear stresses, with increases in steps between 0.35 and 15 dyn/cm2 every 30 s. Images were taken, and the adherent cells were counted in four fields for every condition.
Cell Isolation
For CFU-A assays, bone marrow was obtained from 8- to 12-week-old female B6D2F1 mice (Charles River Laboratories, Sulzfeld, Germany) that had been subcutaneously treated with 60 mg/kg phenylhydrazine at 7, 6, and 4 days prior to sacrifice. Lin– or Lin–/Sca1+ cells were prepared from bone marrow cells of 6- to 10-week-old C57BL/6 mice (Charles River Laboratories) treated with 150 mg/kg 5-Fluorouracil (5-FU) 5 days prior to sacrifice. Femurs were flushed with IMDM. Bone marrow cells were incubated with monoclonal antibodies against Mac-1, Gr-1, TER 119, B220, CD4 and CD8, and subsequently labeled with magnetic beads coupled to secondary anti-rat IgG antibody and passaged over MACS-type CS columns according to the manufacturer's instructions (Miltenyi Biotec, Bergisch-Gladbach, Germany). The Lin– enriched cells were stained with FITC-conjugated LY-6A/E monoclonal antibody E13–161.7 (BD PharmingenTM; BD Biosciences, Heidelberg, Germany), phycoerythrin counterstained using antibodies against Mac-1, Gr-1, TER 119, B220, CD4, and CD8, and sorted for Lin–/Sca1+ cells on FACSAria (BD Biosciences). Sca1+ cells represented 3–5% of Lin– cells; upon reanalysis, the purity of Lin–/Sca1+ cells was 87–92%.
CFU-A Assay
Aliquots of 5 × 104 bone marrow cells isolated from regenerating bone marrow were incubated in 0.3% agar suspended in IMDM supplemented with 10% fetal calf serum (FCS) (GE Healthcare Life Sciences HyClone Laboratories, South Logan, UT, USA), 250 pg/ml murine GM-CSF, and 100 U/ml human M-CSF (R&D Systems). CCL15(1–92) (Bachem Biochemica, Heidelberg, Germany), CCL15(27–92), or CCL3 (R&D Systems) were added to the cell suspensions before plating. In some experiments, Lin–/Sca1+ bone marrow cells purified from regenerating bone marrow were used. Culture dishes were incubated in a humidified atmosphere containing 5% CO2, 95% air for 11 days. Colonies were subsequently stained with 0.8 mmol/l p-iodonitrotetrazolium violet (Sigma, Munich, Germany), and colonies with an overall diameter equal to or greater than 2 mm were scored as CFU-A [19].
Cell Cycle Status
For cell cycle analysis bone marrow was obtained from 6 to 10-week-old female C57BL/6 mice that had been intraperitoneally treated with 150 mg/kg 5-FU 5 days prior to sacrifice. Bone marrow was obtained from 4 mice and pooled. Aliquots of 1 × 106 bone marrow cells were treated with either 1,000 ng/ml CCL15(1-92), CCL15(27–92), CCL3 or phosphate-buffered saline (PBS) as controls for 6 h at 37 ° C. Subsequently cells were washed with PBS/0.5% BSA. The bone marrow cells were stained with PeCy7-CD177 (clone: ACK2; eBioscience, San Diego, CA, USA) and PE-Ly-6A/E (clone: E13–161.7, BD Biosciences), and fixed and permabilized with Fix/Perm Diluent/Fix Perm Concentrate (3: 1; eBioscience). Next, cells were stained with eFluor450-Ki-67 (clone: SolA15, eBioscience), washed, and finally stained with APC-lineage Antibody Cocktail (BD Biosciences). After RNase digestion (25 μg RNaseA/ml permeabilization buffer (eBioscience), 500 μl/sample, 37 °C, 30 min) cells were stained with 7-AAD for 10 min and immediately measured by FACS.
Competitive Hematopoietic Repopulation in Mice
Bone marrow cell suspensions were prepared from C57BL/6 CD45.1 and CD45.2 mice (Charles River Laboratories) as single cell suspensions by rinsing through 23G needles. Freshly isolated CD45.1 bone marrow cells were suspended at 5 × 106/ml in IMDM/10% FCS and pre-treated with 3,000 ng/ml CCL15(1–92), CCL15(27–92), or PBS as controls for 30 min, washed twice by centrifugation in PBS, and counted. 2 × 106 cells were injected intravenously into 8.5 Gy pre-irradiated BL6 CD45.2 recipient mice, together with 5 × 106 freshly isolated but untreated CD45.2 bone marrow cells. Regeneration of CD45.1 and CD45.2 repopulating cells was determined 4–5 weeks later by flow-cytometric analysis of bone marrow cell suspension using phycoerythrin-conjugated anti-Ly 5.1 (clone A20) and FITC-conjugated anti-Ly5.2 (clone 104) antibodies (BD Biosciences).
Statistical Analysis
Data were compared by the two sided, paired Student's t test, and p values < 0.05 were considered to be significant.
Results
CCL15 Plasma Concentrations in G-CSF-Treated Stem Cell Donors
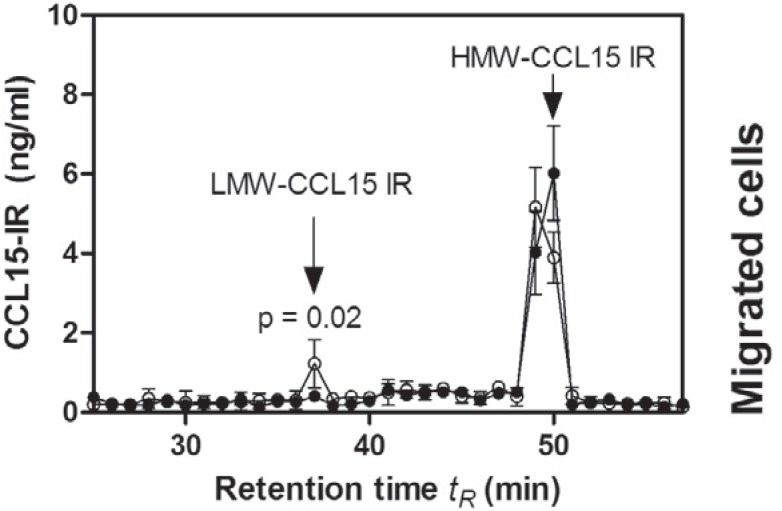
To differentiate CCL15(1-92) and N-terminally truncated CCL15 isoforms in blood, a standardized RP chromatographic method was used [10]. The CCL15-IR elution profile from plasma of G-CSF-treated stem cell donors obtained during leukapheresis revealed a peak with a tR of 37 min and a peak with a tR of 49–50 min. The tR of resynthesized CCL15(27–92), CCL15(24–92) and CCL15(1–92) were 36, 38 and 51 min, respectively (fig. 1). Recently, we could show that CCL15-IR detected with a tR of 37 min represents N-terminally truncated CCL15 isoforms (LMW-CCL15-IR), whereas CCL15-IR with a tR of 51 min represents CCL15(1–92) (high-molecular-weight CCL15-IR (HMW-CCL15-IR)) [10, 20]. This strongly suggests that early eluted CCL15-IR material is proteolytically processed CCL15. The detected plasma concentration of HMW-CCL15 is 9,2 ± 0,9 ng/ml in G-CSF-treated patients, 8,8 ± 0,5 ng/ml in G-CSF-treated stem cell donors, and 10,0 ± 2,2 ng/ml in untreated volunteers. The plasma concentration of LMW-CCL15 in G-CSF-treated stem cell donors is 1.1 ± 0.1 ng/ml, and 0.4 ± 0.1 ng/ml in untreated volunteers (p = 0.02).
Fig. 1.
CCL15-IR detected in plasma of G-CSF-treated peripheral stem cell donors obtained during leukapheresis. Elution profile of CCL15 in blood plasma of peripheral stem cell donors treated with G-CSF (O) or plasma from healthy volunteer donors (•) using the standardized RP-chromatography described in Material and Methods. The determined elution positions of LMW-CCL15 and HMW-CCL15 are indicated by arrows. Data from one of three experiments were pooled. Significance was calculated with an unpaired, two-sided t-test.
CCL15 Increases Migration and Adhesion of HPCs
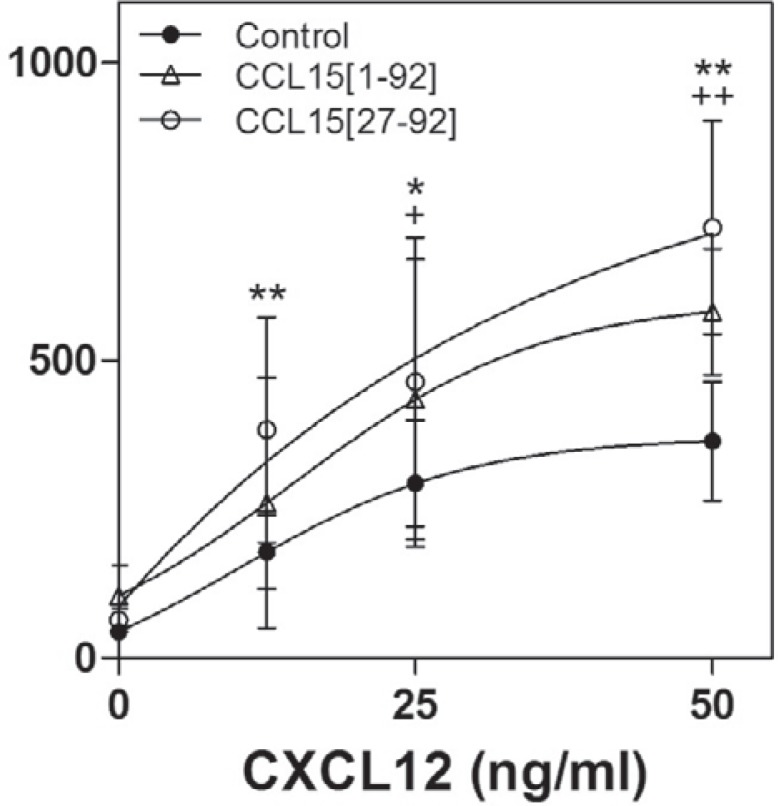
To date, CXCL12 has been described as the main potent inducer of HPC migration and adhesion. When CCL15(1–92) or CCL15(27–92) was placed into the upper wells of a transwell chamber, CXCL12-induced transmigration of murine Lin–/Sca1+ HPCs was significantly enhanced (fig. 2), indicating that both CCL15(1–92) and CCL15(27–92) amplify the CXCL12-induced HPC migration. CCL15(27–92) revealed a higher efficacy to increase the CXCL12-induced transmigration (increase of 91 ± 24%) compared to CCL15(1–92) (increase of 51 ± 5%).
Fig. 2.
The effect of CCL15 on HPC migration in transwell chambers. 20,000 Lin-/Sca1+ cells were placed in upper wells of migration chambers, and their transmigration capacity was assessed against a gradient of CXCL12 in the lower wells. CCL15(1-92) or CCL15(27–92) were added at 1,000 ng/ml to both wells. Statistical significance of CCL15(27–92) versus control: ** p < 0.01; * p < 0.05; CCL15(1–92) versus control: + p < 0.05; ++ p < 0.01. The results of 3 independent experiments were pooled. Each concentration was tested in triplicate in each experiment. The values shown represent the means ± SD.
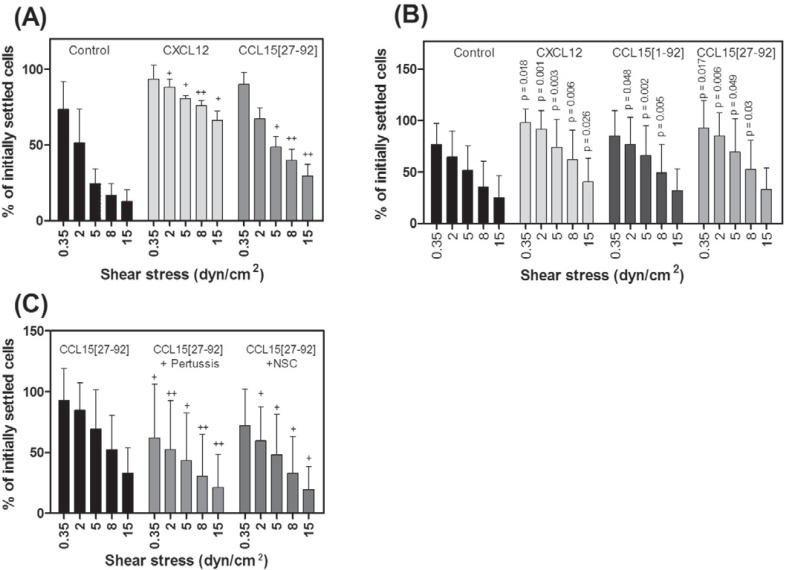
CXCL12 has been ascribed a role in inducing shear stress-dependent adhesion of HPCs to integrin ligands [21]. We therefore asked whether CCL15 could also stimulate adhesion of HPCs to the integrin ligand VCAM-1. We employed a shear stress-dependent adhesion assay using a parallel plate flow chamber on immobilized VCAM-1-Fc, the ligand for the HPC homing molecule VLA-4/integrin α4β1. FDCP-mix cells or bone marrow-derived HPCs were allowed to settle on VCAM-1 for 3 min in the presence or absence of immobilized chemokines CXCL12 or CCL15(27–92). Then, stepwise increasing shear forces were applied. As shown in figure 3A and B, CXCL12 effectively strengthened adhesion of both FDCP-mix and Lin– bone marrow HPCs to VCAM-1. Although somewhat less effective, CCL15(27–92) also significantly strengthened VCAM-1-induced adhesion on Lin– bone marrow HPCs and on FDCP-mix cells. Also CCL15(1–92) improved the adhesion of Lin– bone marrow HPCs to VCAM-1. A global comparison of adhesion strengthening, considering the results for all shear stresses, showed a significantly increased adhesion of HPCs induced by CXCL12, CCL15(27–92), and CCL15(1–92) by 49.5 ± 16.5%, 33.3 ± 8.7%, and 24.5 ± 9.3%, respectively (control vs. CXCL12: p < 0.0001; control vs. CCL15(27–92): p < 0.0001; control vs. CCL15(1–92): p < 0.0001). In addition, on FDCP-mix cells global comparison revealed a significant increase of adhesion strengthening induced by CXCL12 (45.1 ± 14.8%; p < 0.0001) and CCL15(27–92) (19.2 ± 3.6%; p < 0.0001). For CXCL12, a significant increase of adherent Lin– HPCs was found at almost all tested shear forces, whereas CCL15(27–92) significantly strengthened adhesion of HPCs at low shear force rates up to 8 dyn/cm2 and revealed a tendency to increase VCAM-1-induced adhesion at 15 dyn/cm2 (p = 0.09).
Fig. 3.
Influence of CCL15 on HPC adhesion strength. FDCP-mix A or freshly isolated Lin– bone marrow HPCs B, C were allowed to settle on immobilized VCAM-1 with or without chemokines for 3 min, before defined shear stress was applied, increasing stepwise every 30 s, as indicated. The percentages of remaining attached cells are given (100% correspond to initially settled cells). A Induction of adhesion in FDCP Mix HPCs or B in Lin– bone marrow cells by CXCL12, CCL15(27–92), or CCL15(1–92), (chemokine vs. control: + p < 0.05; ++ p < 0.01). C HPCs were pretreated with 200 μg/ml Bordetella Pertussis Toxin (Pertussis) or 100 μmol/l Rac inhibitor NSC 23766 for 2 h. Subsequently cells to immobilized to VCAM-1 plus CCL15(27–92) (CCL15(27–92) vs.CCL15(27–92) + Pertussis toxin + p < 0.05; ++ p < 0.01; CCL15(27–92) vs.CCL15(27–92) + NSC 23766: + p < 0.05; ++ p < 0.01). Results from 3 different experiments were pooled. In each experiment 4 microscopic fields were scored for each step of shear stress. Values are means ± SD.
The pre-incubation of HPCs with pertussis toxin, which is known to disrupt Gαi-dependent signaling, suppressed the adhesion induced by CCL15(27–92), as did pre-incubation of HPCs with NSC23766, an inhibitor of the Rac small GTPases (fig. 3C). Taken together, these data show that CCL15 induces shear stress-dependent adhesion of HPCs, and suggest that a Gαi-mediated, Rac-dependent signaling process is involved in CCL15(27–92)-induced integrin activation and shear stress-resistant adhesion in HPC.
CCL15(27–92) Modulates CFU-A Colony Formation
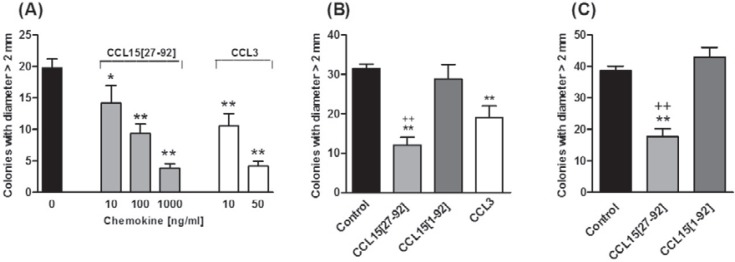
Since the structurally related chemokine CCL3 was shown to modulate CFU-A colony formation, we tested CCL15 in the CFU-A from murine whole bone marrow and Lin-/Sca1+ enriched bone marrow cells [21]. Pre-incubation with either CCL3 or CCL15(27–92) dose-dependently suppressed the formation of >2 mm colonies from whole bone marrow (fig. 4A). As shown in Fig. 4B, CCL15(27–92), but not its full-length form CCL15(1–92), potently reduced CFU-A colony growth from whole bone marrow. Similarly, CCL15(27–92), but not CCL15(1–92), also suppressed CFU-A colony formation from Lin-/Sca1+ enriched bone marrow cells (fig. 4C).
Fig. 4.
The effect of CCL15 on CFU-A colony formation. CFU-A assays were performed with murine bone marrow cells in the presence of 250 pg/ml mouse GM-CSF and 100 U/ml human M-CSF. A CFU-A colony formation (> 2 mm diameter) in the presence of various concentrations of CCL15(27–92) or CCL3 after seeding 50,000 whole bone marrow cells. B CFU-A colony formation in the presence of 1,000 ng/ml CCL15(27–92), its precursor, 1,000 ng/ml CCL15(1–92) after seeding 50,000 whole bone marrow cells and C after seeding of 5,000 Lin–/Sca1+ enriched cells. CCL3 was added at 10 ng/ml in (B). Each bar represents the mean ± SD of triplicate determinations. Data from one of three representative experiments are shown. * p < 0.05 vs. control, ** p < 0.01 vs. control; ++ p < 0.01 vs. CCL15(1–92).
Cell Cycle Analysis
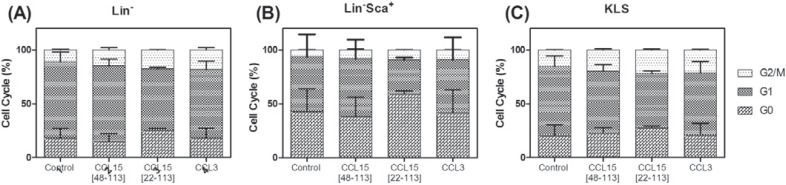
The impact of CCL15(1–92) and CCL15(27–92) on the cell cycle of murine HPCs was tested with regenerating bone marrow of female C57BL/6 mice that had been subcutaneously treated with 150 mg/kg 5-FU. In the performed experiments, Lin– HPCs, the Lin–/Sca1+, and the KLS cells did not reveal a reduced cell cycling activity after treatment with CCL15(1–92) and CCL15(27–92) (fig. 5).
Fig. 5.
CCL15(1–92) and CCL15(27–92) and cell cycle of Lin–, Lin–/Sca1+, and KLS cells. Treatment of regenerating bone marrow from C57BL/6 mice with CCL15(1–92), CCL15(27–92), or CCL3 revealed no change in cell cycle of Lin– or KLS cells. Bone marrow was treated with 1,000 ng/ml CCL15(1–92), 1,000 ng/ml CCL15(27–92), or 1,000 ng/ml CCL3 for 6 h, and subsequently the cell cycle status of (A) Lin–, Lin–/Sca1+, and (C) KLS cells was analyzed.
Induction of Increased Donor-Type Hematopoietic Repopulation in Mice by Pre-Incubation of Bone Marrow Cells with CCL15
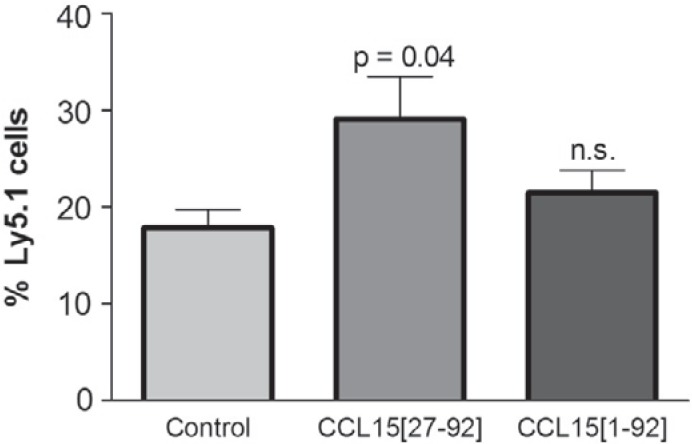
The ability of CCL15(27–92) to modulate SDF-1/CXCL12-dependent migration of HPCs, and to induce integrin-mediated adhesion led us to test for its ability to improve regeneration behavior of transplanted HPCs after pre-treatment with CCL15(27–92). Bone marrow cells of Ly5.1 mice were exposed to both CCL15 isoforms for 30 min and intravenously transplanted with congenic Ly5.2 competitor cells. Three weeks later, regeneration of donor and recipient cells was analyzed by flow cytometry. This revealed a significant, approximately 50% increased content of Ly5.1 cells derived from the CCL15(27–92)-exposed cells compared to controls (fig. 6). CCL15(1–92) only partly induced this effect, which did not reach statistical significance.
Fig. 6.
Effect of CCL15 on hematopoietic repopulation in mice. Bone marrow cells from Ly5.1 mice (5 × 106) were pre-treated with the indicated CCL15 isoforms for 30 min or not in IMDM/10% FCS, washed by centrifugation, admixed with a threefold excess of untreated Ly5.2 cells and transplanted into 8.5 Gy pre-irradiated Ly5.2 recipient mice in groups of 3–5 mice. Mice were sacrificed 3–4 weeks later, and bone marrow cells were analyzed by flow cytometry for expression of Ly5.1 and Ly5.2. Values are means ± SD from three experiments with a total of 8 (controls with untreated bone marrow cells), 9 (CCL15(1–92)-treated bone marrow cells) and 11 mice (CCL15(27–92)-treated bone marrow cells). *p < 0.05 versus control.
Taken together, our results show a significant impact of the truncated form of CCL15, CCL15(27–92) in both in vitro and in vivo behavior of HPCs.
Discussion
Our study shows increased plasma concentrations of LMW-CCL15 in stem cell donors treated with G-CSF. The presence of activated CCL15 in blood plasma of stem cell donors is in accordance with the increased activation of neutrophils induced by the G-CSF treatment. G-CSF treatment strongly induces proliferation and activation of neutrophils with the release of the serine-proteases elastase and cathepsin G [22, 23], which were shown to activate CCL15 [10]. In recent investigations, we could demonstrate that LMW-CCL15 represents N-terminally truncated CCL15 isoforms with an increased potency to induce adhesion and chemotactic migration of monocytes. In the standardized chromatographic method [10], LMW-CCL15 in plasma of the G-CSF-treated stem cell donors and patients eluted with a tR of 37 min, which resides between the elution time of CCL15(27–92) (tR of 36 min) and CCL15(24–92) (tR of 38 min). This divergence may be correlated to additional C- and N-terminally processed CCL15 forms, taking place in the presence of neutrophils. In a recent study we could show that, in addition, neutrophils generate CCL15(22–92), CCL15(24–91), CCL15(25–92), and CCL15(29–92). These different isoforms slightly vary in the hydrophobic and hydrophilic amino acid composition, which may explain the divergence in the elution time. Importantly, all the identified resynthesized CCL15 isoforms generated by neutrophils as well as neutrophil supernatants incubated with CCL15(1–92) were shown to generate calcium flux and chemotactic migration of monocytes and CCR1-trans-fected cell lines [10, 24], suggesting that processing of CCL15 by neutrophils in vivo will cause an activation of CCL15 and is biologically relevant [10, 25].
Although the concentration of activated LMW-CCL15 in plasma does not reach functionally active concentrations, local concentrations at the endothelium may be significantly higher. Our recent results suggest that activation of CCL15 is a quantum proteolytic event taking place in the close proximity of activated neutrophils which may cause biological relevant CCL15 concentrations before the active CCL15 is diluted in the blood plasma and eliminated due to a short half-life, which was suggested for CCL15 and for other chemokines [20, 26, 27].
Functional analysis CCL15 was performed with murine HPCs, in order to build the basis for further investigations in different stem cell transplantation and regenerative medicine models with non-immunocompromised mice [15, 16, 17]. There are a number of facts indicating that mouse models are sufficient to perform an initial preclinical evaluation of CCL15. In mice, the orthologue for CCL15 is CCL9. Both chemokines reveal structural and functional similarities. Both CCL9 and CCL15 possess an additional N-terminal domain of 16–20 amino acids and two additional precisely positioned cysteines that form a third disulfide bridge. They are ligands for the CCR1 receptor and circulate in blood plasma, and proteolytic processing of the N-terminus of both chemokines significantly increases their biological potency [14, 20, 25]. On HPCs both were shown to act on immature myeloid cells [11, 28, 29].
To investigate if activated CCL15 affects HPCs, we first assessed the role of CCL15 in regulating adhesion and migration. Few chemokines have been described to induce robust migration and adhesion responses in HPCs. Most of these results have been obtained with CXCL12, which acts mainly through the CXCR4 receptor. However, mature monocytes or granulocytes can be induced to migrate in chemotaxis assays by several other chemokines including CCL15 (monocytes) or IL-8 family chemokines (granulocytes). We found that CCL15 acts on HPCs, in which it can directly activate adhesion in approximately half of the cells that are recruited by an optimal concentration of CXCL12. The difference in CXCL12- and CCL15(27–92)-induced adhesion strengthening and the predominant increase of adhesion strengthening at low shear force rates induced by CCL15(27-92) may be correlated to a different expression of the corresponding receptors CXCR4 (CXCL12) and CCR1 or CCR3 (CCL15), with a dominant mRNA expression found for CXCR4 in human and murine HSC [30]. In addition, CCR1, CCR3, and CXCR4 are expressed on different HPC populations. In humans, high CXCR4 expression is found in Lin– cells and in the CD34+/CD38–/Lin– and CD34–/CD38–/Lin– subpopulations [31, 32] which are enriched for cells with repopulating ability, demonstrating that CXCL12 is important for primitive hematopoietic cells [33, 34, 35, 36, 37]. CCR3 is expressed in CD34+ progenitors which develop into eosinophils and support the migration of CD34+ progenitors to the site of allergic inflammation [38, 39, 40]. CCR1 is expressed in approximately 30% of human CD34+ bone marrow cells [41], whereby granulocyte/macrophage progenitors are primarily positive for CCR1, [42, 43]. But CD34+/CCR1+ cells also reveal the capacity to induce high levels of engraftment in NOD/SCID mice, indicating that CCR1+ HPCs also contain more primitive HPCs [33, 44, 45, 46].
Adhesion activation by CCL15 is mediated by Gαi, since pretreatment with pertussis toxin strongly interfered with the ability of CCL15 to induce increased HPC adhesion. Similar findings have been made for substances that act through membrane-bound G protein-coupled receptors. The observed inhibition of CCL15-induced HPC adhesion by the Rac-specific inhibitor NSC23766 suggests that CCL15(27–92) acts by stimulating the Rac GTPase pathway, which has been shown to play a key role not only in CXCL12-induced migration and CXCL12-mediated adhesion of HPCs [47, 48].
In contrast to our adhesion assay results, where CCL15 acted alone, we did not detect activation of chemotactic migration of HPCs by CCL15 alone. Since a cooperation of CCR1 and CXCR4 ligands has been shown in monocyte chemotaxis [49], we tested whether CCL15 increases CXCL12-induced chemotaxis of HPCs. In these assays we could clearly show that CCL15 amplifies the CXCL12-mediated migration of highly purified Lin–/Sca1+ HPCs, pointing to a direct effect of CCL15 on these cells. Notably, both CCL15(27–92) and CCL15(1–92) revealed a significant cooperation with CXCL12 in the chemotaxis assays, whereby CCL15(27–92) displayed a tendency for a stronger CXCL12-cooperating activity. This finding is in accordance with chemotaxis assays performed with monocytes, in which CCL15(1–92) revealed a significant chemotactic activity, whereas this isoform revealed only a marginal calcium mobilization activity [10].
Next, we investigated the impact of CCL15 on colony growth in the CFU-A assay, an assay which was shown to be modulated by CCL3. We found that CCL15(27–92) significantly reduces the formation of macroscopic colonies with diameters > 2 mm (CFU-A colonies), whereas the growth of smaller colonies was not affected by CCL15. It was shown by Pragnell et al. [21, 50] that CFU-A colonies are formed by more primitive HPCs which reveal the capacity to repopulate the bone marrow of lethally irradiated mice. This reduced growth of colonies with a diameter > 2 mm may be correlated with altered adhesion and migration properties of the cells forming the colonies, and with inhibition of their cell cycling. Although HPC cell cycling inhibition by CCL15 with myeloprotective potential was postulated by Kim et al. [28] who intavenously injected CCL15 into mice, we could not detect inhibition of cell cycling by CCL15 in liquid cultures. The result in liquid culture is also contradictory to own results obtained with CFU-A assays and CFU-S assays using cell cycle blockade by cytosine arabinoside in the presence and absence of CCL15 isoforms (data not shown). However, recent research demonstrates that HPCs are maintained in niches, suggesting that direct cell contact is of importance to regulate stem cell proliferation and to protect from differentiation and from loss of stemness by induction of quiescence in a process regulated by CXCL12 [51]. HPCs leaving the niche enter into the transitional amplifying pool of committed progenitors [52]. Thus, it is possible that in the animal models, and in the in vitro assays, the cell cycle is modulated secondary to changed adhesion mechanisms and due to cell-cell contacts.
Although the CFU-A assay does not allow to differentiate into further colony formations other studies showed growth suppression of granulocyte-erythroid-megakaryocyte-macrophage (CFUGEMM) colonies, granulocyte-macrophage (CFU-GM) colonies, and erythroid (BFU-E) colonies from multipotential cord blood CD34+ cells as well as human bone marrow cells [53, 54]. These results are in accordance with our results on CFU-C assays performed with CD34+ human bone marrow cells. We identified a 80% reduction of CFU-C (CFU-M, CFU-GM, CFU-G) in the presence of CCL15(27–92) (data not shown) which is in accordance with results in our CFU-A assays. CFU-A colony growth is dependent on the combination of the cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF): M-CSF regulates the survival, proliferation, and differentiation of mononuclear phagocytic cells and is the primary regulator of mononuclear phagocyte production [55, 56], and picogram concentrations of GM-CSF enhance the responsiveness of HPCs to M-CSF [57], indicating that CFU-A cells are derived from HPCs which primarily develop into mononuclear phagocytic cells. However, the combination of the cytokines M-CSF and GM-CSF was shown to support to grow colonies from more primitive HPCs with increased replating efficacy of HPCs [21, 58], also suggesting that CCL15 regulates more primitive HPCs.
The results of the CFU-A assays show that relatively higher concentrations of CCL15(27-92) are needed to achieve activity comparable to that of CCL3, but that CCL15(27-92) suppresses CFU-A colony formation to the same degree as CCL3. This could be attributed to different affinities of CCL15(27–92) and CCL3 to the murine CCR1 or CCR3 receptors, or to the ability of CCL3 to stimulate more chemokine receptors at the same time, e.g., both chemokines activate the CCR1 and CCR3 receptors, but only CCL3 stimulates CCR4 and CCR5 [59, 60].
To our knowledge, this is the first report showing that activation of CCL15 takes place during human stem cell mobilization and that CCL15 modulates HPC adhesion and migration as well as growth of CFU-A colonies in vitro, and reveal the capacity to improve regeneration of HPCs. In this context, the proteolytic activation of CCL15 by neutrophil serine proteases, which we have described previously, could lead to enhancement of CXCL12-directed migration and the strengthening of HPC adhesion to endothelial ligands such as VCAM-1, which have all been shown to be centrally involved in the regulation of HPC mobilization. Thus, CCL15 provides means to modulate adhesion/migration of HPCs with the potency to improve HPC regeneration in stem cell transplantation and regenerative medicine. Further investigations are warranted to evaluate if CCL15 acts on primitive, long-term regenerating HPCs or exclusively improves regeneration of lineage-specific progenitors.
Disclosure Statement
The authors declared no conflict of interest.
Acknowledgements
We gratefully acknowledge the excellent technical assistance of V. Lang and S. Kummerow. This work has been supported by research grants from the Deutsche Forschungsgemeinschaft SFB T/R23, the Bundesministerium for Bildung und Forschung (BMBF) 0312683 and the DFG Excellence Cluster Cardio-Pulmonary System (ECCPS).
References
- 1.Hoggatt J, Mohammad KS, Singh P, Hoggatt AF, Chitteti BR, Speth JM, Hu P, Poteat BA, Stilger KN, Ferraro F, Silberstein L, Wong FK, Farag SS, Czader M, Milne GL, Breyer RM, Serezani CH, Scadden DT, Guise TA, Srour EF, Pelus LM. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature. 2013;495:365–369. doi: 10.1038/nature11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorrance AM, De VS, Radu M, Reddy PN, McGuinness MK, Harris CE, Mathieu R, Lane SW, Kosoff R, Milsom MD, Chernoff J, Williams DA. The Rac GTPase effector p21-activated kinase is essential for hematopoietic stem/progenitor cell migration and engraftment. Blood. 2013;121:2474–2482. doi: 10.1182/blood-2012-10-460709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SY, Wolfram P, Canty K, Harley B, Nombela-Arrieta C, Pivarnik G, Manis J, Beggs HE, Silberstein LE. Focal adhesion kinase regulates the localization and retention of pro-B cells in bone marrow microenvironments. J Immunol. 2013;190:1094–1102. doi: 10.4049/jimmunol.1202639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapid K, Glait-Santar C, Gur-Cohen S, Canaani J, Kollet O, Lapidot T. Egress and mobilization of hematopoietic stem and progenitor cells: a dynamic multifacet process; in StemBook. Cambridge (MA), Harvard Stem Cell Institute, 2013. www.stembook.org/node/762. [PubMed] [Google Scholar]
- 5.Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. Mobilization and homing of hematopoietic stem cells. Adv Exp Med Biol. 2012;741:152–170. doi: 10.1007/978-1-4614-2098-9_11. [DOI] [PubMed] [Google Scholar]
- 6.Sahin AO, Buitenhuis M. Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adh Migr. 2012;6:39–48. doi: 10.4161/cam.18975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 9.Pelus LM, Fukuda S. Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp Hematol. 2006;34:1010–1020. doi: 10.1016/j.exphem.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Richter R, Bistrian R, Escher S, Forssmann W-G, Vakili J, Henschler R, Spodsberg N, Frimpong-Boateng A, Forssmann U. Quantum proteolytic activation of chemokine CCL15 by neutrophil granulocytes modulates mononuclear cell adhesiveness. J Immunol. 2005;175:1599–1608. doi: 10.4049/jimmunol.175.3.1599. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura T, Fujishita T, Loetscher P, Revesz L, Hashida H, Kizaka-Kondoh S, Aoki M, Taketo MM. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc Natl Acad Sci U S A. 2010;107:13063–13068. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Bacon KB, Oldham ER, Schall TJ. Molecular cloning and functional characterization of human MIP-1 delta, a new C-C chemokine related to mouse CCF-18 and C10. J Clin Immunol. 1998;18:214–222. doi: 10.1023/a:1020535106684. [DOI] [PubMed] [Google Scholar]
- 13.Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- 14.Forssmann U, Magert HJ, Adermann K, Escher SE, Forssmann WG. Hemofiltrate CC chemokines with unique biochemical properties: HCC-1/CCL14a and HCC-2/CCL15. J Leukoc Biol. 2001;70:357–366. [PubMed] [Google Scholar]
- 15.Cheng L, Ramesh AV, Flesken-Nikitin A, Choi J, Nikitin AY. Mouse models for cancer stem cell research. Toxicol Pathol. 2010;38:62–71. doi: 10.1177/0192623309354109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nevozhay D, Opolski A. Key factors in experimental mouse hematopoietic stem cell transplantation. Arch Immunol Ther Exp (Warsz) 2006;54:253–269. doi: 10.1007/s00005-006-0030-2. [DOI] [PubMed] [Google Scholar]
- 17.Del CC, Campos de Carvalho AC. Cell therapy in dilated cardiomyopathy: from animal models to clinical trials. Braz J Med Biol Res. 2011;44:388–393. doi: 10.1590/S0100-879X2011007500035. [DOI] [PubMed] [Google Scholar]
- 18.Escher SE, Sticht H, Forssmann WG, Rosch P, Adermann K. Synthesis and characterization of the human CC chemokine HCC-2. J Pept Res. 1999;54:505–513. doi: 10.1034/j.1399-3011.1999.00125.x. [DOI] [PubMed] [Google Scholar]
- 19.Graham GJ, Wright EG, Hewick R, Wolpe SD, Wilkie NM, Donaldson D, Lorimore S, Pragnell IB. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature. 1990;344:442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- 20.Richter R, Forssmann U, Henschler R, Escher S, Frimpong-Boateng A, Forssmann WG. Increase of expression and activation of chemokine CCL15 in chronic renal failure. Biochem Biophys Res Commun. 2006;345:1504–1512. doi: 10.1016/j.bbrc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 21.Pragnell IB, Wright EG, Lorimore SA, Adam J, Rosendaal M, DeLamarter JF, Freshney M, Eckmann L, Sproul A, Wilkie N. The effect of stem cell proliferation regulators demonstrated with an in vitro assay. Blood. 1988;72:196–201. [PubMed] [Google Scholar]
- 22.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 23.Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 24.Lee JK, Kim HS, Im SA, Kim K, Kwon BS, Lee CK. Identification of NH(2)-terminal amino acid residues essential for the biological activity of leukotactin-1. Immunol Lett. 2005;99:63–68. doi: 10.1016/j.imlet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Berahovich RD, Miao Z, Wang Y, Premack B, Howard MC, Schall TJ. Proteolytic activation of alternative CCR1 ligands in inflammation. J Immunol. 2005;174:7341–7351. doi: 10.4049/jimmunol.174.11.7341. [DOI] [PubMed] [Google Scholar]
- 26.Hepburn TW, Hart TK, Horton VL, Sellers TS, Tobia LP, Urbanski JJ, Shi W, Davis CB. Pharmacokinetics and tissue distribution of SB-251353, a novel human CXC chemokine, after intravenous administration to mice. J Pharmacol Exp Ther. 2001;298:886–893. [PubMed] [Google Scholar]
- 27.Laterveer L, Lindley IJ, Heemskerk DP, Camps JA, Pauwels EK, Willemze R, Fibbe WE. Rapid mobilization of hematopoietic progenitor cells in rhesus monkeys by a single intravenous injection of interleukin-8. Blood. 1996;87:781–788. [PubMed] [Google Scholar]
- 28.Kim WY, Broxmeyer HE, Han IS, Park DH, Lee KM, Vinay DS, Kwon BS. Effect of leukotactin-1 on the protection in vivo of myeloid progenitor cells against cytotoxic chemotherapeutics. J Hematother Stem Cell Res. 2003;12:107–113. doi: 10.1089/152581603321210181. [DOI] [PubMed] [Google Scholar]
- 29.Youn BS, Jang IK, Broxmeyer HE, Cooper S, Jenkins NA, Gilbert DJ, Copeland NG, Elick TA, Fraser MJ, Jr, Kwon BS. A novel chemokine, macrophage inflammatory protein-related protein-2, inhibits colony formation of bone marrow myeloid progenitors. J Immunol. 1995;155:2661–2667. [PubMed] [Google Scholar]
- 30.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosu-Myles M, Khandaker M, Wu DM, Keeney M, Foley SR, Howson-Jan K, Yee IC, Fellows F, Kelvin D, Bhatia M. Characterization of chemokine receptors expressed in primitive blood cells during human hematopoietic ontogeny. Stem Cells. 2000;18:374–381. doi: 10.1634/stemcells.18-5-374. [DOI] [PubMed] [Google Scholar]
- 32.Kassmer SH, Niggemann B, Punzel M, Mieck C, Zanker KS, Dittmar T. Cytokine combinations differentially influence the SDF-1alpha-dependent migratory activity of cultivated murine hematopoietic stem and progenitor cells. Biol Chem. 2008;389:863–872. doi: 10.1515/BC.2008.099. [DOI] [PubMed] [Google Scholar]
- 33.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- 35.Wang JC, Lapidot T, Cashman JD, Doedens M, Addy L, Sutherland DR, Nayar R, Laraya P, Minden M, Keating A, Eaves AC, Eaves CJ, Dick JE. High level engraftment of NOD/SCID mice by primitive normal and leukemic hematopoietic cells from patients with chronic myeloid leukemia in chronic phase. Blood. 1998;91:2406–2414. [PubMed] [Google Scholar]
- 36.Cashman JD, Lapidot T, Wang JC, Doedens M, Shultz LD, Lansdorp P, Dick JE, Eaves CJ. Kinetic evidence of the regeneration of multilineage hematopoiesis from primitive cells in normal human bone marrow transplanted into immunodeficient mice. Blood. 1997;89:4307–4316. [PubMed] [Google Scholar]
- 37.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Stirling RG, van Rensen EL, Barnes PJ, Chung KF. Interleukin-5 induces CD34(+) eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med. 2001;164:1403–1409. doi: 10.1164/ajrccm.164.8.2010002. [DOI] [PubMed] [Google Scholar]
- 39.Sehmi R, Baatjes AJ, Denburg JA. Hemopoietic progenitor cells and hemopoietic factors: potential targets for treatment of allergic inflammatory diseases. Curr Drug Targets Inflamm Allergy. 2003;2:271–278. doi: 10.2174/1568010033484007. [DOI] [PubMed] [Google Scholar]
- 40.Southam DS, Widmer N, Ellis R, Hirota JA, Inman MD, Sehmi R. Increased eosinophil-lineage committed progenitors in the lung of allergen-challenged mice. J Allergy Clin Immunol. 2005;115:95–102. doi: 10.1016/j.jaci.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 41.Su SB, Mukaida N, Wang J, Nomura H, Matsushima K. Preparation of specific polyclonal antibodies to a C-C chemokine receptor, CCR1, and determination of CCR1 expression on various types of leukocytes. J Leukoc Biol. 1996;60:658–666. doi: 10.1002/jlb.60.5.658. [DOI] [PubMed] [Google Scholar]
- 42.de Wynter EA, Heyworth CM, Mukaida N, Jaworska E, Weffort-Santos A, Matushima K, Testa NG. CCR1 chemokine receptor expression isolates erythroid from granulocyte-macrophage progenitors. J Leukoc Biol. 2001;70:455–460. [PubMed] [Google Scholar]
- 43.Anderson MW, Zhao S, Ai WZ, Tibshirani R, Levy R, Lossos IS, Natkunam Y. C-C chemokine receptor 1 expression in human hematolymphoid neoplasia. Am J Clin Pathol. 2010;133:473–483. doi: 10.1309/AJCP1TA3FLOQTMHF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Wynter EA, Heyworth CM, Mukaida N, Matsushima K, Testa NG. NOD/SCID repopulating cells but not LTC-IC are enriched in human CD34+ cells expressing the CCR1 chemokine receptor. Leukemia. 2001;15:1092–1101. doi: 10.1038/sj.leu.2402146. [DOI] [PubMed] [Google Scholar]
- 45.Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–3924. [PubMed] [Google Scholar]
- 46.Larochelle A, Vormoor J, Hanenberg H, Wang JC, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao XL, Kato I, Williams DA, Dick JE. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 47.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 48.Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 49.Gouwy M, Struyf S, Berghmans N, Vanormelingen C, Schols D, Van DJ. CXCR4 and CCR5 ligands cooperate in monocyte and lymphocyte migration and in inhibition of dual-tropic (R5/X4) HIV-1 infection. Eur J Immunol. 2011;41:963–973. doi: 10.1002/eji.201041178. [DOI] [PubMed] [Google Scholar]
- 50.McNiece IK, Robinson BE, Quesenberry PJ. Stimulation of murine colony-forming cells with high proliferative potential by the combination of GM-CSF and CSF-1. Blood. 1988;72:191–195. [PubMed] [Google Scholar]
- 51.Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 53.Han IS, Ra JS, Kim MW, Lee EA, Jun HY, Park SK, Kwon BS. Differentiation of CD34+ cells from human cord blood and murine bone marrow is suppressed by C6 beta-chemokines. Mol Cells. 2003;15:176–180. [PubMed] [Google Scholar]
- 54.Youn BS, Zhang SM, Lee EK, Park DH, Broxmeyer HE, Murphy PM, Locati M, Pease JE, Kim KK, Antol K, Kwon BS. Molecular cloning of leukotactin-1:a novel human beta-chemokine, a chemoattractant for neutrophils, monocytes, and lymphocytes, and a potent agonist at CC chemokine receptors 1 and 3. J Immunol. 1997;159:5201–5205. [PubMed] [Google Scholar]
- 55.Lord BI, Spooncer E. Isolation of haemopoietic spleen colony forming cells. Lymphokine Res. 1986;5:59–72. [PubMed] [Google Scholar]
- 56.Visser JW, Bauman JG, Mulder AH, Eliason JF, de Leeuw AM. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984;159:1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caracciolo D, Shirsat N, Wong GG, Lange B, Clark S, Rovera G. Recombinant human macrophage colony-stimulating factor (M-CSF) requires subliminal concentrations of granulocyte/macrophage (GM)-CSF for optimal stimulation of human macrophage colony formation in vitro. J Exp Med. 1987;166:1851–1860. doi: 10.1084/jem.166.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 59.Broxmeyer HE, Cooper S, Hangoc G, Gao JL, Murphy PM. Dominant myelopoietic effector functions mediated by chemokine receptor CCR1. J Exp Med. 1999;189:1987–1992. doi: 10.1084/jem.189.12.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ottersbach K, Cook DN, Kuziel WA, Humbles A, Lu B, Gerard C, Proudfoot AE, Graham GJ. Macrophage inflammatory protein-1alpha uses a novel receptor for primitive hemopoietic cell inhibition. Blood. 2001;98:3476–3478. doi: 10.1182/blood.v98.12.3476. [DOI] [PubMed] [Google Scholar]