Abstract
This review focuses on two different topics: (a) iodine and autoimmune thyroid disease (AITD) and (b) AITD and papillary thyroid carcinoma (PTC). Iodine intake modifies the expression of thyroid diseases and has been associated with induction of AITD. Thyroglobulin (Tg) is an important target in iodine-induced autoimmune response due to post-translational modifications of iodinated Tg, as suggested in animal models. We have shown that the unmasking of a cryptic epitope on Tg contributes to iodine-induced thyroid autoimmunity in humans. The relationship between AITD and PTC has been suggested in many studies. The presence of two different mechanisms has been hypothesized, one typical of AITD and the other of an immune reaction to PTC. We have shown that in AITD, the pattern of Tg recognition by anti-Tg antibodies (TgAb) is ‘restricted’ to the immunodominant regions of Tg, while in patients with non-AITD, such as nodular goiter and PTC devoid of thyroid lymphocytic infiltration at histology, TgAb show a less restricted epitopic pattern and bind also to other regions of Tg. Thyroid function may also affect the frequency of PTC, the risk of cancer increasing with serum TSH levels. We have shown that this mechanism, rather than thyroiditis per se, plays a major role in the association of PTC with Hashimoto's thyroiditis, as a consequence of the autoimmune process leading to a progressive increase of serum TSH in these patients.
Key Words: Iodine, Hashimoto's thyroiditis, Thyroid autoimmunity, Papillary thyroid cancer
Introduction
This review will focus on two different, related topics: (a) iodine and thyroid autoimmunity and (b) thyroid autoimmunity and papillary thyroid carcinoma (PTC). We will review the latest data on these issues and describe our more recent contributions to these fields.
Iodine and Thyroid Disease
Iodine Nutrition and the Spectrum of Thyroid Diseases
The iodine dietary intake is insufficient in over 2 billion of people and, therefore, deficiency of iodine is one of the main issues of public health worldwide [1]. The term iodine deficiency disorders (IDD) refers to the several consequences that iodine deficiency imposes on individuals, including goiter, which is the most common clinical manifestation, and cerebral impairment ranging from mild cognitive defects to cretinism. The frequency and severity of IDD are proportional to the magnitude of iodine deficiency [2] and can be prevented by improving iodine nutrition through salt iodization [3].
Only few countries (Switzerland, some Scandinavian countries, Australia, the USA and Canada) were completely iodine sufficient before 1990. Since then, iodized salt (IS) programs have been implemented in many countries worldwide, and two thirds of the world's population (71%) are nowadays estimated to be covered by IS [2].
Iodine supplementation affects the epidemiology and eventually the natural history of thyroid diseases. In old Chinese medical writings of 3600 B.C., a decrease in goiter size was reported upon ingestion of seaweed and overcooked sea sponge [4]. A large number of studies have shown the effect of iodine supplementation on the epidemiology of goiter [5,6,7]. In two surveys conducted in Pescopagano [8,9], an Italian village with a previous moderate iodine deficiency, before and after the introduction of a iodine prophylaxis program on a voluntary basis, a significant reduction of the prevalence of goiter was observed, mainly due to the reduction of diffuse goiter in 2010 compared to the previous survey of 1995 (10.3 vs. 34.0%). The overall prevalence of nodular goiter was not significantly different in the two surveys, but when age was taken into account, its frequency was significantly lower in 2010 than in 1995 in subjects aged 26-35 years (3.8 vs. 11.3%), while no difference was observed in subjects >35 years old. The absence of a significant difference in the frequency of nodular goiter in subjects older than 35 years may be explained by the fact that in the majority of the subjects of this class of age the process of goiter development was initiated already before the increase of iodine intake.
After the introduction of iodine prophylaxis, an increased incidence of hyperthyroidism was observed [10]. This is due to the development of iodine-induced thyrotoxicosis in subjects with preexisting autonomous multinodular goiter. This condition is transient and correlated to the level of iodine deficiency and the amount of iodine administered. In 1980, when in Switzerland the salt iodine content was increased from 7.5 to 15 mg/kg, doubling iodine intake, the incidence of toxic nodular goiter increased by 12% in the first 2 years, but then declined to only 25% of the initial incidence [11]. In 2013, the WHO indicated that 10 countries have iodine intakes above 300 μg/l, the threshold of ‘excessive’ iodine intake [1]. These data emphasize the importance of regular monitoring of iodine status to detect both low and excessive intakes of iodine. In the survey conducted in Pescopagano, after the introduction of an iodine prophylaxis program on a voluntary basis [9], thyroid autonomy was not observed in younger subjects and was associated with a significant reduction in the frequency of hyperthyroidism due to toxic nodular goiter in older age. We did not observe the increased frequency of hyperthyroidism after the beginning of iodine prophylaxis that has been reported in other studies [12,13], conceivably because of the lower level of iodine supplementation in this population. Taken as a whole, these data indicate that iodine prophylaxis is associated with a reduced frequency of thyroid autonomy in younger subjects and of toxic nodular goiter in older patients. This phenomenon may have a relevant impact on the health of this population, thyrotoxicosis being associated with arrhythmias and mortality in older people.
Iodine Nutrition and Thyroid Autoimmunity
A potential side effect of iodine supplementation is the induction or the worsening of autoimmune thyroiditis [14,15]. Cross-sectional studies of populations with different iodine intakes in Great Britain [16], in Denmark and in Iceland [17] showed that the frequency of thyroid autoantibodies (TAb) and hypothyroidism is higher in iodine-replete than in iodine-deficient populations. Boukis et al. [18] showed that in goitrous patients from a mildly iodine-deficient area in Greece, TAb became positive in 42.8% of the subjects 3 and 6 months after treatment with iodized oil. Recently, Pedersen et al. [19] found an increased prevalence of TAb after the beginning of a cautious iodization program, supporting the view that even a small increase in iodine supplementation may be associated with an increased thyroid autoimmunity. An increased frequency of hypothyroidism has also been reported after the careful introduction of IS in Denmark [14]. Similar findings were reported in the Pescopagano survey, where an increased frequency of TAb and of Hashimoto's thyroiditis (HT) was observed after the beginning of iodine prophylaxis [20]. This study showed that iodine intake modulates the pattern of thyroid diseases, even in case of slight differences in intake and doses below 150 μg daily recommended for preventing IDD. The role of iodine in inducing thyroid autoimmunity is also strongly supported by animal models. Excessive iodine intake can precipitate spontaneous thyroiditis in genetically predisposed animals by increasing the immunogenicity of thyroglobulin (Tg) [21]. This may be explained by the fact that Tg is the only self-antigen that undergoes post-translational modification as a consequence of the environmental supply of iodine, with the exposure of previously hidden epitopes. In experimental animals, enhanced iodination of Tg facilitates processing and presentation of a cryptic pathogenic peptide [22,23,24,25]. In agreement with this concept is the observation that iodine may play a role in central tolerance, and T cells undergoing thymic selection likely recognize only noniodinated Tg epitopes [21]. Furthermore, in experimental models of autoimmune thyroiditis, it has been shown that breaking B-cell self-tolerance occurs first for Tg and subsequently for thyroperoxidase [26].
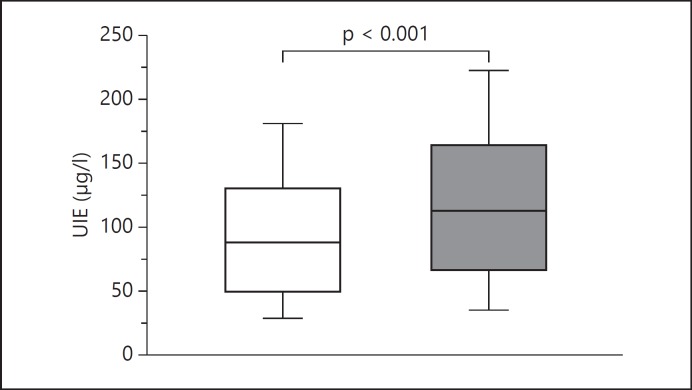
It is not known how Tg modifications induced by posttranslational iodination are involved in the development of spontaneous thyroid autoimmunity in humans. We have approached this matter in the population of Pescopagano. In a second survey conducted in this village in 2010 [20], 70% of subjects declared to routinely use IS (IS users) and 30% never to use IS (IS non-users). Accordingly, median urinary iodine excretion was slightly higher in IS users than in IS non-users (112.0 vs. 86.5 µg/l; p < 0.01; fig. 1). Positive anti-Tg antibodies (TgAb) were more frequently detected in IS users (18.9 vs. 13.6%; p = 0.02), while this difference was not statistically different for anti-thyroperoxidase antibodies (TPOAb; table 1). We, therefore, investigated whether iodine supply was associated with changes in the antigenic characteristics of Tg [20]. To this purpose, we evaluated the epitope pattern of TgAb from IS users and IS non-users. We have previously characterized 4 human recombinant monoclonal TgAb-Fab (A, B, C and D) [27,28]. In agreement with the well-known restriction of TgAb epitope pattern [29,30], the 4 TgAb-Fab recognize 4 different but partially overlapping epitopes, A epitope lying at one pole, B epitope at the other pole and epitopes C and D being on the fringe of the immunodominant region of Tg [28]. By inhibiting TgAb binding to Tg with human recombinant monoclonal TgAb-Fab (fig. 2), we showed that region A was the major component of the immunodominant region on Tg recognized by TgAb from autoimmune thyroid disease (AITD) and non-AITD patients, while epitopic region B was recognized to the greatest extent by HT patients [28,31,32].
Fig. 1.
Box-whisker plot of urinary iodine excretion (UIE) measured in subjects not using (white box) or using (gray box) IS. Results are reported as median values (black lines), interquartile (25th to 75th percentiles) ranges (boxes) and 10th to 90th percentiles (whiskers). UIE was significantly higher in IS users than in non-users (Mann-Whitney U test: p < 0.0001).
Table 1.
Prevalence of TAb according to use of IS
| Users (n = 906) | Non-users (n = 389) | p | |
|---|---|---|---|
| TgAb | 171 (18.9%) | 53 (13.6%) | 0.02 |
| TPOAb | 153 (16.9%) | 51 (13.1%) | n.s. |
n.s. = Not significant.
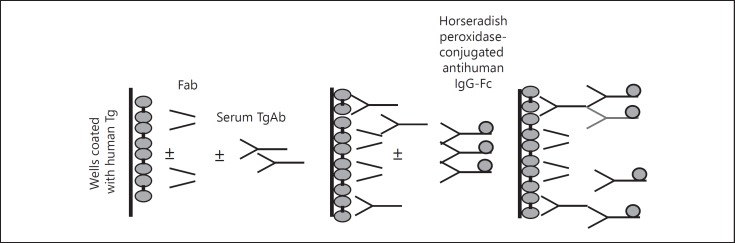
Fig. 2.
The epitope pattern of TgAb was assessed by measuring the inhibition of serum TgAb binding to Tg exerted by recombinant human TgAb-Fab in ELISA. Only sera with high titers (>100 U/ml) of TgAb were evaluated, because they were adequate to perform inhibition-binding experiments. Four TgAb-Fab to 4 different epitope regions (A, B, C and D) on Tg were employed. Wells coated with human Tg were incubated with serial dilutions of sera with high levels of TgAb in the presence of each TgAb-Fab. TgAb binding was detected with a horseradish peroxidase-conjugated antihuman IgG-Fc, which binds to IgG but not to Fab molecules. After the addition of substrate (o-phenylenediamine + H2O2), the optical density was read at 490 nm.
When we compared sera from IS users and IS non-users, we observed a similar pattern of inhibition of Tg binding by TgAb-Fab A, C and D, while inhibition by TgAb-Fab B was higher in IS users than in IS non-users (27.5 vs. 3.0%; p = 0.04). These results suggest that iodine contributes to autoimmunity to Tg by unmasking a cryptic epitope on Tg (fig. 3), showing for the first time in humans the results reported in animal models. In addition, the observation that neither age nor genetic background influence TgAb epitope pattern in HT [33], and that the rise in TgAb levels observed after 131I treatment for Graves' hyperthyroidism [34] is not associated with an epitope spreading of TgAb, underlines that in AITD, TgAb epitope pattern, once established, remains restricted.
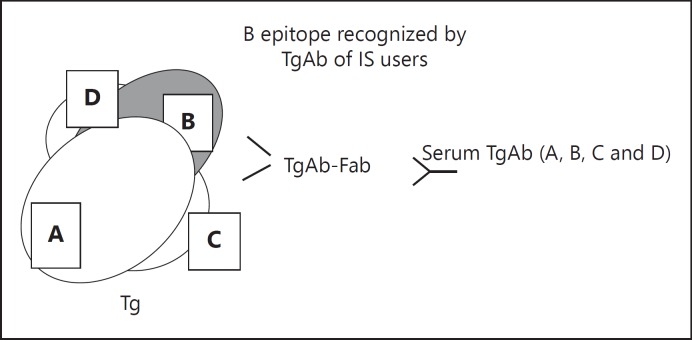
Fig. 3.
Schematic representation of the immunodominant region recognized by human TgAb. Only a portion of the very large Tg molecule is depicted. Four different epitope regions (A, B, C and D) on Tg are recognized by the recombinant human TgAb-Fab. The binding of TgAb to its specific epitope on Tg is inhibited by the relative TgAb-Fab. The levels of inhibition for regions A, C and D were similar in subjects using or not using IS. Interestingly, inhibition by TgAb-Fab to the epitope B was significantly higher in IS users (median 27.5%, interquartile range 6.5-48.3) than in IS non-users (3.0%, interquartile range 0.0-20.5; Mann-Whitney U test: p = 0.047).
Thyroid Autoimmunity and PTC
HT and PTC
The relationship between thyroid autoimmunity and PTC has been suggested in many studies, but its meaning is still debated. The association between these two disorders is appealing, and it has been hypothesized that in HT, the inflammatory response may create a favorable setting for malignant transformation as well established for other cancers [35].
The possible association between HT and PTC was first proposed more than 50 years ago by Dailey et al. [36] who found a high frequency of thyroid carcinomas in patients with HT who underwent thyroidectomy. Hirabayashi and Lindsay [37] examined 9,287 thyroid specimens and found that papillary carcinoma was present in 22.5% of the glands with HT and only in 2.4% of those with no pathological signs of HT. Okayasu et al. [38] and Okayasu [39] found that the prevalence of lymphocytic infiltrates, suggestive of autoimmune thyroiditis, was significantly higher in patients with PTC than in those with adenomatous goiter or follicular adenoma. Ott et al. [40,41] reported a high incidence of thyroid carcinoma (32%) in 146 patients with HT and solitary cold nodules. However, other studies yielded conflicting results, reporting a low incidence of thyroid carcinoma in patients with HT [42,43,44]. Boi et al. [45] found a significant association between HT and PTC in a cytological series and Jankovic et al. [46] reported a significant relationship between HT and PTC in histological, but less consistently in cytological series. Indeed, the average prevalence of PTC in patients with HT was 27.56 and 1.20% in histological versus cytological series, respectively. The authors underline that a selection bias may account for the higher prevalence of PTC in HT in histological series, because in the vast majority of these patients, the indication for surgery is the presence of nodules suspicious for malignancy, while it is more commonly the size of goiter or the presence of compressive symptoms in patients with nodular goiter. These findings were confirmed by Castagna et al. [47] who reported that the prevalence of malignant cytology in patients with nodular HT diagnosed on clinical grounds was similar (4.5%) to that found in nodular goiter with or without TAb and in nodular Graves' disease (GD). When the same analysis was performed in the subgroup of patients submitted to thyroid surgery, the prevalence of PTC was significantly higher in patients with nodular HT (67.8%) compared with the other groups (36.9, 37.2 and 40%, respectively).
The heterogeneity of criteria used to define HT may hamper the drawing of conclusions on this issue. By strict criteria, HT is a histological diagnosis characterized by a widespread lymphocytic infiltration of the thyroid. On the other hand, PTC is often associated with a significant lymphocytic infiltration [38] that may represent a response to the tumor. On clinical grounds, the diagnosis of HT is based on the presence of serum TAb and of spontaneous hypothyroidism that may be present at the initial evaluation or may develop during follow-up [15,16,48]. However, TAb may also be detected in euthyroid subjects in the absence of a diffuse lymphocytic infiltration of the thyroid, especially in nodular goiter. Thyroid ultrasound has been proposed as a tool for the clinical diagnosis of HT, even if this point is not universally accepted. The typical hypoechoic pattern at ultrasound is related to the structural changes caused by the lymphocytic infiltration of the thyroid gland and has been associated with the onset of hypothyroidism [49,50].
We addressed the issue of the relationship between thyroid autoimmunity and PTC in a large series of patients submitted to fine-needle aspiration of thyroid nodules and to thyroid surgery [51]. We showed that the prevalence of TAb was similar in patients with benign nodules as compared to patients with malignant nodules at cytology (38.7 vs. 35.6%, respectively). However, in women with multinodular goiter, TAb were more common in presence of benign nodules than in PTC (40.2 vs. 32.5%, respectively; p = 0.02), while in men with single/isolated thyroid nodules, TAb were more common in PTC than in nodular goiter (31.2 vs. 20.4%, respectively; p = 0.02). At variance with these clinical observations, a moderate to severe lymphocytic infiltration at histology was more common in PTC than in benign nodular thyroid disease, both in patients with multinodular goiter (37.8 vs. 9.2%, respectively; p < 0.01) and in those with single thyroid nodules (58.7 vs. 30.1%, respectively; p < 0.01). Interestingly, in subjects with moderate/severe thyroid lymphocytic infiltration, the frequency of positive serum TAb was significantly higher in patients with benign diseases than in those with PTC (78.2 vs. 49.7%, respectively; p = 0.0002), indicating that several patients with PTC had undetectable serum TAb despite the moderate/severe thyroid lymphocytic infiltration observed at histology. Furthermore, in a group of patients treated with total thyroidectomy for PTC [52], thyroiditis at histology was detected in 40.7% of patients, while a clinical diagnosis of HT or GD was performed in half of these patients (20.3%), supporting the observation that in PTC, lymphocytic thyroiditis on histology is more common than clinically diagnosed AITD. Based on these data, (1) we confirmed that PTC was not predicted by positive serum TAb; (2) we concluded that PTC was associated with TAb only in the subgroups of patients with a lower prevalence of thyroid autoimmunity (i.e. men vs. women); (3) we showed that a moderate to severe lymphocytic thyroiditis was much more common in PTC than in nodular goiter and (4) we demonstrated that the correlation between histological thyroiditis and serum TAb was lower in PTC than in benign nodules.
Thyroid-Stimulating Hormone, Thyroid Autoimmunity and PTC
Thyroid function may also affect the frequency of clinically detectable PTC [53]. In the last few years, it has been reported that in patients with nodular thyroid diseases, the risk of thyroid malignancy increases with increasing concentrations of serum thyroid-stimulating hormone (TSH), and even within normal ranges, higher TSH values are associated with a higher frequency and more advanced stage of thyroid cancer [54,55,56,57,58,59,60,61]. In HT patients, the progressive reduction of thyroid function as a consequence of the autoimmune process leads to the progressive increase in serum TSH. Therefore, it is possible to hypothesize that increased TSH levels may also play a role in the development of PTC in patients with nodular HT. To verify this hypothesis [62], we have evaluated the relationship between TSH and PTC in 9,824 untreated patients with nodular HT (n = 893) or nodular goiter (n = 8,931). Patients were diagnosed as affected by nodular HT if they had a high titer of TAb, i.e. had both TgAb and TPOAb higher than 100 IU/ml, or a low level of TAb, but had hypothyroidism or a clear hypoechoic ‘thyroiditis’ pattern at thyroid ultrasound. Patients with nodular goiter had no hypoechoic pattern at thyroid ultrasound, were euthyroid and had either undetectable serum TAb (TAb-nodular goiter; n = 6,571) or low-level serum TAb (TAb+ nodular goiter; n = 2,360). The frequency of PTC in patients with nodular HT (9.4%) was significantly higher compared to patients with TAb- nodular goiter (6.4%) and TAb+ nodular goiter (6.5%). Altogether, these data suggest that the high frequency of PTC in patients with nodular HT is mainly due to the higher TSH levels compared to patients with nonnodular goiter. The results of the statistical analysis were confirmed in a second group of patients with nodular HT (n = 638) or nodular goiter (n = 3,276) under treatment with L-thyroxine (LT4) [62]. This therapy was inhomogeneous in the patients included in the study, the purpose of LT4 treatment being to reduce TSH levels to lower values of a normal range in patients with nodular goiter and to correct hypothyroidism, bringing serum TSH within the range of normal values in more than half of the patients with nodules in HT. As a result of this uneven treatment, higher levels of TSH and a higher frequency of PTC were observed in nodular HT with respect to nodular goiter in the whole series of LT4-treated patients. However, when only patients with serum TSH levels below the median value (0.90 mIU/l) were taken into account, no significant difference in PTC frequency was found, supporting the hypothesis that TSH is the main factor associated with the risk of PTC independently of the diagnosis of nodular HT or goiter (fig. 4).
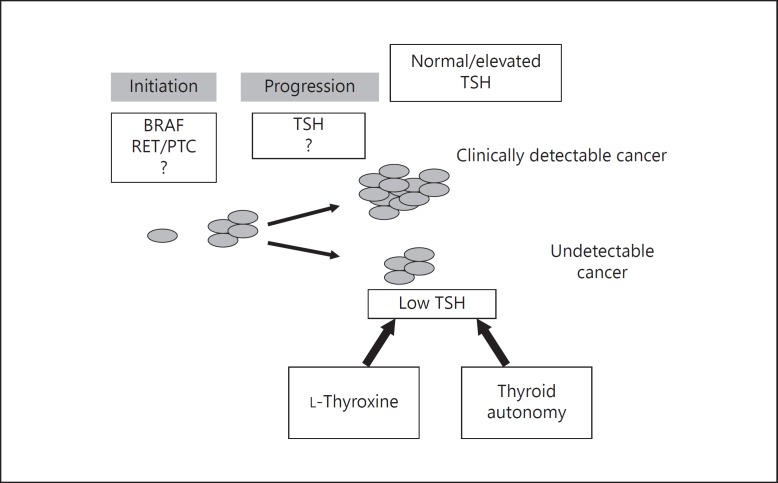
Fig. 4.
A schematic representation of the role of TSH in the development of PTC. TSH is a factor involved in the progression of thyroid cancer, and the risk of clinically detectable thyroid malignancy increases with serum TSH concentrations; even within normal ranges, higher TSH values are associated with a higher frequency of clinically detectable cancers. Thyroid diseases that affect the function of the gland, influencing pituitary secretion of TSH, are associated with a different risk of PTC, the likelihood of thyroid malignancy being reduced when TSH is lower, as in thyroid autonomy, and increased when TSH is higher, as in HT. Moreover, in patients with nodular thyroid disease, LT4 treatment, which reduces serum TSH, is associated with a significantly lower risk of developing clinically detectable thyroid cancer.
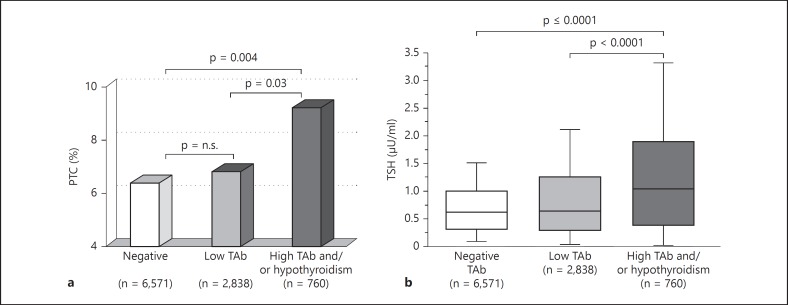
As thyroid hypoechogenicity at ultrasound is not a widely accepted criterion for the diagnosis of HT, we analyzed our data taking into account only TAb level and hypothyroidism. In untreated patients, the frequency of PTC was significantly higher (9.3%) in those with hypothyroidism and/or high serum levels of TAb compared to patients with both low serum levels of TAb (6.8%) and negative serum TAb (6.4%; fig. 5a). Patients with hypothyroidism and/or high serum levels of TAb also had higher TSH levels compared to patients with lower serum levels of TAb or negative serum TAb (fig. 5b). The question at this point was whether autoimmunity per se or rather autoimmunity as a cause of increased serum TSH was responsible for the increased frequency of PTC. A multivariate analysis was performed to identify the variables independently associated with an increased probability of PTC. According to a forward stepwise binary logistic regression model, serum TSH was the most significant factor associated with PTC, TPOAb as well as the clinical diagnosis of HT were unrelated with PTC, whereas TgAb showed a slight association with PTC (table 2). These data may be explained by the hypothesis that patients with PTC have circulating antibodies to Tg unrelated to the classical HT. In a recent paper, Boi et al. [63] found that both TAb and serum TSH levels represented independent risk factors of thyroid carcinoma in patients with thyroid nodules submitted to fine-needle aspiration. The discrepancy between these data and our results may be due to different methods for TAb detection and/or different genetic predisposition of thyroid autoimmunity of the patients studied. It is actually conceivable that serum TAb levels in the series of Boi et al. [63] were more accurate markers of lymphocytic thyroiditis than serum TAb detected in the studies by Fiore et al. [61,62]. Indeed, this could explain the variable association between serum TAb and risk of thyroid cancer reported in several studies, as compared to the consistent association observed with HT.
Fig. 5.
a Frequency of PTC according to serum levels of TAb and hypothyroidism. Patients with high (both TgAb and TPOAb >100 U/l) serum TAb or with hypothyroidism (black column) showed a significantly higher frequency of PTC compared to subjects with lower serum TAb levels (gray column) or negative TAb (white column). b Box-whisker plot of serum TSH (μU/ml) in patients with high serum TAb levels or hypothyroidism (black box), lower serum TAb levels (gray box) or negative TAb (white box). Results are reported as median values (black lines), interquartile (25th to 75th percentiles) ranges (boxes) and 10th to 90th percentiles (whiskers). The statistical difference between groups was evaluated using the Mann-Whitney U test. Patients with high serum TAb also showed serum TSH (1.16 μU/ml, interquartile range 0.52-2.00) compared to both patients with low TAb levels (0.75 μU/ml, interquartile range 0.39-1.34; Mann-Whitney U test: p < 0.001) and patients with negative TAb (0.72 μU/ml, interquartile range 0.40-1.10; Mann-Whitney U test: p = < 0.001). n.s. = Not significant.
Table 2.
Independent predictors of the diagnosis of PTC in patients with nodular goiter or HT
| Variable | p | OR |
|---|---|---|
| TSH | <0.0001 | 66.5 |
| TgAb | 0.0003 | 2.0 |
| TPOAb | n.s. | – |
| Clinical diagnosis | n.s. | – |
OR = Cumulative odds ratio; n.s. = not significant.
Epitope Pattern of TgAb and PTC
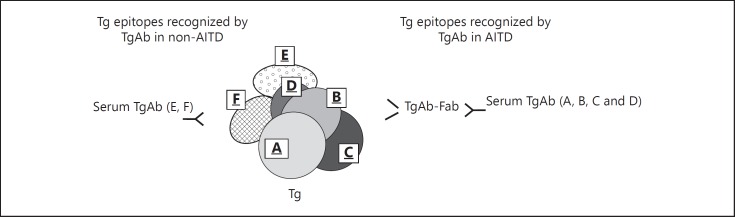
The presence of two different autoimmune mechanisms, one typical of AITD and the other of an immune reaction to PTC, was demonstrated by our study on the epitope pattern of TgAb [31], using the same method as described in the previous paragraph and in figure 2. In this study, we evaluated the inhibition exerted by monoclonal TgAb-Fab A, B, C and D on the binding to Tg of sera from patients with AITD, namely HT and GD, from patients with nodular goiter and circulating TAb (non-AITD), but devoid of tissue lymphocytic infiltration at histology, and from patients with PTC and circulating TAb subdivided in those without (PTC-no-thyroiditis) or with associated thyroiditis at histology (PTC-thyroiditis). The pattern of AITD patients differed from that of non-AITD patients, because the latter group showed a significantly lower inhibition by monoclonal TgAb-Fab A, B, C and D. Sera from patients with PTC-thyroiditis showed an epitope recognition pattern similar to that of AITD, while those with PTC-no-thyroiditis had a pattern similar to patients with nodular goiter, indicating that in these conditions, TgAb are not restricted to the epitopes identified by the 4 TgAb-Fab that are dominant in AITD and, therefore, are characterized by a less restricted and more heterogeneous epitope pattern (fig. 6).
Fig. 6.
Schematic representation of the immunodominant region recognized by human TgAb. Only a portion of the very large Tg molecule is depicted. Four immunodominant epitopes (A, B, C and D) are recognized by recombinant human TgAb-Fab directed to a specific epitope. The binding of TgAb to these epitopes on Tg is inhibited by the relative TgAb-Fab. In patients with AITD (including both HT and GD), the pattern of Tg recognition by TgAb was ‘restricted’ to the immunodominant regions of Tg, while in patients with non-AITD (including nodular goiter and PTC), TgAb bind also to other regions of Tg. Sera from patients with PTC associated with thyroiditis show an epitope recognition pattern similar to that of AITD, while those with PTC and devoid of severe lymphocytic infiltration at histology show a pattern similar to that in non-AITD patients.
Therefore, in PTC patients, the pattern of TgAb epitopes is quite variable, being of a non-AITD type in the absence of lymphocytic infiltration and AITD-like in its presence. Discrepancies of Tg molecules could cause the different TgAb patterns of AITD and non-AITD. Another hypothesis for this discrepancy is the existence of different mechanisms of antigen presentation or processing of Tg in AITD and non-AITD.
Conclusion
Iodine intake modulates the pattern of thyroid diseases, and iodine supplementation is associated with an increased frequency of thyroid autoimmunity. As far as breaking of self-tolerance is concerned, the iodine effect is correlated with the intrinsic characteristics of Tg, namely its modifications induced by post-translational iodination. In the presence of genetic susceptibility, iodine, which is essential for normal thyroid function, induces thyroid autoimmunity by unmasking a cryptic epitope on Tg.
Thyroid autoimmunity is associated with an increased risk of PTC; however, different factors should be taken into account when evaluating this association. On histological grounds, the lymphocytic infiltration of the thyroid is the typical hallmark of HT, but PTC may be associated with a significant lymphocytic infiltration in the absence of the characteristic signs of autoimmune thyroiditis, as an immune response to the tumor. On clinical grounds, the diagnosis of HT is based on the presence of TAb, but these antibodies may be detected in euthyroid subjects in the absence of a diffuse lymphocytic infiltration of the thyroid, and PTC is associated with serum TAb only in patients with a lower prevalence of thyroid autoimmunity (i.e. men vs. women). The different epitope patterns of TgAb in AITD and non-AITD patients support the hypothesis that two different autoimmune mechanisms may be involved, one typical of AITD and the other of an immune reaction to PTC. Thyroid function may also affect the frequency of PTC, the risk of cancer increasing with serum TSH levels. We have shown that this mechanism, rather than thyroiditis per se, plays a major role in the association of PTC with HT, as a consequence of the autoimmune process leading to a progressive increase in serum TSH in these patients, even if the direct involvement of immunological mechanisms cannot be excluded.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid. 2013;23:523–528. doi: 10.1089/thy.2013.0128. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30:376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet. 2008;372:1251–1262. doi: 10.1016/S0140-6736(08)61005-3. [DOI] [PubMed] [Google Scholar]
- 4.Reseinfeld L. Discovery and early use of iodine. J Chem Educ. 2000;77:984–987. [Google Scholar]
- 5.Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr. 2012;142:744–750. doi: 10.3945/jn.111.149393. [DOI] [PubMed] [Google Scholar]
- 6.Vanderpump MP, Lazarus JH, Smyth PP, Laurberg P, Holder RL, Boelaert K, Franklyn JA. Iodine status of UK schoolgirls: a cross-sectional survey. Lancet. 2011;377:2007–2012. doi: 10.1016/S0140-6736(11)60693-4. [DOI] [PubMed] [Google Scholar]
- 7.Aghini-Lombardi F, Vitti P, Antonangeli L, Fiore E, Piaggi P, Pallara A, Consiglio E, Pinchera A. The size of the community rather than its geographical location better defines the risk of iodine deficiency: results of an extensive survey in Southern Italy. J Endocrinol Invest. 2013;36:282–286. doi: 10.1007/BF03347103. [DOI] [PubMed] [Google Scholar]
- 8.Aghini-Lombardi F, Antonangeli L, Martino E, Vitti P, Maccherini D, Leoli F, Rago T, Grasso L, Valeriano R, Balestrieri A, Pinchera A. The spectrum of thyroid disorders in an iodine-deficient community: the Pescopagano survey. J Clin Endocrinol Metab. 1999;84:561–566. doi: 10.1210/jcem.84.2.5508. [DOI] [PubMed] [Google Scholar]
- 9.Aghini LF, Fiore E, Tonacchera M, Antonangeli L, Rago T, Frigeri M, Provenzale AM, Montanelli L, Grasso L, Pinchera A, Vitti P. The effect of voluntary iodine prophylaxis in a small rural community: the Pescopagano survey 15 years later. J Clin Endocrinol Metab. 2013;98:1031–1039. doi: 10.1210/jc.2012-2960. [DOI] [PubMed] [Google Scholar]
- 10.Leung AM, Braverman LE, Pearce EN. History of US iodine fortification and supplementation. Nutrients. 2012;4:1740–1746. doi: 10.3390/nu4111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgi H, Kohler M, Morselli B. Thyrotoxicosis incidence in Switzerland and benefit of improved iodine supply. Lancet. 1998;352:1034. doi: 10.1016/S0140-6736(05)60076-1. [DOI] [PubMed] [Google Scholar]
- 12.Todd CH, Allain T, Gomo ZA, Hasler JA, Ndiweni M, Oken E. Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe. Lancet. 1995;346:1563–1564. doi: 10.1016/s0140-6736(95)92095-1. [DOI] [PubMed] [Google Scholar]
- 13.Connolly RJ, Vidor GI, Stewart JC. Increase in thyrotoxicosis in endemic goitre area after iodation of bread. Lancet. 1970;1:500–502. doi: 10.1016/s0140-6736(70)91582-5. [DOI] [PubMed] [Google Scholar]
- 14.Laurberg P, Bülow Pedersen I, Knudsen N, Ovesen L, Andersen S. Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid. 2001;11:457–469. doi: 10.1089/105072501300176417. [DOI] [PubMed] [Google Scholar]
- 15.Vanderpump MPJ. Philadelphia: JB Lippincott-Raven; 2005. The epidemiology of thyroid diseases; in Braverman LE, Utiger RD (eds): Werner and Ingbar's The Thyroid: A Fundamental and Clinical Text; pp. 398–406. [Google Scholar]
- 16.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley EJ, Hasan DM, Rodgers H, Tunbridge F. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 17.Laurberg P, Pedersen KM, Hreidarsson A, Sigfusson N, Iversen E, Knudsen PR. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab. 1998;83:765–769. doi: 10.1210/jcem.83.3.4624. [DOI] [PubMed] [Google Scholar]
- 18.Boukis MA, Koutras DA, Souvatzoglou A, Evangelopoulou A, Vrontakis M, Moulopoulos SD. Thyroid hormone and immunological studies in endemic goiter. J Clin Endocrinol Metab. 1983;57:859–862. doi: 10.1210/jcem-57-4-859. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen IB, Knudsen N, Carlé A, Vejbjerg P, Jorgensen T, Perrild H, Ovesen L, Rasmussen LB, Laurberg P. A cautious iodization program bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin Endocrinol (Oxf) 2011;75:120–126. doi: 10.1111/j.1365-2265.2011.04008.x. [DOI] [PubMed] [Google Scholar]
- 20.Latrofa F, Fiore E, Rago T, Antonangeli L, Montanelli L, Ricci D, Provenzale MA, Scutari M, Frigeri M, Tonacchera M, Vitti P. Iodine contributes to thyroid autoimmunity in humans by unmasking a cryptic epitope on thyroglobulin. J Clin Endocrinol Metab. 2013;98:E1768–E1774. doi: 10.1210/jc.2013-2912. [DOI] [PubMed] [Google Scholar]
- 21.Carayanniotis G. Recognition of thyroglobulin by T cells: the role of iodine. Thyroid. 2007;17:963–973. doi: 10.1089/thy.2007.0199. [DOI] [PubMed] [Google Scholar]
- 22.Dai YD, Rao VP, Carayanniotis G. Enhanced iodination of thyroglobulin facilitates processing and presentation of a cryptic pathogenic peptide. J Immunol. 2002;168:5907–5911. doi: 10.4049/jimmunol.168.11.5907. [DOI] [PubMed] [Google Scholar]
- 23.Li HS, Jiang HY, Carayanniotis G. Modifying effects of iodine on the immunogenicity of thyroglobulin peptides. J Autoimmun. 2007;28:171–176. doi: 10.1016/j.jaut.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Jiang HY, Li HS, Carayanniotis K, Carayanniotis G. Variable influences of iodine on the T-cell recognition of a single thyroglobulin epitope. Immunology. 2007;121:370–376. doi: 10.1111/j.1365-2567.2007.02584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi L, Bi M, Yang R, Zhou J, Zhao S, Fan C, Shan Z, Li Y, Teng W. Defective expression of regulatory B cells in iodine-induced autoimmune thyroiditis in non-obese diabetic H-2(h4) mice. J Endocrinol Invest. 2014;37:43–50. doi: 10.1007/s40618-013-0013-1. [DOI] [PubMed] [Google Scholar]
- 26.Chen CR, Hamidi S, Braley-Mullen H, Nagayama Y, Bresee C, Aliesky HA, Rapoport B, McLachlan SM. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology. 2010;151:4583–4593. doi: 10.1210/en.2010-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latrofa F, Pichurin P, Guo J, Rapoport B, McLachlan SM. Thyroglobulin-thyroperoxidase autoantibodies are polyreactive, not bispecific: analysis using human monoclonal autoantibodies. J Clin Endocrinol Metab. 2003;88:371–378. doi: 10.1210/jc.2002-021073. [DOI] [PubMed] [Google Scholar]
- 28.Latrofa F, Phillips M, Rapoport B, McLachlan SM. Human monoclonal thyroglobulin autoantibodies: epitopes and immunoglobulin genes. J Clin Endocrinol Metab. 2004;89:5116–5123. doi: 10.1210/jc.2003-032173. [DOI] [PubMed] [Google Scholar]
- 29.Prentice L, Kiso Y, Fukuma N, Horimoto M, Petersen V, Grennan F, Pegg C, Furmaniak J, Rees SB. Monoclonal thyroglobulin autoantibodies: variable region analysis and epitope recognition. J Clin Endocrinol Metab. 1995;80:977–986. doi: 10.1210/jcem.80.3.7533775. [DOI] [PubMed] [Google Scholar]
- 30.Estienne V, McIntosh RS, Ruf J, Asghar MS, Watson PF, Carayon P, Weetman AP. Comparative mapping of cloned human and murine antithyroglobulin antibodies: recognition by human antibodies of an immunodominant region. Thyroid. 1998;8:643–646. doi: 10.1089/thy.1998.8.643. [DOI] [PubMed] [Google Scholar]
- 31.Latrofa F, Ricci D, Grasso L, Vitti P, Masserini L, Basolo F, Ugolini C, Mascia G, Lucacchini A, Pinchera A. Characterization of thyroglobulin epitopes in patients with autoimmune and non-autoimmune thyroid diseases using recombinant human monoclonal thyroglobulin autoantibodies. J Clin Endocrinol Metab. 2008;93:591–596. doi: 10.1210/jc.2007-1199. [DOI] [PubMed] [Google Scholar]
- 32.Latrofa F, Ricci D, Montanelli L, Altea MA, Pucci A, Pinchera A, Vitti P. Thyroglobulin autoantibodies of patients with subacute thyroiditis are restricted to a major B cell epitope. J Endocrinol Invest. 2012;35:712–714. doi: 10.1007/BF03345804. [DOI] [PubMed] [Google Scholar]
- 33.Latrofa F, Ricci D, Vitti P, Prinzis A, Cambuli VM, Ghiani M, Pilia S, Carta D, Loche S, Pinchera A, Mariotti S. Characterization of thyroglobulin epitopes in Sardinian adults and juveniles with Hashimoto's thyroiditis: evidence against a major effect of age and genetic background on B-cell epitopes. Clin Endocrinol (Oxf) 2010;73:110–113. doi: 10.1111/j.1365-2265.2009.03748.x. [DOI] [PubMed] [Google Scholar]
- 34.Chiovato L, Fiore E, Vitti P, Rocchi R, Rago T, Dokic D, Latrofa F, Mammoli C, Lippi F, Ceccarelli C, Pinchera A. Outcome of thyroid function in Graves' patients treated with radioiodine: role of thyroid-stimulating and thyrotropin-blocking antibodies and of radioiodine-induced thyroid damage. J Clin Endocrinol Metab. 1998;83:40–46. doi: 10.1210/jcem.83.1.4492. [DOI] [PubMed] [Google Scholar]
- 35.Guarino V, Castellone MD, Avilla E, Melillo RM. Thyroid cancer and inflammation. Mol Cell Endocrinol. 2010;321:94–102. doi: 10.1016/j.mce.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70:291–297. doi: 10.1001/archsurg.1955.01270080137023. [DOI] [PubMed] [Google Scholar]
- 37.Hirabayashi RN, Lindsay S. The relation of thyroid carcinoma and chronic thyroiditis. Surg Gynecol Obstet. 1965;121:243–252. [PubMed] [Google Scholar]
- 38.Okayasu I, Fujiwara M, Hara Y, Tanaka Y, Rose NR. Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer. 1995;76:2312–2318. doi: 10.1002/1097-0142(19951201)76:11<2312::aid-cncr2820761120>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Okayasu I. The relationship of lymphocytic thyroiditis to the development of thyroid carcinoma. Endocr Pathol. 1997;8:225–230. doi: 10.1007/BF02738789. [DOI] [PubMed] [Google Scholar]
- 40.Ott RA, Calandra DB, McCall A, Shah KH, Lawrence AM, Paloyan E. The incidence of thyroid carcinoma in patients with Hashimoto's thyroiditis and solitary cold nodules. Surgery. 1985;98:1202–1206. [PubMed] [Google Scholar]
- 41.Ott RA, McCall AR, McHenry C, Jarosz H, Armin A, Lawrence AM, Paloyan E. The incidence of thyroid carcinoma in Hashimoto's thyroiditis. Am Surg. 1987;53:442–445. [PubMed] [Google Scholar]
- 42.Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601–604. doi: 10.1056/NEJM198503073121001. [DOI] [PubMed] [Google Scholar]
- 43.Walker RP, Paloyan E. The relationship between Hashimoto's thyroiditis, thyroid neoplasia, and primary hyperparathyroidism. Otolaryngol Clin North Am. 1990;23:291–302. [PubMed] [Google Scholar]
- 44.Villagelin DG, Santos RB, Romaldini JH. Is diffuse and peritumoral lymphocyte infiltration in papillary thyroid cancer a marker of good prognosis? J Endocrinol Invest. 2011;34:e403–e408. doi: 10.3275/7870. [DOI] [PubMed] [Google Scholar]
- 45.Boi F, Lai ML, Marziani B, Minerba L, Faa G, Mariotti S. High prevalence of suspicious cytology in thyroid nodules associated with positive thyroid autoantibodies. Eur J Endocrinol. 2005;153:637–642. doi: 10.1530/eje.1.02020. [DOI] [PubMed] [Google Scholar]
- 46.Jankovic B, Le KT, Hershman JM. Clinical review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013;98:474–482. doi: 10.1210/jc.2012-2978. [DOI] [PubMed] [Google Scholar]
- 47.Castagna MG, Belardini V, Memmo S, Maino F, Di Santo A, Toti P, Carli AF, Caruso G, Pacini F. Nodules in autoimmune thyroiditis are associated with increased risk of thyroid cancer in surgical series but not in cytological series: evidence for selection bias. J Clin Endocrinol Metab. 2014;99:3193–3198. doi: 10.1210/jc.2014-1302. [DOI] [PubMed] [Google Scholar]
- 48.Walsh JP, Bremner AP, Feddema P, Leedman PJ, Brown SJ, O'Leary P. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab. 2010;95:1095–1104. doi: 10.1210/jc.2009-1977. [DOI] [PubMed] [Google Scholar]
- 49.Marcocci C, Vitti P, Cetani F, Catalano F, Concetti R, Pinchera A. Thyroid ultrasonography helps to identify patients with diffuse lymphocytic thyroiditis who are prone to develop hypothyroidism. J Clin Endocrinol Metab. 1991;72:209–213. doi: 10.1210/jcem-72-1-209. [DOI] [PubMed] [Google Scholar]
- 50.Rago T, Chiovato L, Grasso L, Pinchera A, Vitti P. Thyroid ultrasonography as a tool for detecting thyroid autoimmune diseases and predicting thyroid dysfunction in apparently healthy subjects. J Endocrinol Invest. 2001;24:763–769. doi: 10.1007/BF03343925. [DOI] [PubMed] [Google Scholar]
- 51.Fiore E, Rago T, Scutari M, Ugolini C, Proietti A, Di Cosico G, Provenzale MA, Berti P, Grasso L, Mariotti S, Pinchera A, Vitti P. Papillary thyroid cancer, although strongly associated with lymphocytic infiltration on histology, is only weakly predicted by serum thyroid auto-antibodies in patients with nodular thyroid diseases. J Endocrinol Invest. 2009;32:344–351. doi: 10.1007/BF03345725. [DOI] [PubMed] [Google Scholar]
- 52.Latrofa F, Ricci D, Montanelli L, Rocchi R, Piaggi P, Sisti E, Grasso L, Basolo F, Ugolini C, Pinchera A, Vitti P. Lymphocytic thyroiditis on histology correlates with serum thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: impact on detection of serum thyroglobulin. J Clin Endocrinol Metab. 2012;97:2380–2387. doi: 10.1210/jc.2011-2812. [DOI] [PubMed] [Google Scholar]
- 53.Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab. 2012;97:1134–1145. doi: 10.1210/jc.2011-2735. [DOI] [PubMed] [Google Scholar]
- 54.Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006;91:4295–4301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 55.Jonklaas J, Nsouli-Maktabi H, Soldin SJ. Endogenous thyrotropin and triiodothyronine concentrations in individuals with thyroid cancer. Thyroid. 2008;18:943–952. doi: 10.1089/thy.2008.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polyzos SA, Kappaita M, Efstathiadou Z, Poulakos P, Slavakis A, Sofianou D, Flaris N, Leontsini M, Kourtis A, Avramidis A. Serum thyrotropin concentration as a biochemical predictor of thyroid malignancy in patients presenting with thyroid nodules. J Cancer Res Clin Oncol. 2008;134:953–960. doi: 10.1007/s00432-008-0373-7. [DOI] [PubMed] [Google Scholar]
- 57.Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen H. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–814. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 2009;71:434–439. doi: 10.1111/j.1365-2265.2008.03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, Di Coscio G, Berti P, Grasso L, Elisei R, Pinchera A, Vitti P. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16:1251–1260. doi: 10.1677/ERC-09-0036. [DOI] [PubMed] [Google Scholar]
- 60.Jin J, Machekano R, McHenry CR. The utility of preoperative serum thyroid-stimulating hormone level for predicting malignant nodular thyroid disease. Am J Surg. 2010;199:294–297. doi: 10.1016/j.amjsurg.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 61.Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, Di Coscio G, Miccoli P, Grasso L, Pinchera A, Vitti P. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer. 2010;17:231–239. doi: 10.1677/ERC-09-0251. [DOI] [PubMed] [Google Scholar]
- 62.Fiore E, Rago T, Latrofa F, Provenzale MA, Piaggi P, Delitala A, Scutari M, Basolo F, Di Coscio G, Grasso L, Pinchera A, Vitti P. Hashimoto's thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with L-thyroxine. Endocr Relat Cancer. 2011;18:429–437. doi: 10.1530/ERC-11-0028. [DOI] [PubMed] [Google Scholar]
- 63.Boi F, Minerba L, Lai ML, Marziani B, Figus B, Spanu F, Borghero A, Mariotti S. Both thyroid autoimmunity and increased serum TSH are independent risk factors for malignancy in patients with thyroid nodules. J Endocrinol Invest. 2013;36:313–320. doi: 10.3275/8579. [DOI] [PubMed] [Google Scholar]