Abstract
Cervical cancer is on the declining trend in India according to the population-based registries; yet it continues to be a major public health problem for women in India. Multifactorial causation, potential for prevention, and the sheer threat it poses make cervical cancer an important disease for in-depth studies, as has been attempted by this paper. This paper attempts to review the available knowledge regarding the epidemiology and pattern of cervical cancer; types of HPV (human papilloma virus) prevalent among cervical cancer patients and among women in general, high-risk groups such as commercial sex workers, and HIV (human immunodeficiency virus)-positive women; and the role of the national program on cancer in control efforts. The peak age of incidence of cervical cancer is 55–59 years, and a considerable proportion of women report in the late stages of disease. Specific types of oncogenic HPV-16, 18 have been identified in patients with cervical cancer. Other epidemiological risk factors are early age at marriage, multiple sexual partners, multiple pregnancies, poor genital hygiene, malnutrition, use of oral contraceptives, and lack of awareness. A multipronged approach is necessary which can target areas of high prevalence identified by registries with a combination of behavior change communication exercises and routine early screening with VIA. Sensitizing the people of the area, including menfolk, is necessary to increase uptake levels. Vaccination against types 16 and 18 can also be undertaken after taking into confidence all stakeholders, including the parents of adolescent girls. Preventing and treating cervical cancer and reducing the burden are possible by targeting resources to the areas with high prevalence.
Keywords: cervical cancer, HPV, screening, prevention, epidemiology, India
Introduction
Cervical cancer is the commonest cancer cause of death among women in developing countries.1 Mortality due to cervical cancer is also an indicator of health inequities,2 as 86% of all deaths3 due to cervical cancer are in developing, low- and middle-income countries.4
Every year in India, 122,844 women are diagnosed with cervical cancer and 67,477 die from the disease.5 India has a population of 432.2 million women aged 15 years and older who are at risk of developing cancer.5 It is the second most common cancer in women aged 15–44 years.5 India also has the highest age standardized incidence of cervical cancer in South Asia at 22, compared to 19.2 in Bangladesh, 13 in Sri Lanka, and 2.8 in Iran.5 Therefore, it is vital to understand the epidemiology of cervical cancer in India.
India has had a national program for cancer since 1975, when the emphasis was on equipping premier cancer institutions, which, by 1984–1985, shifted to primary prevention and early detection of cancer cases and, by 1990–1991, to the district cancer control program. As of 2008, creation/recognition of new regional cancer centers, strengthening of existing regional cancer centers, development of oncology wings in medical college hospitals, the district cancer control program, and the decentralized NGO scheme were the priorities of the program.6 In 2010, cancer control became a part of a more comprehensive, larger program on noncommunicable diseases called National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Disease and Stroke (NPCDCS) where the common risk factors are addressed in an integrated manner. The present program, initiated on a pilot basis, emphasizes risk reduction and, in addition, promotes opportunistic screening or screening through camps in women above 30 years at different levels in rural areas and in urban slums.7 It also advocates comprehensive cancer care in district-level hospitals and tertiary care centers for strengthening cancer care.
In the absence of a nationwide screening program, there are disparities in screening, treatment, and also survival. An analysis of population-based surveys indicates that coverage of cervical cancer screening in developing countries is 19% compared to 63% in developed countries and ranges from 1% in Bangladesh to 73% in Brazil.8 However, older and poor women who are at the highest risk of developing cancer are least likely to undergo screening. Opportunistic screening in various regions of India varied from 6.9% in Kerala9 to 0.006% and 0.002% in the western state of Maharashtra and southern state of Tamil Nadu, respectively.10,11 Most of the cases (85%) present in advanced and late stages, and more than half (63%–89%) have regional disease at the time of presentation.12 Cervical cancer diagnosis and treatment in the advanced stages makes it a costly exercise, with a poor prognosis resulting in poor compliance. Five-year survival rates in Mumbai population-based cancer registry in 1992–1994 were 47.7% for cervical cancer. Survival was determined by age and the extent of disease, with younger women having longer survival.4 In the 1980s the Bangalore registry reported a 5-year survival of 34.4% and relative survival of 38.3%.13 This registry also observed a significant decline in the proportion of patients presenting in Stage IV from 1982 to 1989, indicating a probable improvement in awareness levels.13
Epidemiology of cervical cancer in India
Distribution of cervical cancer in India and trends
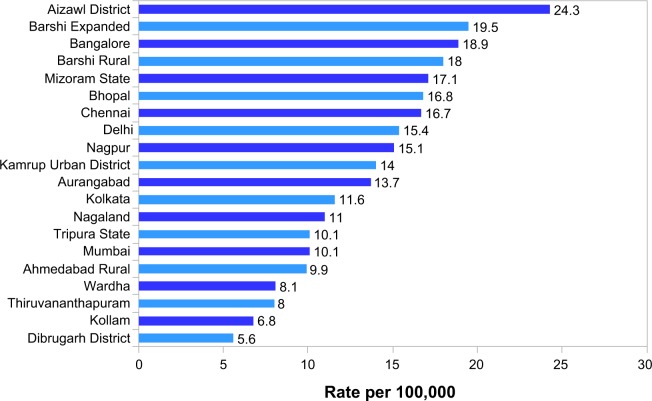
Cancer of the cervix has been the most important cancer among women in the past two decades.13 In India the peak age for cervical cancer incidence is 55–59 years.14 Current data from the National Cancer Registry Program (NCRP) indicates that the most common sites of cancer among women are the breasts and the cervix.13 The recent NCRP data show that between 2009 and 2011 Aizawl district in the north eastern part of India had the highest levels of cervical cancer at an age-adjusted rate of 24.3, followed by Barshi Expanded at 19.5 and Bangalore at 18.9.15 In the Bangalore registry, the age-adjusted rate fell from 32.4 in 1982 to 18.7 in 2009, in Barshi from 22.1 in 1988 to 14.1 in 2010, in Chennai from 41 to 16.7 in 2009, and in Thiruvananthapuram from 9.2 in 2005 to 7.7 in 2011.15 The annual percentage decrease ranged from a minimum of 1.3% in Bhopal to 3.5% in Chennai in the years from 1982 to 2010.16 All the older PBCRs showed a statistically significant decline in age-adjusted rate from the 25–34 age group up to 54, although the Barshi registry showed a decline only up to 44 years.16 The 2010 age-adjusted rate for cervical cancer in the various registries are indicated in Figure 1. The common histological type found in the ectocervix is squamous cell carcinoma and that in the endocervix is adenocarcinoma.2
Figure 1.
Age adjusted incidence rates of cervix uteri-females (rate per 100,000) in the various population based cancer registries.
Note: National Centre for Disease Informatics and Research, National Cancer Registry Programme, Indian Council of Medical Research.16
Abbreviations: PBCR, population based cancer registry; MZ, Mizoram.
All the population-based registries have shown a persistent decline in the age-adjusted rates even in the absence of a control program.13 As early as 1995 the Bangalore Cancer Registry has shown a declining trend.13 The mortality statistics for cervical cancer are incomplete from NCRP, as the cause of death is often incomplete. Great care has been taken to ensure quality of data of incident cases.15 Although good-quality population-based cancer registries are the best indicators of the extent of the problem, hospital-based registries provide information as the outcome of a complex interaction between incidence of disease and health care-seeking behavior. Among hospital-based registries, over a 30-year study period in the Mumbai registry there was an estimated annual percent change in cervical cancer by −1.8% (95% CI: −2.0, −1.6), which was associated with a significant increase in breast cancer.17 Cervical cancer rates among women in the 30–64 age group decreased by 1.8% per year on average but still accounted for 16% of the total female cancer burden.17 Chhabra et al18 found an increase in cervical cancer among outpatients from 0.55% in 1983 to 3.5% in 2007. But over the years from 1983–1987 to 2003–2007 there was a fall in the percentage of all cervical cancers from 43.8% to 37.6%. On the other hand, in Odisha, cancer cervix was the second most common cancer, with an increase of 3.1% from 2001 to 2011.19 In the southern part of India, the north eastern districts of Tamil Nadu show a distinctive pattern with a high incidence of cervical cancer and penile cancer. This may be attributed to infection with human papilloma virus (HPV), which is also implicated in the causation of penile cancer.20 This area also has a high prevalence of human immunodeficiency virus (HIV).21 The high burden of cervical cancer in South and Southeast Asian countries is due to a high prevalence of HPV (more than 10% in women aged more than 30 years) and due to lack of screening.22 In this context it is necessary to understand the prevalence and distribution of HPV in India.
Agent: types in India
HPV has been found to be a necessary but not sufficient cause for cervical cancer.23 Of the more than 100 HPV types, 18 have been categorized as high-risk types, while the rest are low-risk types for cervical cancer.23 HPV prevalence among cervical cancer patients in India has varied from 87.8% to 96.67%.24–27 Molecular studies have shown that HPV-16 and 18 are the two most common highly oncogenic types found in invasive cervical cancer, and out of these two HPV-16 has been found more commonly.28 Prevalence of other high-risk types is very low. On the other hand, genital warts and benign cervical lesions have been associated with 11 low-risk HPV types. Of this, HPV types 6 and 11 cause 90% of the genital warts.29 HPV has also been detected in healthy women and in women with benign cervical cytology.
HPV prevalence among women without cervical cancer varied from 7.5% to 16.9% (Table 1) comparable with a worldwide prevalence of HPV infection between 9% and 13%.29 Hospital-based studies showed a prevalence ranging from 9.9% to 16.6% among women with benign cervical cytology.30–32 The prevalence was higher among high-risk categories such as commercial sex workers at 25%, urban slum in Mumbai at 32.3% and HIV-positive women from 41.7% to 56%.33,34 A meta-analysis by Bhatla et al28 showed that there was no significant difference in the prevalence of HPV infection in North and South India. However, HPV-16 and 45 appeared to be significantly more prevalent in North India, while HPV-35 appeared to be more prevalent in South India. A prospective study in Delhi indicated that persistence was higher for high-risk HPV type and the highest rate of persistence was found in types 16, 45, 67, 31, 51, 59.35 Among the high-risk types, the mean duration of persistence due to HPV-16 was 12.5 months.35 This is the same as reported by Moscicki36 among adolescents in the US. Prevalence of HPV-18 was greater than that of HPV-16, although HPV-16 was associated more frequently with high-grade squamous intraepithelial lesion cytology.37 Among healthy women in a slum in Delhi, the incidence rate for HPV infection was found to be 5 per 1,000 woman-months. In young, HPV-negative women, the cumulative incidence of a first HPV infection has been estimated at 32% at 24 months and 43% at 36 months.35
Table 1.
The extent of distribution of HPV and its types among healthy women/women with gynecological morbidities other than cancer in India
| Authors | Sample size, age group (years) | HPV types measured in study | HPV types detected | Prevalence of infection |
|---|---|---|---|---|
| Community-based studies | ||||
| Franceschi et al,65 2005 | Dindigul District, Tamil Nadu 2,000 Age 16–59 |
Enzyme immunoassay using two HPV oligoprobe cocktails that, together, detect 44 HPV types | High-risk: 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 73, 82 Low-risk: 6, 30, 32, 40, 42, 43, 44, 54, 55, 67, 69, 70, 81, 83, 84, 85, CP6108, JC9710 |
16.9% HPV prevalence; high-risk HPV types 12.5% and low-risk types 6.0%. Most commonly found in either single- or multiple-type infections were HPV-16 (3.8%), HPV-42 (a low-risk type, 2.2%) |
| Sowjanya et al,24 2005 | Medchal, Andhra Pradesh 185 Age >30 |
Primary screening for high-risk HPV, followed by PCR-based line blot for genotype determination | 52, 16 | 10.3% |
| Sarkar et al,66 2008 | High-risk group commercial sex workers, West Bengal 229 | Oncogenic HPV was detected by real-time PCR | 16, 18 (no data about other types) | Prevalence of oncogenic HPV: 25% in a subset (n=112) HPV-16 in 10%, 18 in 7%, both 16 and 18 in 7% |
| Datta et al,67 2010 | Slum in Delhi 1,300 Age 16–24 | HPV genotyping on samples positive by either hybrid capture 2 or PCR | High-risk: 16, 52, 51 Low-risk: 62, 84, 89 |
7% 20 high-risk types and 11 low-risk types were found |
| Laikangbam et al,68 2011 | Manipur (n=692) and Sikkim (n=415) in Northeast India and West Bengal (n=1,112) in eastern India Age 14–80 |
16, 18 | 16, 18 | HPV prevalence in Manipur (7.4%), Sikkim (12.5%), West Bengal (12.9%). HPV-18 was predominant in Manipur (2.03%) and strikingly lower (0.2%) in Sikkim and West Bengal (0.9%), while the reverse was true for HPV-16 |
| Catherine et al,69 2011 | Maharashtra 27,192 Age 30–59 |
Second-generation hybrid capture for high-risk types HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 | 10.3% | |
| Dutta et al37 2012 | Eastern India 2,501 Age 25–65 |
Prevalence of any HPV type and type-specific prevalence of HPV-16/18 | 16, 18 | 9.9% HPV-18 (1.4%) and HPV-16 (0.6%) |
| Beegum et al,70 2014 | Mumbai slum 1,013 couples Age >18 |
– | – | 32.2% |
| Hospital-based studies | ||||
| Arora et al,71 2005 | New Delhi 160 Age 20–60 |
Type-specific primers in PCR for HPV types 16 and 18 | 16, 18 | High-risk HPV (type 16 and 18) prevalence by PCR was found to be 10% (16/160) |
| Aggarwal et al,32 2006 | North India, women with benign cervical cytology 472 Age 19–75 |
Samples were subjected to PCR, using consensus primers for low- and high-risk HPV (types 6, 11, 16, 18, 31, and 33) | 16, 18, 31, 33 | 36.8% women tested positive for HPV DNA, 8.2% positive for high-risk HPV |
| Gupta et al,31 2009 | Delhi 769 Age 18–45 |
High-risk: 16, 18 Low-risk: 6, 11 |
16, 18 | HPV prevalence was 16.6%. HR-HPV-16 was detected in 10.1%, whereas HPV-18 was detected in 1% of women |
| Sahasrabuddhe et al,33 2010 | Hospital based, Pune 303 HIV-infected women Age 30 |
High-risk HPV by hybrid capture method | Not mentioned | 41.7% of high-risk HPV DNA |
| Sarkar et al,72 2011 | Hospital based, West Bengal 93 known HIV-positive females and 1,106 HIV-negative females as controls |
HPV test for 16/18 in all samples and the following genotypes 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 66, 68, 73, 82, 83, and 84 for HIV-positive | 16, 18, 31, 33, 35, 39, 45, 52, 56, 58, 59, 68, 73 | HPV among HIV-positive was found to be 56%, and prevalence of HPV-16 and/or 18 was 32.2%. Among HIV-negative study population, prevalence of HPV-16 and/or 18 was 9.1% |
| Srivastava et al,73 2012 | aEastern Uttar Pradesh 2,424 | All samples were first analyzed for HPV positivity by PCR using L1 consensus primer sets, and then the amplified regions were sequenced to detect the genotypes | Twenty-six different HPV genotypes were detected, including 15 high-risk, 10 low-risk, and 1 intermediate-risk types. 16, 31, 6, 81, 33 in the order of decreasing frequency | 9.9% |
Note:
This study was hospital and camp based (in villages).
Abbreviations: HPV, human papilloma virus; PCR, polymerase chain reaction; HR, high risk.
In Maharashtra, high-risk HPV was associated with increasing age, low education level, manual work, early age at first sexual intercourse, and widowhood/separation.38 The risk of HPV infection in eastern India was found to be higher in married women and in women with parity >4.37 HPV infection was found to significantly decrease with age, whereas other infections increased with the age of women. As the age of cohabitation increased, the HPV infection decreased significantly.39 In Indian women HPV infection is common at 26–35 years of age, which is a decade later than that in developed countries, and cancer occurs between 45 and 59 years of age.29 Hence, there is a long gap between infection and invasive cancer, which gives ample scope for preventive activities.29
The current cervical carcinogenesis model includes three steps of HPV infection, progression to high-grade preinvasive lesions, and invasion.40 More than 95% of infections, including those with cytological abnormalities, resolve spontaneously, returning to HPV DNA negativity with seropositivity.41
HPV testing has attracted a lot of attention in the recent times as a primary screen to be used in conjunction with cytological screening approaches. This approach stems from two observations: HPV testing is 25% more sensitive but has 10% lower specificity; a combination of HPV testing with cytology gives a negative predictive value of nearly 100%.42
HPV infection worldwide
HPV is associated with 50,000 new cases of cervical cancer and 250,000 associated cervical cancer deaths worldwide each year.36 It also causes vulvar, vaginal, anal, and penile cancers as well as precancerous lesions of vulva/vagina, genital warts, and respiratory papilomatosis.36,43,44 HPV infections are asymptomatic, and generally, individuals are not aware of being infected, thus facilitating the spread easily and unknowingly.36
At least 50% of men and women will acquire genital HPV infection during their lifetime.45 All sexually active women are infected with HPV at least once during their lifetime, and the highest prevalence is seen soon after the onset of sexual activities.46,47 Fortunately, in the US, an important proportion of the burden of HPV disease is due to nononcogenic HPV-6 and 11.44 However, due to its contagious nature48 and recurrence,49 it causes morbidity and high levels of anxiety. A majority of episodes of type-specific HPV infection resolve spontaneously within 2 years, but this may be followed by an infection with a new type.44 HPV transmission exclusively occurs following skin-to-skin contact with an infected partner. Sexual intercourse is not necessary, and the virus can be transmitted through sexual foreplay.36 HPV can only replicate in stratified squamous epithelium. HPV infection is the most common sexually transmitted diseases.49 The major risk factor for HPV infection is sexual behavior, including early age of onset of sexual activity, multiple sexual partners, and coinfection with HIV.50 Although the determinants of risk for persistent infection and progression to invasive diseases are not fully understood, persistence appears to be related to HPV type and concurrent infection with multiple virus types.50 The prevalence and distribution of HPV types in the general population as well as in cervical neoplasia vary with geographic region and by the grade of disease.51 Cervical cancer is highly amenable to screening, although early detection of dysplasia has failed women in developing countries, as indicated by the large number of people who report in late stages.
Screening for cervical cancer
Secondary prevention involves screening for precancerous lesions and treating them. The three screening modalities are cytology, visual inspection, and HPV test. In cytology, cells are scraped from the squamo-columnar junction of the cervix and fixed on a glass slide for reading by a trained cytologist.52 This method, commonly called Pap smear, has several limitations, such as high false-negative rates, low sensitivity, subjective interpretation, and low predictive value, as one-third of women who progressed to cervical cancer had a normal Pap smear.53 The notification of results to women as well as the visits required for cytologic screening pose programmatic and logistic challenges.53 In this context, visual screening was developed based on the principle that a higher concentration of intracellular proteins leads to a dense aceto-whitening effect. Its advantage is that it is an easy-to-learn, inexpensive method that requires minimum equipment.53 The sensitivity of VIA to detect CIN2 and 3 lesions and invasive cervical cancer varied from 49% to 96% and the specificity from 49% to 98%.53 Recently, visual inspection with Lugol’s iodine (VILI) was evaluated in cross-sectional studies in India and Africa. The pooled sensitivity and specificity to detect high-grade CIN were 92% and 85%, respectively, for VILI versus 77% and 86% for VIA, indicating a higher sensitivity for VILI but a similar specificity for VILI and VIA.54 Apart from the validity of the tests, the cost-effectiveness of a variety of cervical cancer screening strategies in India, Kenya, Peru, South Africa, and Thailand showed that a onetime screening at 35 years with a 1-visit or 2-visit screening and treatment strategy involving VIA reduced the lifetime risk of cancer by approximately 25%–36% and cost less than US $500 per year of life saved.55
The newer test detecting HPV DNA was conducted at various places in India, and the sensitivity of the test varied from 45.7% to 80.9% for detection of cervical intraepithelial neoplasia grade 2 or worse.56 However, Khan et al57 argue that one should allow for the learning curve of the newly trained technicians and that the 85% sensitivity recorded at one of the centers of the study is in all probability replicable all over India. In another cluster randomized study by Sankaranarayanan et al10 the death rate and incidence rate of cervical cancer was four times more in the cytological, VIA, and control groups compared to the HPV testing group. HPV testing is expensive and requires sophisticated laboratory infrastructure, although it is the most reproducible of all cervical screening tests.53
Other than the availability of screening tests, there are numerous other factors that influence uptake. Age, education, marital status, income, number of children, use of contraception, lack of knowledge about screening of cervical cancer and its prevention, personal and lifestyle factors, attitudes, limited family support, ease of access, and lack of patient-friendly health services are factors affecting screening.9
Other determinants of cervical cancer
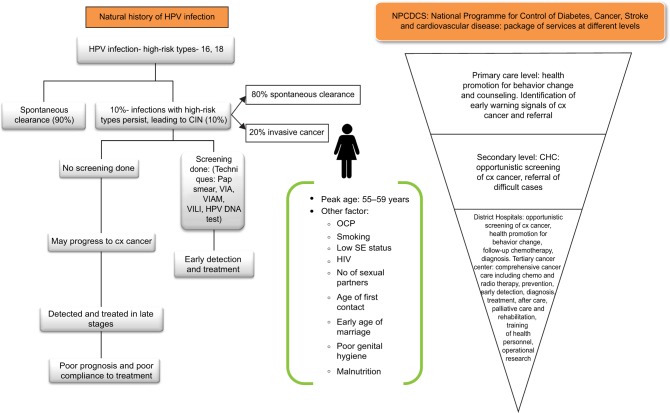
The long interval between initial infection and disease indicates that there are other factors involved, such as sexual habits, reproductive factors, other sexually transmitted diseases, coinfection with HIV, smoking, nutritional deficiency, genetic susceptibility, use of hormonal contraceptives, and high parity.29 Specific religious practices also modify the risk of developing cervical cancer in women following HPV infection.29 In a population-based prospective cohort study in the southern part of India, there was a 2.5-fold increase in risk among women aged 50–59 years compared to those aged 30–39. There was a higher risk with more children and lack of education.58 Similarly, a hospital-based susceptibility study in North India found an increase in cytopathological abnormalities with increasing age and parity.59 Viral sexually transmitted agents such as herpes simplex virus have also been associated with squamous intraepithelial lesions.59 A meta-analysis of social inequality and the risk of cervical cancer showed a 100% increased risk in the low-social-class categories for the development of invasive cervical cancer. Although this difference was observed in all countries, it was stronger in low- and middle-income countries.60 The different factors affecting the development and progression of cervical cancer among women and the provisions under the national program are presented diagrammatically in Figure 2.
Figure 2.
Epidemiology of cervical cancer.
Abbreviations: CHC, community health center; HPV, human papilloma virus; VIA, visual inspection with acetic acid; VIAM, VIA with magnification; VILI, Visual inspection with lugols iodine; CIN, cervical intraepithelial neoplasia; OCP, oral contraceptive pill; SE, socioeconomic status; Cx-cervix.
Postdiagnosis
A retrospective study in a tertiary care center in the eastern part of India showed that even after diagnosis only 38.8% had completed treatment consisting of external radiation and brachytherapy. The reasons mentioned were old age, having many children at home, and inability to travel. Thus, the postdiagnosis scenario is also bleak for thousands of poor women.
Prevention of cervical cancer
HPV is necessary for the development of cervical cancer. Therefore, preventing HPV infection can prevent cervical cancer. This can be achieved by complete abstinence from sexual activity or by a vaccine.1 Primary prevention involves a risk reduction approach through behavioral intervention for sexual and health care-seeking behavior or through mass immunization against high-risk HPV.61
The objective of cervical screening/secondary prevention is to prevent invasive cervical cancer from developing by detecting and treating women with CIN2/3 lesions, and the effectiveness is determined by reduction in incidence and mortality. The critical components of a screening program are an acceptable good-quality screening test, prompt diagnostic investigations, appropriate treatment, and posttreatment follow-up.53 There is strong support from nonexperimental studies62 in developed countries such as Denmark and Finland that the incidence and mortality of cervical cancer can be reduced by screening.63 Ensuring high levels of participation and sufficient health care infrastructure and human resources are important for a screening program to succeed.64 It is also important for screening to be guided by equity considerations for those who are more vulnerable or with lesser access to health care services because of social, economic, or demographic factors.60
Conclusion
Thus, the peak age of occurrence of cervical cancer in India is between 55 and 59 years, and the highest age-adjusted rates are in Aizawl in the north eastern part of India at 24.3 per 100,000 women. Mortality statistics and trends in cervical cancer are lacking due to inadequate and incomplete information on deaths. HPV infection prevalence is 87.8%–96.67% among women with cervical cancer and 9.9%–36.8% among women with no cancer or other gynecological morbidities. There is evidence that cervical cancer incidence is greater among women of lower classes, those less educated, and those with a larger number of children. Screening levels are low in the general population. In order to increase this, it is necessary to carry out specific health education sessions for men and women to facilitate care seeking. The national program NPCDCS has a plan of implementation at the primary, secondary, and tertiary levels where the screening is opportunistic. There are resource limitations to establishing cervical cancer screening program as a priority program all over the country. Simultaneous behavior change communication exercises and routine screening in registry areas with a high incidence can perhaps accelerate the decline. In addition to this, prudent measures to vaccinate adolescent girls can be carried out after getting consent. Research needs to be carried out in making HPV tests cheaper and accessible to the entire population through the national program.
Acknowledgments
The authors are thankful to Dr Helen Angel and Dr Tina Mary for their assistance in editing the manuscript. Aswathy Sreedevi is supported by Fogarty International Centre, National Institutes of Health, under Award Number: D43TW008332 (ASCEND Research Network). The contents of this publication is solely the responsibility of the author(s) and does not necessarily represent the official views of the National Institutes of Health or the ASCEND Research Network. The authors would also like to thank National Cancer Registry programme, India for the permission to use the graph on age adjusted rates of Cervical cancer.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Denny L. Cervical cancer: prevention and treatment. Discov Med. 2012;14:125–131. [PubMed] [Google Scholar]
- 2.Satija A. Cervical cancer in India. South Asia centre for chronic disease. [Accessed February16, 2014]. Available from: http://sancd.org/uploads/pdf/cervical_cancer.pdf.
- 3.Arbyn M, Castellsague X, DeSanjose S, et al. Worldwide burden of cervical cancer. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 4.Yeole BB, Kumar AV, Kurkureet A, Sunny L. Population-based survival from cancers of breast, cervix and ovary in women in Mumbai. Asian Pac J Cancer Prev. 2004;5:308–315. [PubMed] [Google Scholar]
- 5.ICO Information Centre on HPV and cancer (Summary Report 2014-08-22).Human Papillomavirus and Related Diseases in India. 2014 [Google Scholar]
- 6.National Cancer Control Programme. [Accessed July 16, 2014]. Available from: http://www.mohfw.nic.in/showfile.php?lid=324.
- 7.National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Disease and Stroke. [Accessed July 15, 2014]. Available from: http://www.nrhmhp.gov.in/sites/default/files/files/NCD_Guidelines.pdf.
- 8.Gakidou E, Stella N, Ziad O. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PloS Med. 2009;5:e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aswathy S, Quereshi MA, Kurian B, Leelamoni K. Cervical cancer screening: current knowledge and practice among women in a rural population of Kerala, India. Indian J Med Res. 2012;136(2):205–210. [PMC free article] [PubMed] [Google Scholar]
- 10.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 11.Sankaranarayanan R, Esmy PO, Ramkumar R, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 12.Dutta S, Biswas N, Mukheriee G. Evaluation of sociodemographic factors for non compliance to treatment in locally advanced cases of cancer cervix in a rural medical college hospital in India. Indian J of Palliat Care. 2013;19(3):158–165. doi: 10.4103/0973-1075.121530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nandakumar A, Ramnath T, Chaturvedi M. The magnitude of cancer cervix in India. Indian J Med Res. 2009;130(3):219–221. [PubMed] [Google Scholar]
- 14.World – both sexes estimated incidence by age. [Accessed October 30, 2014]. Available from: http://www.globocan.iarc.fr/old/age_specific_table_r.asp?
- 15.National Centre for Disease Informatics Research, National Cancer Registry Programme, ICMR Three Year Report of Population Based Registries, 2009–2011 Bangalore, India: NCDIR-NCRP (ICMR; )2014 [Google Scholar]
- 16.National Centre for Disease Informatics Research, National Cancer Registry Programme, ICMR Time Trends in Cancer Incidence Rates, 1982–2010 Bangalore, India: NCDIR-NCRP (ICMR; )2013 [Google Scholar]
- 17.Dhillon PK, Yeole BB, Dikshit R, et al. Trends in breast, ovarian and cervical cancer incidence in Mumbai, India over a 30-year period, 1976–2005: an age-period-cohort analysis. Br J Cancer. 2011;105(5):723–730. doi: 10.1038/bjc.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhabra S, Bhavani M, Mahajan N, et al. Cervical cancer in Indian rural women: trends over two decades. J Obstet Gynaecol. 2010;30(7):725–728. doi: 10.3109/01443615.2010.501412. [DOI] [PubMed] [Google Scholar]
- 19.Hussain MA, Pati S, Swain S, et al. Pattern and trends of cancer in Odisha, India: a retrospective study. Asian Pac J Cancer Prev. 2012;13(12):6333–6336. doi: 10.7314/apjcp.2012.13.12.6333. [DOI] [PubMed] [Google Scholar]
- 20.Smith PG, Kinlen LJ, White GC, et al. Carcinoma of penis and cervix. Lancet. 1980;2(8191):417. doi: 10.1016/s0140-6736(80)90454-7. [DOI] [PubMed] [Google Scholar]
- 21.Solomon S, Kumaraswamy N, Ganesh AK, et al. Prevalence and risk factors of HIV-1 and HIV-2 infection in rural and urban areas of Tamil Nadu, India. Int J STD AIDS. 1998;9:98–103. doi: 10.1258/0956462981921756. [DOI] [PubMed] [Google Scholar]
- 22.Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programs for cervical cancer in low and middle income developing countries. Bull World Health Organ. 2001;79:954–962. [PMC free article] [PubMed] [Google Scholar]
- 23.International agency for research on Cancer . Human Papilloma Virus. Vol. 90. Lyon: International Agency for Research on Cancer; 2007. IARC monographs on the evaluation of carcinogenic risks to humans. [Google Scholar]
- 24.Sowjanya AP, Jain M, Poli UR, et al. Prevalence and distribution of high-risk human papilloma virus (HPV) types in invasive squamous cell carcinoma of the cervix and in normal women in Andhra Pradesh, India. BMC Infect Dis. 2005;5:116. doi: 10.1186/1471-2334-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni SS, Kulkarni SS, Vastrad PP, et al. Prevalence and distribution of high risk human papillomavirus (HPV) Types 16 and 18 in Carcinoma of cervix, saliva of patients with oral squamous cell carcinoma and in the general population in Karnataka, India. Asian Pac J Cancer Prev. 2011;12(3):645–648. [PubMed] [Google Scholar]
- 26.Gheit T, Vaccarella S, Schmitt M, et al. Prevalence of human papillomavirus types in cervical and oral cancers in central India. Vaccine. 2009;27(5):636–639. doi: 10.1016/j.vaccine.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 27.Basu P, Roychowdhury S, Bafna UD, et al. Human papillomavirus genotype distribution in cervical cancer in India: results from a multi-center study. Asian Pac J Cancer Prev. 2009;10(1):27–34. [PubMed] [Google Scholar]
- 28.Bhatla N, Lal N, Bao YP, et al. A meta-analysis of human papillomavirus type-distribution in women from South Asia: implications for vaccination. Vaccine. 2008;26(23):2811–2817. doi: 10.1016/j.vaccine.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 29.Human Papilloma Virus ICMR: High power Committee to Evaluate Performance of ICMR, 2012–2013. New Delhi, India: ICMR; 2014. Disease Specific Documents for XII plan. [Google Scholar]
- 30.Srivastava S, Gupta S, Roy JK. High prevalence of oncogenic HPV-16 in cervical smears of asymptomatic women of eastern Uttar Pradesh, India: a population-based study. J Biosci. 2012;37(1):63–72. doi: 10.1007/s12038-012-9181-y. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Sodhani P, Sharma A, et al. Prevalence of high-risk human papillomavirus type 16/18 infection among women with normal cytology: risk factor analysis and implications for screening and prophylaxis. Cytopathology. 2009;20(4):249–255. doi: 10.1111/j.1365-2303.2008.00611.x. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal R, Gupta S, Nijhawan R, et al. Prevalence of high-risk human papillomavirus infections in women with benign cervical cytology: a hospital based study from North India. Indian J Cancer. 2006;43(3):110–116. doi: 10.4103/0019-509x.27932. [DOI] [PubMed] [Google Scholar]
- 33.Sahasrabuddhe VV, Ramesh AB, Smita NJ, et al. Prevalence and predictors of colposcopic-histopathologically confirmed cervical intraepithelial neoplasia in HIV-infected women in India. PLoS One. 2010;5(1):e8634. doi: 10.1371/journal.pone.0008634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamalesh S, Reshmi P, Baishali B, Bibhuti S, Subhasish B. Oncogenic HPV among HIV infected female population in West Bengal, India. BMC Infect Dis. 2011;11:72. doi: 10.1186/1471-2334-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta P, Bhatla N, Pandey RM, et al. Type-specific incidence and persistence of HPV infection among young women: a prospective study in North India. Asian Pac J Cancer Prev. 2012;13(3):1019–1024. doi: 10.7314/apjcp.2012.13.3.1019. [DOI] [PubMed] [Google Scholar]
- 36.Moscicki AB. Impact of HPV infection in adolescent populations. J Adolesc Health. 2005;37(6 Suppl):S3–S9. doi: 10.1016/j.jadohealth.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Dutta S, Begum R, Mazumder Indra D, et al. Prevalence of human papillomavirus in women without cervical cancer: a population-based study in Eastern India. Int J Gynecol Pathol. 2012;31(2):178–183. doi: 10.1097/PGP.0b013e3182399391. [DOI] [PubMed] [Google Scholar]
- 38.Sauvaget C, Nene B, Jayant K, et al. Prevalence and determinants of high-risk human papillomavirus infection in middle-aged Indian women. Sex Transm Dis. 2011;38(10):902–906. doi: 10.1097/OLQ.0b013e318223be5f. [DOI] [PubMed] [Google Scholar]
- 39.Shahina B, Naik DO, Nair S, et al. Mobilising women from a low income community to attend cervical cancer screening camps: insights from a study in an urban slum of Mumbai. Gynecol Obstet. 2014;(4):197. [Google Scholar]
- 40.Planning Appropriate Cervical Cancer Prevention Programs. 2nd ed. Seattle WA: Program for Appropriate Technology in Health; 2000. Natural history of cervical cancer; pp. 7–10. [Google Scholar]
- 41.Hildescheim A, Schiffman MH, Gravit PE, et al. Persistence of type specific human pappiloma virus infections among cytologically normal women. J Infect Dis. 1996;(174):927–936. [Google Scholar]
- 42.Monsonego J, Bosch FX, Coursaget P, et al. Cervical cancer control, priorities and new directions. Int J Cancer. 2004;108:329–333. doi: 10.1002/ijc.11530. [DOI] [PubMed] [Google Scholar]
- 43.Bosch FX, Manos M, Muñoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer Study Group. J Natl Cancer Inst. 1995;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 44.Paavonen J. Human papillomavirus infection and the development of cervical cancer and related genital neoplasias. Int J Infect Dis. 2007;11(Suppl 2):S3–S9. doi: 10.1016/S1201-9712(07)60015-0. [DOI] [PubMed] [Google Scholar]
- 45.Department of Health and Human Services Centers for disease control and prevention. Genital HPV infection fact sheet; 2004. [Accessed September 2013]. Available from: http://www.cdc.gov/std/hpv/stdfact-hpv.htm.
- 46.Brown DR, Shew ML, Qadari B, et al. A longitudinal study of genital pappilomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2006;191:182–192. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobs MV, Walboomers JM, Snijders PJ, et al. Distribution of 37 mucosotropic HPV types in women with cytologically normal cervical smears: the age-related patterns for high-risk and low-risk types. Int J Cancer. 2000;87:221–227. [PubMed] [Google Scholar]
- 48.Soper DE. Genitourinary infections and sexually transmitted diseases. In: Berek JS, editor. Novak’s Gynaecology. Philadelphia, PA: Lipincott Williams and Wilkins; 2002. pp. 453–470. [Google Scholar]
- 49.Taylor CA, Keller ML, Egan JJ. Advice from affected persons about living with human papilloma virus infection. Image J Nurs Sch. 1997;29(1):27–32. doi: 10.1111/j.1547-5069.1997.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 50.Human Papilloma Viruses. Vol. 90. Lyon: International agency for research on cancer; 2006. IARC monographs on the evaluation of carcinogenic risks to humans. [Google Scholar]
- 51.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papilloma virus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 52.Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12(3):242s–247s. [PubMed] [Google Scholar]
- 53.Denny L, Sankaranarayanan R. Secondary prevention of cervical cancer. Int J Gynaecol Obstet. 2006;94(Suppl 1):S65–S70. doi: 10.1016/S0020-7292(07)60012-5. [DOI] [PubMed] [Google Scholar]
- 54.Denny L, Quinn M, Sankaranarayanan R. Chapter 8: screening for cervical cancer in developing countries. Vaccine. 2006;24(Suppl 3):S3, 71–77. doi: 10.1016/j.vaccine.2006.05.121. [DOI] [PubMed] [Google Scholar]
- 55.Goldie S, Gaffikin L, Goldhaber-Fiebert J, et al. Cost effectiveness of cervical screening in five developing countries. N Eng J Med. 2005;(353):2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 56.Sankaranarayanan R, Chatterji R, Shastri SS, et al. Accuracy of human papillomavirus testing in primary screening of cervical neoplasia: results from a multicenter study in India. Int J Cancer. 2004;112(2):341–347. doi: 10.1002/ijc.20396. [DOI] [PubMed] [Google Scholar]
- 57.Khan MJ, Mark S, Jose J. Accuracy of human papillomavirus testing in primary screening of cervical neoplasia: results from a multicenter study in India. Int J Cancer. 2005;(116):230–231. doi: 10.1002/ijc.21084. [DOI] [PubMed] [Google Scholar]
- 58.Jissa VT, Nea M, Matti H, et al. Sociodemograhic and reproductive risk factors for cervical cancer-a large prospective cohort study from Rural India. Asian Pac J Cancer Prev. 2012;13:2991–2995. doi: 10.7314/apjcp.2012.13.6.2991. [DOI] [PubMed] [Google Scholar]
- 59.Misra JS, Srivastava S, Singh U, et al. Risk-factors and strategies for control of carcinoma cervix in India: hospital based cytological screening experience of 35 years. Indian J Cancer. 2009;46(2):155–159. doi: 10.4103/0019-509x.49155. [DOI] [PubMed] [Google Scholar]
- 60.Seema P, Paul B, Boffetta P. Meta analysis of social inequality and the risk of cervical cancer. Int J Cancer. 2003;105:687–691. doi: 10.1002/ijc.11141. [DOI] [PubMed] [Google Scholar]
- 61.Sehgal A, Singh V. Human papillomavirus infection (HPV) and screening strategies for cervical cancer. Indian J Med Res. 2009;130:234–240. [PubMed] [Google Scholar]
- 62.Hakama M, Miller AB, Day NE. Screening for Cancer of the Uterine Cervix. Lyon, France: IARC Press; 1986. (IARC Scientific Publications No 76). [Google Scholar]
- 63.Hakama M, Rasanen-Virtanen U. Effectiveness of mass screening program on the risk of cervical cancer. Am J Epidemiol. 1989;(17):173–204. doi: 10.1093/oxfordjournals.aje.a112253. [DOI] [PubMed] [Google Scholar]
- 64.Alliance for cervical cancer prevention (ACCP) Planning and Implementing Cervical Cancer Prevention and Control Programs: A Manual for Managers. Seattle, WA, USA: ACCP; 2004. [Google Scholar]
- 65.Franceschi S, Rajkumar R, Snijders PJF, et al. Papillomavirus infection in rural women in southern India. Br J Cancer. 2005;92(3):601–606. doi: 10.1038/sj.bjc.6602348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarkar K, Bhattacharya S, Bhattacharyya S, et al. Oncogenic human papilloma virus and cervical pre-cancerous lesions in brothel-based sex workers in India. J Infect Public Health. 2008;1(2):121–128. doi: 10.1016/j.jiph.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Datta P, Bhatla N, Dar L, et al. Prevalence of human papillomavirus infection among young women in North India. Cancer Epidemiol. 2010;34(2):157–161. doi: 10.1016/j.canep.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 68.Laikangbam P, Sengupta S, Bhattacharya P, et al. A comparative profile of the prevalence and age distribution of human papillomavirus type 16/18 infections among three states of India with focus on northeast India. Int J Gynecol Cancer. 2007;17(1):107–117. doi: 10.1111/j.1525-1438.2007.00827.x. [DOI] [PubMed] [Google Scholar]
- 69.Sauvaget C, Nene BM, Jayant K, et al. Prevalence and determinants of high-risk human papillomavirus infection in middle-aged Indian women. Sex Transm Dis. 2011;38(10):902–906. doi: 10.1097/OLQ.0b013e318223be5f. [DOI] [PubMed] [Google Scholar]
- 70.Beegum S, Nair S, Donta B, et al. Prevalence of unmet need for contraception in urban slum communities, Mumbai. Int J Reprod Contracept Obstet Gynecol. 2014;3(3):627–630. [Google Scholar]
- 71.Arora R, Kumar A, Prusty BK, et al. Prevalence of high-risk human papillomavirus (HR-HPV) types 16 and 18 in healthy women with cytologically negative Pap smear. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):104–109. doi: 10.1016/j.ejogrb.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 72.Sarkar K, Pal R, Bal B, et al. Oncogenic HPV among HIV infected female population in West Bengal, India. BMC Infect Dis. 2011;11:72. doi: 10.1186/1471-2334-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Srivastava S, Gupta S, Roy JK. High prevalence of oncogenic HPV-16 in cervical smears of asymptomatic women of eastern Uttar Pradesh, India: a population-based study. J Biosci. 2012;37(1):63–72. doi: 10.1007/s12038-012-9181-y. [DOI] [PubMed] [Google Scholar]