Abstract
Aim:
The aim of the research was to compare the relationship between inflammatory biomarkers and procoagulants with kidney function assessed by using cystatin C, serum creatinine, and eGFR and determine the sensitivity of cystatin C, serum creatinine and eGFR to total cardiovascular morbidity in patients with CKD stages 1-4.
Methods:
The research included 120 patients older than 18 years with CKD stages 1-4 monitored over a period of 24 months.
Results:
Serum cystatin C correlates with fibrinogen (p<0.01), serum albumin (p<0.01), D-dimer (p<0.05), antithrombin III (p<0.01) strongly in relation to the evaluation of kidney function based on serum creatinine and eGFR. By following cystatin C, creatinine and eGFR with comparison of ROC to total cardiovascular morbidity, the highest sensitivity in relation to the presence of cardiovascular morbidity shows cystatin C, then eGFR and the lowest, creatinine, with a significant difference between cystatin C and serum creatinine (p<0.05).
Conclusion:
Serum cystatin C is more strongly correlated with some biomarkers (fibrinogen, serum albumin, D-dimer, antithrombin III), while simultaneously showing a stronger sensitivity in relation to total cardiovascular morbidity compared with the assessment of kidney function based on serum creatinine and eGFR.
Keywords: inflammatory biomarkers, procoagulants, cystatin C, cardiovascular morbidity
1. INTRODUCTION
Cardiovascular diseases (CVD) are the leading cause of morbidity and mortality in patients with chronic kidney disease (CKD), and these people have about twenty times higher risk of cardiac death compared to people of the same sex and age without CKD (1). Some studies have shown that among older patients with CKD, traditional Framingham risk factors were strongly associated with cardiovascular (CV) morbidity in relation to new risk factors (2). On the other hand, some authors point out that with the progression of kidney disease, traditional risk factors only partly explained increased CV risk (3). Cardiovascular and CKD are closely associated and there is expected interest in biomarkers that could predict CV morbidity and mortality (4).
Inflammation and CVD are associated with CKD (5). In general population inflammation is one firmly established risk marker of cardiovascular morbidity, where C-reactive protein (CRP) and leucocytosis are independently associated with adverse cardiovascular outcome (6). We should take into consideration different influences of inflammatory cytokines on renal and cardiac function in evaluation of cardio-renal syndrome (7).
Cystatin C as a marker of kidney function can be associated with inflammation. Some studies suggest that cystatin C shows a stronger linear correlation with cardiovascular morbidity and mortality in comparison with serum level of creatinine (8). The results of ‘’The Heart and Soul Study’’ have indicated that the association of cystatin C with cardiovascular diseases may be attributed to inflammation (9). Shlipak confirmed that cystatin C levels had a linear association with the GFR range. This included patients with GFRs of 60–90 mL/min/1.73 m2, a group that previously we had described as “preclinical kidney disease”(10).
2. MATERIAL AND METHODS
This prospective study included 120 patients older than 18 years, examined and monitored over a period of 24 months. Thirty patients in every stage of CKD from 1-4 were included, wherein the stages of CKD are determined according to KDIGO (International Kidney Disease: Improving Global Outcomes) classification (12): 30 patients in stage 1 of CKD, which is characterized by glomerular filtration rate > 90 ml/min/1.73 m² and the presence of albuminuria, proteinuria or pathological urine sediment; 30 patients in stage 2 of CKD, which is characterized by mildly decreased glomerular filtration rate of 60-89 ml/min/1.73 m² and the presence of albuminuria, proteinuria or pathological urine sediment; 30 patients in stage 3 of CKD with moderate reduction of glomerular filtration rate of 30-59 ml/min/1.73 m² and 30 in stage 4 (progressed) of CKD with a glomerular filtration rate of 15-29 ml/min/1.73m².
The research did not include: patients with acute renal failure, patients with kidney transplant, heavier mental state, patients with severe liver failure and liver cirrhosis, with malignant disease, severe infection and sepsis, with primary hematologic disease, type I diabetes, pregnant and lactating women. In the methodological approach in this research we used personal, demographic and anamnesis data, physical examination and methods of laboratory, electrocardiographic and echocardiographic diagnosis.
The cardiovascular morbidity
Total CV morbidity is classified as hypertension, left ventricular hypertrophy (LVH), ischemic heart disease, heart failure, arrhythmias, other vascular diseases (cerebrovascular insult–CVI, pulmonary thromboembolism–PTE, deep vein thrombosis–DVT). Information about heart attack and coronary revascularization, stenosis and some vascular incident (DVT, PTE) within 6 months from the start of the research were obtained from patient’s medical records. Blood pressure was measured in all patients.
The last three measurements were taken into account with their average value calculated. It is thought that the patient is hypertensive if his systolic blood pressure was higher than 140 mmHg, as well as diastolic blood pressure > 90 mmHg. The diagnosis of diabetes was based on positive anamnesis, elevated levels of blood sugar and HbA1C, or based on information about taking insulin or peroral antidiabetic therapy.
Electrocardiography was used for recognition of arrhythmias and acute coronary syndrome while the assessment of systolic LV weaknesses was based on echocardiographic measurement of ejection fraction (EFLV). Echocardiography (ECG) and coronary angiography were used to confirm diagnosis of ischemic coronary disease.
Laboratory diagnostics
The research included determining the following laboratory parameters: serum creatinine, serum cystatin C, serum albumin and C-reactive protein, leukocytes in the blood, plasma fibrinogen, D-dimer, antithrombin III, coagulation factors VII (FC VII) and VIII (FC VIII). Glomerular filtration rate was calculated using MDRD formula (Modification of Diet in Renal Disease), while classification of chronic renal disease was made on the basis of KDIGO criteria (11).
Statistical analysis
The study results were statistically analyzed by using descriptive statistics and Student- t test, with the acceptance of statistical significance at p<0.05. For determining normal distribution of the examined variables was used Kolmogorov-Smirnov test. The statistical differences in mean values between the examined groups was determined by using ANOVA method with Turkeys post hoc test for variables with normal distribution or Kruskal-Wallis and Mann-Whitney post hoc test for variables that do not have a normal distribution. For testing relation between two variables was used correlation coefficient. ROC (receiver operator curve) was used to assess the sensitivity and specificity of the observed parameters.
3. RESULTS
Analysis of the correlation coefficient of relation of cystatin C value towards other variables indicates that the statistically significant positive correlation was recorded to the group, age, creatinine, fibrinogen, D-dimer, FC VIII and total cardiovascular morbidity. Statistically significant negative correlation was recorded only by the values of eGFR, antithrombin III and serum albumins.
The strongest correlation between cystatin C value is achieved towards creatinine, eGFR, FC VIII moderately strong correlation towards cardiovascular morbidity, followed by age, fibrinogen, antithrombin III, serum albumins and the lowest towards the D-dimer. Significant correlation of cystatin C value has not been achieved towards the number of leukocytes, CRP and FC VII.
Analysis of correlation coefficient of relation of eGFR values towards other variables, indicates that the statistically significant negative correlation was recorded and according to age, creatinine, cystatin C, fibrinogen, coagulation factor VIII and total cardiovascular morbidity.
The strongest correlation of eGFR value was achieved towards serum creatinine, cystatin C, moderate towards age, FC VIII, total cardiovascular morbidity and weak towards fibrinogen. Significant correlation of eGFR towards leukocytes, CRP, serum albumin, D-dimer, antithrombin III, FC VII has not been achieved.
Value of mean creatinine statistically significant negative correlated with eGFR values, while positive correlation was confirmed towards cystatin C, FC VIII and total cardiovascular morbidity.
The strongest correlation of serum creatinine value was achieved towards eGFR values, cystatin C, moderate to total cardiovascular morbidity and the lowest towards FC VIII. Significant correlation of serum creatinine value towards leukocytes, CRP, serum albumin, D-dimer, antithrombin III, FC VII has not been proven (Table 1). Analysis of area under the ROC for cystatin C, creatinine and eGFR in relation to the presence of cardiovascular morbidity suggests that cystatin C has the highest sensitivity, followed by eGFR and, finally, the lowest creatinine, with a statistically significant difference between cystatin C and creatinine (p = 0.04), but without statistically significant differences between cystatin C and eGFR (p >0.05) (Figure 1).
Table 1.
Corelation of serum cystatin C, serum creatinine and eGFR with the observed variables. ** Correlation is significant at p <0.01; * Correlation is significant at p <0.05; rho–Person’s coefficient of correlation; CRP–C reactive protein; FC VII–coagulation factor VII; FC VIII–coagulation factor VIII.
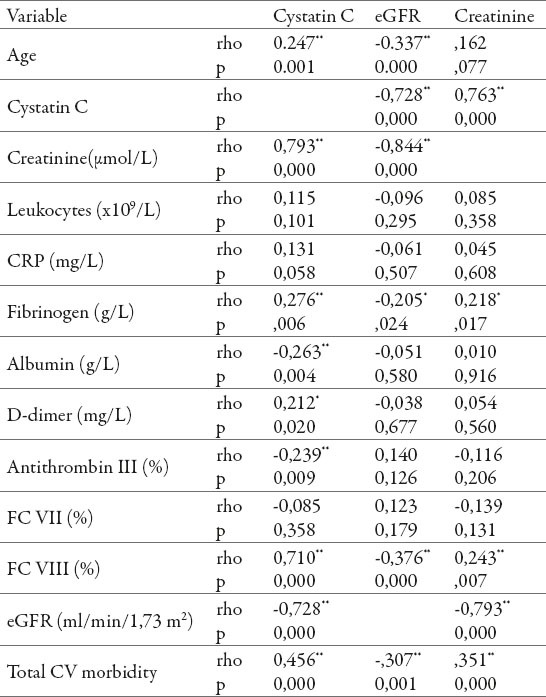
Figure 1.
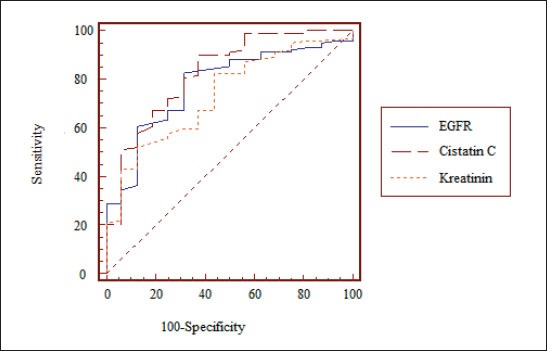
ROC curve of sensitivity and specificity for cystatin C, creatinine and eGFR in relation to the presence of cardiovascular morbidity Notes: The area under the ROC curve -AUC for eGFR is 0,779 (standard error is 0,0711, confidence interval 95% CI -0,694 to 0,849) vs. AUC for serum creatinine of 0,736 (0,0581 with a standard error, confidence interval 95% CI -0,648 to 0,812) vs. AUC for cystatine C of 0,825 (a standard error 0,0447, confidence interval 95%Cl-0,745 to 0,889).
4. DISCUSSION
The paper follows the relationship of renal function assessed through serum creatinine, cystatin C and eGFR with inflammatory and procoagulant markers on one side and the overall cardiovascular morbidity on the other. The connection of inflammation with disorder of renal function and increased cardiovascular morbidity and mortality is an important subject of research by many authors. Earlier studies have shown an association of CKD with elevated CRP, as well as the connection of CRP with cardiovascular risk in patients with ESRD (12). Relation of CV risk and inflammation in patients with milder degree of renal impairment is not sufficiently tested. One recent study showed that inflammation estimated by measuring the levels of CRP correlate with endothelial dysfunction and atherosclerotic changes in patients with CKD (13). Cystatin C is a biomarker of renal function closely associated with the risk of CV morbidity and mortality in patients with and without CKD, as evidenced by a series of prospective studies (14). Cystatin C may be a useful alternative to creatinine for detecting high risk of death and CVD in elderly with CKD (15). Renal dysfunction, on one hand, and inflammatory biomarkers, on the other, are associated with increased cardiovascular risk. Particularly this connection is shown in patients with end stage of renal disease (16).
We found the strongest correlation between cystatin C with age, fibrinogen, serum albumin, antithrombin III, FC VIII and total cardiovascular morbidity (p<0.01), as well as with D-dimer (p<0.05). Significant correlation of serum creatinine and eGFR was confirmed only in relation with FC VIII, overall cardiovascular morbidity (p<0.01) and value of fibrinogen (p <0.05). It is clear that the results of the study point to significantly stronger association of cystatin C with inflammatory biomarkers and procoagulants compared to serum creatinine and renal function assessed by value of eGFR. The correlation of cystatin C is significantly stronger in relation with fibrinogen, serum albumin, antithrombin III and D-dimer.
It would be necessary to give priority to this relationship of cystatin C with inflammation as a prognostic marker in relation to creatinine and eGFR, which in practice can identify the patients of ‘’increased risk’’ from cardiovascular complications. Our results confirmed that cystatin C is the highest sensitive marker for detection of presence of CV morbidity in comparison with other two parameters (serum creatinine and eGFR) using the ROC curve (AUC for cystatin C of 0.825 with a standard error 0.0447, confidence interval 95%Cl -0.745 to 0.889).
This investigation also indicate that serum cystatin C compared to serum creatinine and eGFR is stronger predictor of cardiovascular risk in CKD patients. Previously publised study showed that the value of cystatin C over 1.3 mg/L was strongly associated with increased CV risk (17). Studies that have compared the cystatin C and eGFR for prediction of cardiovascular risk, indicate the possibility that eGFR is not sensitive enough to reflect the connection between mild renal impairment and cardiovascular risk (18). In the Multi–Ethnic Study of Atherosclerosis, serum cystatin C was significantly associated with cardiovascular morbidity in relation to serum creatinine (14). One published study which included an older population of patients also showed that serum cystatin C is a stronger predictor of the risk from cardiovascular morbidity and mortality as compared to values of creatinine and eGFR (2). Correlation between cystatin C and cardiovascular outcome can be a result of other factors which also affect the level of cystatin C, independently from eGFR, such as older age, hypertension, high level of CRP, and together with renal function may affect level of serum cystatin C and increase cardiovascular risk (19). Several studies also indicate how cystatin C could be a better predictor of adverse cardiovascular outcome and all causes of death rather than serum creatinine and eGFR (20).
5. CONCLUSION
Serum cystatin C is strongly correlated with some biomarkers (fibrinogen, serum albumin, D-dimer, antithrombin III), while simultaneously shows a stronger sensitivity in relation to total cardiovascular morbidity compared with the assessment of kidney function based on serum creatinine and eGFR. Cystatin C is important and strong predictor of cardiovascular risk in CKD patients stages 1 to 4 in compared to serum creatinine and eGFR. Stronger correlation of cystatin C with cardiovascular morbidity and simultaneously with inflammatory markers could draw attention to finding new therapeutic options.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Locatelli F, Vigano S. Are natriuretic peptides a reliable marker for mortality in ESRD patients? Nephrol Dial Transplant. 2010;25(2):347–349. doi: 10.1093/ndt/gfp606. [DOI] [PubMed] [Google Scholar]
- 2.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease. Comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel DM, Raggi P, Smits G, Block GA. Factors associated with mortality in patients new to haemodilysis. Nephrol Dial Transp. 2007;22(2):3568–3572. doi: 10.1093/ndt/gfm424. [DOI] [PubMed] [Google Scholar]
- 4.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in Chronic Kidney Disease. Kidney Int. 2011;80(8):806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 5.Upadhyay A, Larson MG, Guo CY, Vasan RS, Lipinska I, ODonnell CJ, Kathiresan S, Meigs JB, Keaney JF, Rong J, Benjamin EJ, Fox CS. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant. 2011;26(3):920–926. doi: 10.1093/ndt/gfq471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno JA, Izquierdo MC, Sanchez-Nino MD, Suarez-Alvarez B, Lopez-Larrea C, Jakubowski A, Blanco J, Ramirez R, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A, Sanz AB. The inflammatory cytokines TWEAK and TNF alfa reduce renal klotho expression through NFkB. J Am Soc Nephrol. 2011;22(7):1315–1325. doi: 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lassus JP, Hariola VP, Peuhkurinen K, Sund R, Mebazaa A, Siirila-Waris K, Miettinen K, Punnonen KR, Melin J, Pulkki K, Nieminen MS. Cystatin C, NT-pro BNP and inflammatory markers in acute heart failure: insights into the cardiorenal syndrome. Biomarkers. 2011;16(4):320–310. doi: 10.3109/1354750X.2011.555822. [DOI] [PubMed] [Google Scholar]
- 8.Singh D, Whooley MA, Joachim HIx, Sadia A, Shlipak MG. Association of Cistatin C and estimated GRF with inflammatory Biomarkers;the Heart and Soul Study. Nephrol Dial Transplant. 2007;22(4):1087–1092. doi: 10.1093/ndt/gfl744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller C, Katz R, Sarnak MJ, Fried LF, Kestenbaum B, Cushman M, Shlipak MG. Inflammatory biomarkers and decline in kidney function in the elderly: Cardiovascular Helth Stady. Nephrol Dial Transplant. 2010;25(1):119–124. doi: 10.1093/ndt/gfp429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shlipak MG, Mattes MD, Peralta CA. Update on Cystatin C. Am J Kidney Dis. 2013;62(3):595–603. doi: 10.1053/j.ajkd.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy AS, Eckardt KU, Tsukamoto Y, eKnoyan G. Definition and classification of chronic kidney disease;A position statement from Kidney Disease;Improving Global Outcomes (KDIGO) Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 12.Vidt DG. Inflammation in renal disease. Am J Cardiol. 2006;97(2A):20A–27A. doi: 10.1016/j.amjcard.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Recio-Mayoral A, Banerjee D, Streather C, Kaski JC. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease-a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011;216(2):446–451. doi: 10.1016/j.atherosclerosis.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Pacold IV, Durazo-Arvizu R, Liu K, Shilipak MG, Goff DC, Jr, Tracy RP, Kramer H. The Effect of Including Cystatin C or Creatinine in a Cardiovascular Risk Model for Asymptomatic Individuals. The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2011;174(8):949–945. doi: 10.1093/aje/kwr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta CA, Lee A, Odden MC, Lopez L, Zeki Al Hazzouri A, Neuhaus J, Haan MN. Association between chronic kidney disease detected using creatinine and Cystatin C and death and cardiovascular events in elderly Mexican Americans: the Secramento Area latino Study on Aging. J Am Geriatr Soc. 2013;61(1):90–95. doi: 10.1111/jgs.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mielniczuk LM, Pfeffer MA, Lewis EF, Blazing M, DeLemos JA, Mohanavelu S, Califf R, Braunwald E. Estimated glomerular filtration rate, inflammation and cardiovascular events following an acute coronary syndrome. Am Heart J. 2008;155(4):725–731. doi: 10.1016/j.ahj.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Lee M, Saver LJ, Huang WH, Chrow J, Chang KH, Ovbiagele B. Impact of elevated cystatin C level on cardiovascular disease risk in predominantly high cardiovascular risk populations: a meta- analysis. Circ Cardiovasc Qual Outcomes. 2010;3(6):675–683. doi: 10.1161/CIRCOUTCOMES.110.957696. [DOI] [PubMed] [Google Scholar]
- 18.Menon V, Shlipak MG, Wang X, Coresh J, Greene T, Stevens L, Kusek JW, Beck GJ, Collins AJ, Levey AS, Sarnak MJ. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007;147(1):19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 19.Stevens LA, Levey AS. Chronic kidney disease in the elderly: How to assess risk. N Engl J Med. 2005;352(20):2122–2124. doi: 10.1056/NEJMe058035. [DOI] [PubMed] [Google Scholar]
- 20.Shlipak MG, Wassel Fyr CL, Chertow GM, Harris TB, Kritchevsky SB, Tylavsky FA, Satterfield S, Cummings SR, Newman AB, Fried LF. Cystatin C and mortality risk in the elderly: the health, aging and body composition. J Am Soc Nephrol. 2006;17(1):254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]


