Abstract
Introduction:
Increased levels of C-Reactive Protein are found in 30-60% on hemodialysis patients and it is closely associated with the progression of atherosclerosis, cardiovascular morbidity and mortality. Non enzymatic antioxidants are antioxidants which primarily retain potentially dangerous ions of iron and copper in their inactive form and thereby prevent its participation in the production of free radicals.
Aim:
The aim of the study was to examine the relationship of CRP and non enzymatic antioxidants (albumin, ferritin, uric acid and bilirubin) i.e. examine the importance of CRP as a serum biomarker in assessing the condition of inflammation and its relationship to antioxidant protection in patients on hemodialysis.
Methods:
The study was cross-sectional, clinical, comparative and descriptive. The study involved 100 patients (non diabetic) on chronic hemodialysis. The control group consisted of 50 subjects without subjective and objective indicators of chronic renal disease. In all patients, the concentration of CRP as well as concentrations of non enzymatic antioxidants were determined.
Results:
In the group of hemodialysis patients 60% were men and 40% women. The average age of hemodialysis patients was 54.13 ± 11.8 years and the average age of the control group 41.72 ± 9.8 years. The average duration of hemodialysis treatment was 91.42 ± 76.2 months. In the group of hemodialysis patients statistically significant, negative linear correlation was determined between the concentration of CRP in and albumin concentration (rho = -0.251, p = 0.012) as well as negative, statistics insignificant, linear correlation between serum CRP and the concentration of uric acid (r = -0.077, p = 0.448). Furthermore, the positive, linear correlation was determined between serum CRP and ferritin (r = 0.159, p = 0.114) and positive linear correlation between CRP and total serum bilirubin (r = 0.121, p = 0.230). In the control group was determined a statistically significant, positive, linear correlation between serum CRP and uric acid concentration (rho = 0.438, p = 0.001) and statistically significant, positive, linear correlation between serum CRP and total serum bilirubin (rho = 0.510, p = 0.0001) A statistically significant, negative linear correlation was determined between CRP and albumin concentration (rho= -0.393, p = 0.005) as well as statistically significant, negative linear correlation between serum CRP and ferritin control group (rho = -0.391, p = 0.005).
Conclusion:
Elevated CRP level is a strong and independent predictor of low levels of serum albumin, which indicates that the hypoalbuminemia in hemodialysis patients could be more due to inflammation than malnutrition. There was no statistically significant correlation between CRP and other non enzymatic antioxidants (uric acid, ferritin, bilirubin), which shows that indicators of antioxidant defense in hemodialysis patients must be individually measured to determine their actual stocks and activity.
Keywords: C-reactive protein, non-enzymatic antioxidants, hemodialysis
1. INTRODUCTION
Increased levels of C-Reactive Protein are found in 30-60% on hemodialysis patients and it is closely associated with the progression of atherosclerosis, cardiovascular morbidity and mortality (1-5). CRP is an acute phase protein that is produced in the liver in response to tissue damage induced by various stimuli such as trauma, inflammation, infections and malignant tumors. Speed of hepatic synthesis of CRP is the only determinant of its plasma concentrations. CRP is a protein with immunomodulatory anti-inflammatory and pro-inflammatory activity and is a strong indicator of systemic inflammatory status in both the general population and in patients with end-stage renal disease. Non enzymatic antioxidants are antioxidants which primarily retain potentially dangerous ions of iron and copper in their inactive form and thereby prevent its participation in the production of free radicals.
Albumin is an important and widely used nutritional marker in a population of hemodialysis (HD) patients. Also, it is a negative acute phase reactant which attributed the major antioxidant role, because it inhibits lipid peroxidation of erythrocyte membrane in HD patients. Studies have shown that hypoalbuminemia is a strong risk factor for mortality in HD patients, and the concentration of albumin in the serum of the negative correlation with the concentration of CRP. Possible cause of hypoalbuminemia in this group of patients is reduced intake of protein foods, volume overload due to poor dialysis adequacy, loss of amino acids and proteins and/or their increased utilization in the state of uremia, and chronic systemic inflammation, and reduced hepatic synthesis of albumin.
The high concentration of uric acid in HD patients is a common finding and is closely associated with oxidative stress and is considered to be an independent marker of inflammation, cardiovascular morbidity and mortality. The contact of blood and dialysis membranes leads to different cross-linking, such as adhesion, aggregation and activation of cells, protein transformations and like. Having in mind that patients with chronic kidney disease (CKD) are dialyzed for years and using different types of membranes for dialysis, a proven imbalance in the oxidants-antioxidants system can cause damage to cell membranes and disorders of cell functions.
2. THE AIM OF THE STUDY
The aim of this study was to examine the relationship of CRP and non enzymatic antioxidants (albumin, ferritin, uric acid and bilirubin) i.e. examine the importance of CRP as a serum biomarker in assessing the condition of inflammation and its relationship to antioxidant protection in patients on hemodialysis.
3. MATERIALS AND METHODS
The study was cross-sectional, clinical, comparative and descriptive. The study involved 100 patients (non diabetic) on chronic hemodialysis. The control group consisted of 50 subjects without subjective and objective indicators of chronic renal disease. The average age of HD patients was 54.13 ± 11.8 years, and statistically different from the average age of the control group 41.72 ± 9.8 years (p = 0.0001). In the group of HD patients, 60% were male, and 40% female. In the control group of respondents, 36.0% were male and 64.0% female. Majority of patients 63% were dialyzed through hemodiafiltration, while 37% patients were dialyzed through hemodialysis. The most common type of access for performing dialysis in HD patients was arterio-venous fistula (AVF) 79%, then tunneled central venous catheter 12% and temporary central venous catheter 9%.
In all subjects the concentration of CRP concentration and non enzymatic antioxidants (albumin, ferritin, uric acid, and bilirubin) was determined. Blood sampling for patients on hemodialysis was conducted at the Clinic for hemodialysis UKCS before the procedure of hemodialysis, and determining their concentration was performed on Clinical chemistry and biochemistry UKCS. CRP in serum was determined by nephelometric method on the device BN II analyzer (reference range of 0-5 mg/L). Albumin was determined by spectrophotometry using bromocresol green reagent (reference range 35-50 g/L). Serum uric acid concentration was determined by spectrophotometry method with uricase (reference value 155-428 µmol/L). Ferritin was determined by a chemiluminescence immunoassay (CMIA) and the reference area is 4,63-204.00 ng/mL, while the determination of bilirubin was done spectrophotometrically (reference value is 1.7-20.5 µmol/L).
Statistical analysis
The relationship between these variables was determined by the correlation coefficient method according to the Spearman using SPSS computer program for statistical analysis (SPSS version 13.0, Chicago, IL, USA).
4. RESULTS
In the group of hemodialysis patients a statistically significant, negative linear correlation was determined between the concentration of CRP in and albumin concentration (rho = -0.251, p = 0.012)(Figure 1).
Figure 1.
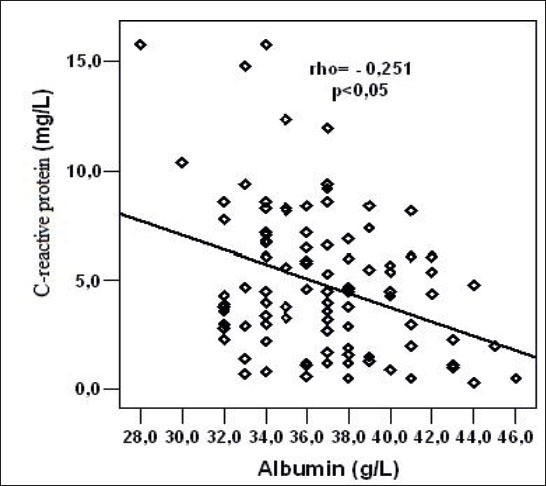
Relation of C-reactive protein and serum albumin concentration in hemodialysis (HD) patients.
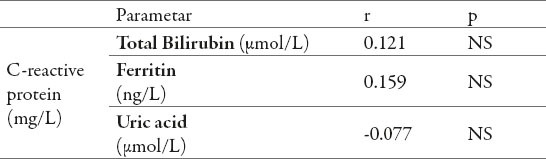
In the group of HD patients was determined a negative, statistically insignificant correlation between the concentration of CRP in serum and serum uric acid levels (r = -0.077, p = 0.448). In the group of HD patients was determined positive, linear correlation between the concentration of CRP in the serum and ferritin concentration in serum (r = 0.159, p = 0.114) also a positive linear correlation between the concentration of CRP and total bilirubin serum concentrations (r = 0.121, p = 0.230). The mentioned positive correlations also were not statistically significant (Table 1).
Table 1.
Relation of C-reactive protein and concentrations of : ferritin, uric acid, and total bilirubin in the serum of hemodialysis (HD) patients.
In the control group a statistically significant, positive, linear correlation was determined between serum CRP and serum uric acid levels (rho = 0.438, p = 0.001)(Figure 2).
Figure 2.
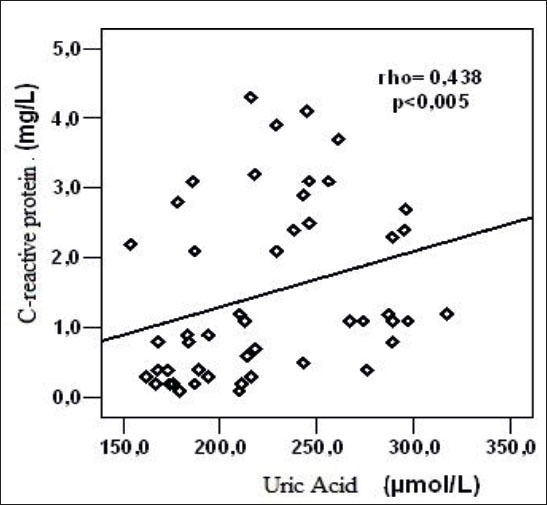
Relation of C-reactive protein and serum uric acid levels of the control group subjects.
There was a statistically significant, positive, linear correlation between serum CRP and serum total bilirubin in serum of control group subjects (rho = 0.510, p = 0.0001)(Figure 3).
Figure 3.
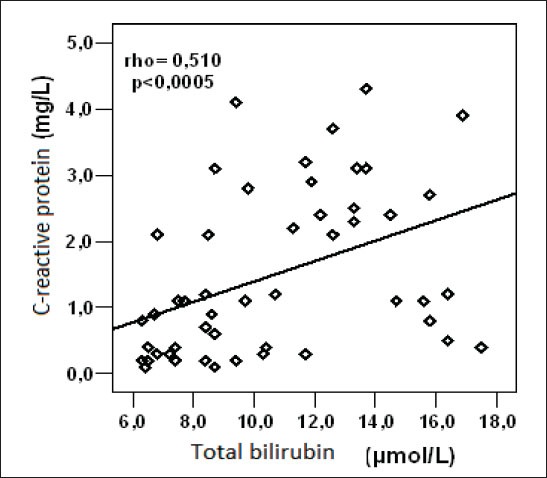
Relation of C-reactive protein and total bilirubin in serum of control group subjects
There was a statistically significant, negative linear correlation between CRP and serum albumin concentration within control group (rho = -0.393, p = 0.005)(Figure 4).
Figure 4.
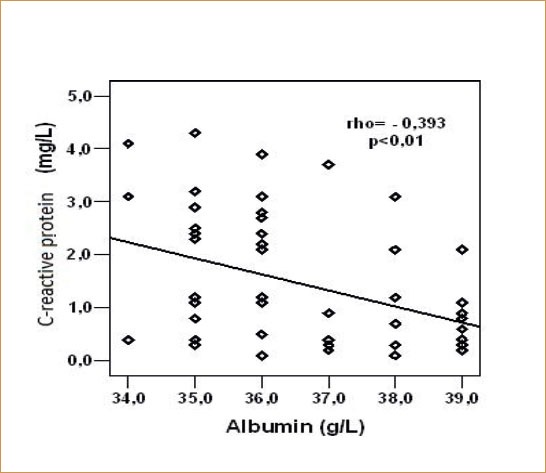
Relation of C-reactive protein and serum albumin concentration within control group
There was a statistically significant, negative linear correlation between serum CRP and ferritin in serum within control group (rho = -0.391, p = 0.005)(Figure 5).
Figure 5.
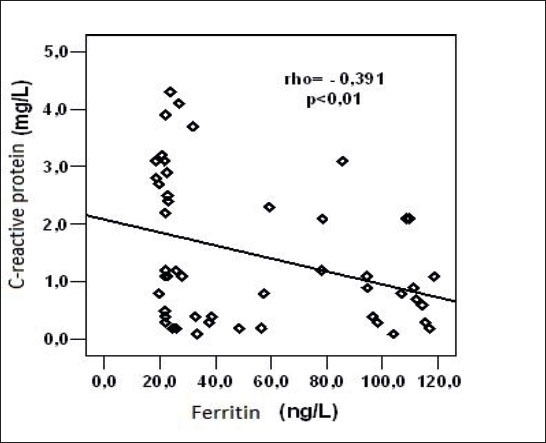
Relation of C-reactive protein and serum ferritin concentration in serum within control group
5. DISCUSSION
Results of our study showed that in both groups, serum albumin concentration was negatively correlated with the concentration of CRP, which is consistent with the results of most of the authors (5). It is already well known that in hemodialysis patients serum levels of CRP and albumin are important predictors of survival (6-11). Meta analysis of Zhang et al., which included 120 studies, summarized the prognostic value of CRP and IL-6 in dialysis patients as the two most common inflammatory markers (1). The analysis found that the elevated levels of one or both markers are significantly associated with total mortality and a higher rate of cardiovascular mortality in dialysis patients, and suggested to be the predictive value of both inflammatory markers used in clinical practice.
The interaction between the blood and the dialysis filter in the course of HD has the potential of activating mononuclear cells, followed by the production of proinflammatory cytokines. The degree of activation depends on the material that is used in the filter, and it is considered an index of biocompatibility. Endoxins from the dialysate and vascular access infections may also boost the production of CRP. Bicarbonate hemodialysis with polysulfone biocompatible membrane and use of ultra pure solution for hemodialysis significantly contribute to reducing CRP. The concentration of CRP is not only based on the type of dialysis membrane, dialysate and vascular access, but also it depends on control of comorbidity, especially diabetes and the presence of infection (7, 12).
In our research, we found a statistically positive correlation between CRP and uric acid only in healthy subjects, whereas in the group hemodialysis subjects referred correlation was negative and non significant. The results of the study showed that the duration of hemodialysis did not significantly affect the change of serum uric acid. Lobo et al. they found significantly higher levels and positive correlation between inflammatory markers and uric acid in HD patients (9). These results suggest that the uric acid may play a role in inflammation and atherosclerosis in these patients.
Hyperuricemia is often associated with chronic renal disease and is considered a marker of kidney dysfunction, and not a factor of disease progression (13).
However, there are controversies regarding the etiological role of uric acid in the development of renal disease. Study Kang and colleagues has provided evidence that hyperuricemia causes hypertension and accelerates the progression of renal disease in rats and that kidney damage is not increased intrarenal deposition of urate crystals (14).
In our study, in the group of hemodialysis patients we did not determine a correlation between the concentration of CRP in serum and non enzymatic antioxidants (bilirubin and ferritin), which can be explained by the fact that the majority of hemodialysis patients are treated with human erythropoietin or intravenous iron that influence the concentration of these two antioxidants, as well as the blood samples were taken immediately before the hemodialysis treatment. Similar results in their study were given by Deira et al. (15).
6. CONCLUSION
Elevated CRP level is a strong and independent predictor of low levels of serum albumin, which indicates that the hypoalbuminemia in hemodialysis patients could be more due to inflammation than malnutrition. There was no statistically significant correlation between CRP and other non enzymatic antioxidants (uric acid, ferritin, bilirubin), which shows that indicators of antioxidant defense in hemodialysis patients must be individually measured to determine their actual stocks and activity.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Zhang W, He J, Zhang F, Huang C, Wu Y, Han Y, Zhang W, Zhao Y. Prognostic role of C-reactive protein and Interleukin-6 in dialysis patients: a systemic review and meta analysis. J Nephrol. 2013;26(2):243–253. doi: 10.5301/jn.5000169. [DOI] [PubMed] [Google Scholar]
- 2.Caglar K, Hakim RM, Ikizler TA. Approaches to the reversal of malnutrition, inflammation and atherosclerosis in end-stage renal disease. Nutr Rev. 2002;60(11):378–387. doi: 10.1301/00296640260385928. [DOI] [PubMed] [Google Scholar]
- 3.Johansen LK, Kaysen AG, Yong SB, Hung MA, da Silva M, Chertowet MG. Longitudinal study of nutritional status, body composition and physical function in hemodialysis patients. Am J Clin Nutr. 2003;77:842–846. doi: 10.1093/ajcn/77.4.842. [DOI] [PubMed] [Google Scholar]
- 4.Friedman AN, Fadem SZ. Reassessment of Albumin as a Nutritional Marker in Kidney Disease. J Am Soc Nephrol. 2010;21(2):223–230. doi: 10.1681/ASN.2009020213. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi I, Ishimura E, Kato Y, Okunoet S, Yamamoto T, Yamakawa T, Mori K, Inaba M, Nishizawa Y. Geriatric Nutritional Risk Index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transplant. 2010;25(10):3361–3365. doi: 10.1093/ndt/gfq211. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg J, Kissova V, Majernikova M, Straussova Z, Boldizsar J. Body composition monitor assessing malnutrition in the hemodialysis population independently predicts mortality. J Ren Nutr. 2014;24(3):172–176. doi: 10.1053/j.jrn.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Bazeley J, Bieber B, Li Y, Morgenstern H, de Sequera P, Combe C, Yamamoto H, Gallagher M, Port FK, Robinson BM. C-reactive protein and prediction of 1-tear mortality in prevalent hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(10):2452–2461. doi: 10.2215/CJN.00710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahrokh S, Heydarian P, Ahmadi F, Saddadi F, Razeghi E. Association of inflammatory bioomarkers with metabolic syndrome in hemodialysis patients. Renal Failure. 2012;34(9):1109–1113. doi: 10.3109/0886022X.2012.713280. [DOI] [PubMed] [Google Scholar]
- 9.Calixto Lobo J, Stockler-Pinto BM, da Nobrega ACI, Carraro-Eduardo JC, Mafra D. Is There Association between Uric Acid and Inflammation in Hemodialysis Patients? Renal Failure. 2013;35(3):361–366. doi: 10.3109/0886022X.2013.764274. [DOI] [PubMed] [Google Scholar]
- 10.Heidari B. C-reactive protein and other markers of inflammation in hemodialysis patients. Caspian J Intern Med. 2013;4(1):611–616. [PMC free article] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Abbot KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 12.Wong KC, Szeto CC, Chan MHM, Leung CB, Li PKT, Lam CWK. Elevation of Pro-Inflammatory Cytokines, C-Reactive Protein and Cardiac Troponin T in Chronic Renal Failure Patients on Dialysis. Immunol Invest. 2007;36(1):47–57. doi: 10.1080/08820130600745505. [DOI] [PubMed] [Google Scholar]
- 13.Coaccioli S, Standoli ML, Biondi R, Panaccione A, Landucci P, Del Giorno R, Paladini A, Standoli M, Puxeddu A. Open comparison study of oxidative stress markers between patients with chronic renal failure in conservative therapy and patients in haemodialysis. Clin Ter. 2010;161(5):435–439. [PubMed] [Google Scholar]
- 14.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13(12):2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 15.Deira J, Diego J, Martinez R, Oyarbide A, Diaz H, Ochoa C, Tabernero J, Grande J. Soluble transferin receptor levels in hemodialysis patients. Nephrol Dial Transplant. 2003;18(9):1945–1946. doi: 10.1093/ndt/gfg273. [DOI] [PubMed] [Google Scholar]


