Abstract
Objective
To investigate the association between physical activity (eg, energy expenditure) and survival over 11 years of follow-up in a large representative community sample of older Brazilian adults with a low level of education. Furthermore, we assessed sex as a potential effect modifier of this association.
Materials and methods
A population-based prospective cohort study was conducted on all the ≥60-year-old residents in Bambuí city (Brazil). A total of 1,606 subjects (92.2% of the population) enrolled, and 1,378 (85.8%) were included in this study. Type, frequency, and duration of physical activity were assessed in the baseline survey questionnaire, and the metabolic equivalent task tertiles were estimated. The follow-up time was 11 years (1997–2007), and the end point was mortality. Deaths were reported by next of kin during the annual follow-up interview and ascertained through the Brazilian System of Information on Mortality, Brazilian Ministry of Health. Hazard ratios (95% confidence intervals [CIs]) were estimated by Cox proportional-hazard models, and potential confounders were considered.
Results
A statistically significant interaction (P<0.03) was found between sex and energy expenditure. Among older men, increases in levels of physical activity were associated with reduced mortality risk. The hazard ratios were 0.59 (95% CI 0.43–0.81) and 0.47 (95% CI 0.34–0.66) for the second and third tertiles, respectively. Among older women, there was no significant association between physical activity and mortality.
Conclusion
It was possible to observe the effect of physical activity in reducing mortality risk, and there was a significant interaction between sex and energy expenditure, which should be considered in the analysis of this association in different populations.
Keywords: physical activity, mortality, sex, elderly
Introduction
The quickly aging population and consequent increases of noncommunicable chronic diseases make physical inactivity a major risk factor among older adults. Worldwide figures show that physical inactivity is responsible for 6% of cardiovascular diseases, 7% of type II diabetes mellitus, 10% of breast cancers, and 10% of bowel cancers, which all contribute to 9% of premature deaths.1 Global strategies by the World Health Organization to promote increases in levels of physical activity2 have been incorporated into the Strategic Action Plan to Combat Noncommunicable Diseases in Brazil.3 Evidence shows that the practice of regular physical activity could reduce the physiological process of aging and increase the survival rate by limiting both the development and progress of chronic diseases and by preserving physical functioning, in addition to psychological and cognitive benefits.4
Some studies have already investigated the association between physical activity and mortality risk among older adults.5,6 However, this association was not consistent in different populations, and it showed sex differences. Despite research showing associations of similar magnitude among men and women,6–8 other studies have shown a reduction in the mortality risk only in older women,9,10 whereas other studies have found a significant association only in men.11,12 Therefore, sex could be a potential effect modifier of this association in older adults.
The prevalence of sedentary behavior increases with age,13–15 and health policies to promote physical activity among older adults are needed. In Brazil, information from the VIGITEL (Telephone-Based Surveillance of Risk and Protective Factors for Chronic Diseases), using a representative sample of adults from 26 state capitals and the Federal District, showed that 32.3% of older adults (aged 65 years and older) were physically inactive considering the following domains: leisure, work, transportation, and household.14 Additional studies conducted in older Brazilian adults found that the prevalence of sedentary behavior related to leisure varied from 71% to 78%.16,17 After all physical activity domains were analyzed, the variation was even greater (26%–69%).13,18,19 Previous findings from the baseline of the Bambuí cohort indicated that the sedentary behavior, considering all physical activities, was approximately 31.2%, and was higher in women (34.4%) than men (26.4%).15
In regard to the effect of physical activity on mortality risk in older adults, it is important to consider that most evidence results from studies conducted in developed countries. There is no similar study that has been conducted in Latin America. Therefore, the present study aimed to investigate the association between energy expenditure, which is an estimate of physical activity level, and survival over 11 years of follow-up in a large representative community sample of older Brazilian adults with a low level of education. Furthermore, we aimed to assess sex as a potential effect modifier of this association.
Materials and methods
Data
Data were collected from the baseline interview (1997) of the Bambuí Cohort Study of Aging, which comprised 1,606 (92% of the total population) people aged 60 years and older in Bambuí (approximately 15,000 inhabitants), Minas Gerais State, southeastern Brazil. This cohort study was designed and developed to investigate the incidence and predictors of adverse health outcomes in an elderly Brazilian population with low education and income levels and in epidemiological transition (that is, with a high prevalence of noncommunicable chronic diseases, but also widespread Trypanosoma cruzi infection, a protozoan that causes Chagas disease, whose main feature is heart involvement). The Bambuí cohort members had a face-to-face interview each year, and there was an additional health examination at baseline and in selected years of follow-up. The Bambuí cohort methodology has been described elsewhere.20 The Bambuí cohort study was approved by the ethics board of the Fundação Oswaldo Cruz, Rio de Janeiro, Brazil. Participants gave full informed consent to participate in this study and authorized death-certificate verification.
Mortality data source
Deaths that occurred from January 1, 1997 to December 31, 2007 were included in this analysis. Deaths were reported by next of kin during the annual follow-up interview and ascertained through the Brazilian System of Information on Mortality, Ministry of Health; death certificates were obtained for 98.9% of individuals. Deaths assigned to any cause were considered in this analysis.
Physical activity assessment
Information regarding physical activity was collected at baseline using a questionnaire with 23 closed questions and two open questions about physical activities performed by the participants in the past 3 months. The questionnaire included type, frequency, and average duration (in minutes) for each physical activity. The following physical activities were included: walking leisurely (2.5 mph [4 km/h]), going up stairs at a normal speed, going up stairs fast or carrying a load, mopping or scrubbing floors, cleaning windows, swimming (leisurely), dancing, rhythmic dancing, cycling (<10 mph, for leisure or to work), home repairs (painting), volleyball, tennis, basketball, football, walking fast (3.5 mph, brisk level, on a firm surface, walking for exercise), aerobics/gym workout, running/jogging, gardening (digging with a spade), sawing wood, horse riding (racing, galloping, trotting), shuttlecock (volleyball), cycling quickly, and cycling a steep hill. The physical activity data were carefully coded by a qualified physiotherapist, who is an author of this manuscript.
The level of physical activity was calculated based on the level of oxygen consumed for each physical activity. This method allowed the research team to quantify the energy expenditure in MET (metabolic equivalent task) according to the Compendium of Physical Activity.21 One MET represents the energy expenditure by a resting individual with an oxygen consumption of 3.5 mL/kg of body weight per minute (3.5 mL·O2·kg−1·min−1). Therefore, the intensity of the physical activities was classified into three groups (light, moderate, and vigorous) according to the MET values.22
The assessment of energy expenditure was calculated by multiplying the MET (intensity of the physical activity performed) by time (duration in minutes) and by frequency (how many times a week), considering only moderate and vigorous activities and a duration of 10 minutes or longer.22,23 The outcome variable of this study was the energy expenditure measured in MET-minute/week. In this study, tertiles of the MET variable at baseline were used. Further details about the physical activity assessment used in the Bambuí cohort have been described elsewhere.15
Confounders
The potential confounding variables were selected based on previous research on the association between physical activity and mortality risk.6–8 The confounders included sociodemographics (age, sex, and years of education), health behaviors (smoking, alcohol consumption, and body mass index [BMI], kg/m2), and health status measures: systolic blood pressure (mmHg), total cholesterol (mg/dL), fasting glucose (mg/dL), B-type natriuretic peptide (BNP) (pg/dL), stroke, angina, infarct, and the Mini-Mental State Examination (MMSE).
Current smokers were those who had smoked at least 100 cigarettes during their lifetime and were currently smoking. Alcohol consumption was measured by the amount of alcohol consumed in the past 12 months before the interview. BMI was calculated using the standard formula (kg/m2), and was used as a continuous variable. The plasma levels of BNP, which is an important predictor of mortality risk among older adults infected by T. cruzi,24 were measured by an immunoassay using microparticles (MEIA/AxSYM; Abbott Laboratories, Chicago, IL, USA). A medical history of infarct was assessed by a single question and a history of stroke,25 and angina26 by standardized instruments. Cognitive function was evaluated by the MMSE.
The interviews were conducted at participants’ homes and answered by the older adult him/herself or by a proxy (4.8%) for those with a cognitive deficit or a very serious health condition. Blood pressure and anthropometric measures together with collection of blood samples were performed at the project’s health clinic, except if the participants were not able to leave their homes. For the blood samples, participants were asked to fast for at least 12 hours. All research procedures were conducted by trained interviewers and well-qualified technicians. Further details can be found elsewhere.20
Statistical analysis
The univariate analyses of the variables associated with energy expenditure at baseline were based on Pearson’s χ2 test and analysis of variance with multiple Bonferroni comparisons. The mortality rates were calculated using person-years at risk (pyrs) as the denominator for each sex and tertile of energy expenditure.
Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between energy expenditure and all-cause mortality risk during the 11-year follow-up period were estimated by Cox proportional-hazard models, after confirming that the assumption of proportionality among the hazards was met based on Schoenfeld residuals.
Three models were built in this analysis with progressive inclusion of confounders. The first model included tertiles of energy expenditure, age, sex, and years of education. At this stage, there was a significant interaction (P<0.02) between sex and energy expenditure, and the results are presented for each sex. It was decided to keep the interaction term in the following models. There was no significant interaction between physical activity and age (P>0.30). Smoking, alcohol consumption, and BMI were included in the second model. In the final model, the variables related to health status were added.
Survival curves were computed to show the interaction between sex and energy expenditure in both sexes. These curves were stratified by the physical activity level (tertiles of energy expenditure), and adjusted for all potential confounding variables selected in this study. The continuous variables were centralized around their mean values, and the categorical variables were fixed in the reference category.
Two sensitivity analyses were conducted: 1) excluding deaths that occurred in the first 2 years of follow-up, assuming that this group could have already been seriously ill, which would compromise the participant’s ability to do any physical activity,6,9,27 and 2) excluding those participants who reported having at least one chronic disease at baseline (stroke, angina, infarct, diabetes, and hypertension) that would compromise the participant’s ability to do any physical activity and increase mortality risk.9 Statistical analyses were conducted using version 13.0 of Stata statistical software (StataCorp, College Station, TX, USA).
Results
Of the 1,606 eligible participants at baseline, 1,378 (85.8%) had available data for all study variables that were included in the analyses. During an average period of 8.9 years, 498 participants died (36.1%), and 90 (6.5%) were lost (that is, their vital status could not be assessed), yielding 12,255 pyrs of observation. The lost participants were slightly younger (mean age 67.3 years, standard deviation [SD] 5.9) compared to those included in the analyses (mean age 68.9, SD 7.0) (P=0.025), and both groups had similar numbers of men and women (P=0.592).
Table 1 shows the sample characteristics at baseline by tertiles of energy expenditure. The highest tertile of energy expenditure was younger, with fewer women and fewer older adults with a lower level of education. This group also consumed more alcohol in the last year, had a lower prevalence of stroke, had higher MMSE scores, and had lower plasma levels of BNP.
Table 1.
Selected baseline characteristics of participants by physical activity level
| Characteristics | Total | Energy expenditure in MET-minute/week
|
P-value | ||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||
| Age in years, mean (SD) | 68.8 (7.0) | 70.9 (7.4)a | 68.8 (7.0)b | 67.2 (6.0)c | <0.001 |
| Women, n (%) | 833 (60.5) | 274 (68.2) | 307 (63.4) | 252 (51.2) | <0.001 |
| <4 education years, n (%) | 872 (63.3) | 255 (63.4) | 329 (68.0) | 288 (58.5) | 0.009 |
| Current smoking, n (%) | 244 (17.7) | 70 (17.4) | 88 (18.2) | 86 (17.5) | 0.944 |
| Alcohol consumption in the last year, n (%) | 302 (21.9) | 58 (14.4) | 93 (19.2) | 151 (30.7) | <0.001 |
| Body mass index, kg/m2, mean (SD) | 25.1 (4.9) | 25.1 (5.6) | 25.2 (4.7) | 25.1 (4.5) | 0.929 |
| Systolic blood pressure, mmHg, mean (SD) | 137.4 (22.4) | 139.1 (24.9) | 137.6 (21.1) | 135.8 (21.2) | 0.088 |
| Total cholesterol, mg/dL, mean (SD) | 233.9 (48.7) | 232.5 (49.3) | 236.3 (49.4) | 232.6 (47.7) | 0.385 |
| Fasting glucose in mg/dL, mean (SD) | 108.9 (42.9) | 109.7 (37.0) | 110.2 (45.3) | 106.9 (44.8) | 0.443 |
| Stroke, n (%) | 45 (3.3) | 24 (6.0) | 9 (1.9) | 12 (2.4) | 0.001 |
| Angina, n (%) | 125 (9.1) | 38 (9.5) | 42 (8.7) | 45 (9.2) | 0.921 |
| Infarct, n (%) | 66 (4.8) | 25 (6.2) | 26 (5.4) | 15 (3.1) | 0.066 |
| Mini-Mental State Examination (MMSE) score, mean (SD) | 24.7 (4.5) | 23.7 (5.2)a | 24.7 (4.3)b | 25.4 (3.8)c | <0.001 |
| B-type natriuretic peptide (BNP), pg/dL, mean (SD) | 125.4 (166.0) | 141.1 (214.7)a | 131.3 (156.6)a,b | 106.6 (122.3)b | 0.005 |
Notes: Tertile 1: 0 to 515.0 MET-minute/week; Tertile 2: 515.1 to 1,529.9 MET-minute/week; Tertile 3: 1,530.0 to 16,765.5 MET-minute/week. Pearson chi-square test for comparisons between proportion and analysis of variance (with Bonferroni adjustment) for comparison between means; the letters a, b, c refer to the statistic differences in the analysis of variance; different letters show significant differences.
Abbreviations: MET, metabolic equivalent task; n, number enrolled in group; SD, Standard Deviation.
The all-cause mortality rate was 40.6 per 1,000 pyrs, and it was higher in men (49.5 per 1,000 pyrs) compared to women (35.2 per 1,000 pyrs). The mortality rates decreased with increases in energy expenditure. This tendency was observed in both sexes (Table 2).
Table 2.
Number of deaths and mortality rates over 11 years, by tertiles of energy expenditure at baseline
| Energy expenditure in MET-minute/week | Total
|
Men
|
Women
|
|||
|---|---|---|---|---|---|---|
| Number of deaths | Mortality rate per 1,000 pyrs | Number of deaths | Mortality rates per 1,000 pyrs | Number of deaths | Mortality rates per 1,000 pyrs | |
| Tertile 1 | 195 | 58.6 | 86 | 92.7 | 109 | 45.4 |
| Tertile 2 | 175 | 40.5 | 76 | 50.4 | 99 | 35.1 |
| Tertile 3 | 128 | 27.8 | 68 | 30.8 | 60 | 25.1 |
| Total | 498 | 40.6 | 230 | 49.5 | 268 | 35.2 |
Notes: Tertile 1: 0 to 515.0 MET-minute/week; Tertile 2: 515.1 to 1,529.9 MET-minute/week; Tertile 3: 1,530.0 to 16,765.5 MET-minute/week.
Abbreviations: MET, metabolic equivalent task; pyrs, person-year at risk.
Table 3 presents the HRs and 95% CIs for 11-year survival by each energy-expenditure tertile. In men, all models showed that an increase in physical activity was associated with reductions in mortality risk. The HRs for the fully adjusted model were 0.59 (95% CI 0.43–0.81) and 0.47 (95% CI 0.34–0.66) for the second and third tertiles, respectively. In women, there was no association between physical activity and mortality risk. In the final adjusted analysis, HRs were 0.99 (95% CI 0.75–1.31) and 0.86 (95% CI 0.62–1.19) for the second and third tertiles, respectively. A significant interaction (P<0.03) was found between sex and tertiles of energy expenditure in all three models, showing that the effect of physical activity on survival over 11 years differed by sex.
Table 3.
Hazard ratio for 11-year mortality, by tertiles of energy expenditure at baseline
| Energy expenditure in MET-minute/week | Model 1
|
Model 2
|
Model 3
|
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Men | |||
| Tertile 1 | 1.0 | 1.0 | 1.0 |
| Tertile 2 | 0.54 (0.40–0.74) | 0.57 (0.42–0.77) | 0.59 (0.43–0.81) |
| Tertile 3 | 0.37 (0.27–0.52) | 0.41 (0.30–0.57) | 0.47 (0.34–0.66) |
| Women | |||
| Tertile 1 | 1.0 | 1.0 | 1.0 |
| Tertile 2 | 0.93 (0.71–1.23) | 0.92 (0.70–1.21) | 0.99 (0.75–1.31) |
| Tertile 3 | 0.74 (0.54–1.02) | 0.74 (0.53–1.02) | 0.86 (0.62–1.19) |
| P-value for the interaction between sex and energy expenditure | <0.02 | <0.03 | <0.02 |
Notes: Model 1 (n=1,378): adjusted by age, sex, education years and interaction term between tertiles of energy expenditure and sex. Model 2 (n=1,378): model 1 plus current smoking, alcohol consumption, and body mass index. Model 3 (n=1,378): model 2 plus systolic blood pressure, total cholesterol, fasting glucose, stroke, angina, infarct, Mini-Mental State Examination score, and levels of B-type natriuretic peptide. Tertile 1: 0 to 515.0 MET-minute/week; Tertile 2: 515.1 to 1,529.9 MET-minute/week; Tertile 3: 1,530.0 to 16,765.5 MET-minute/week.
Abbreviations: HR (95%CI), Hazard ratio and 95% confidence intervals; MET, metabolic equivalent task.
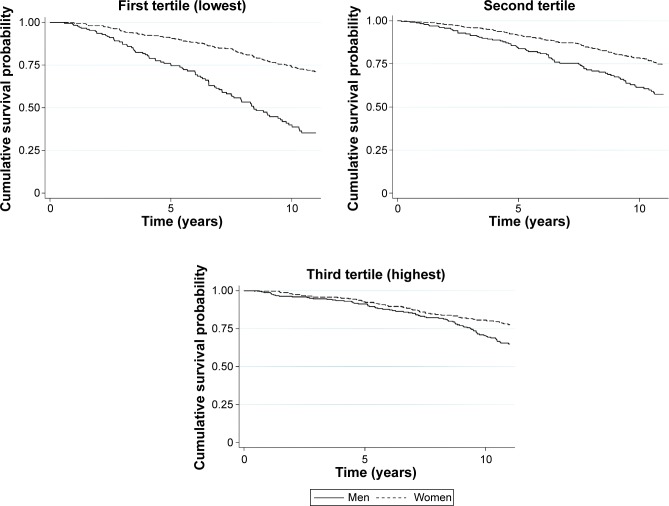
Fully adjusted survival curves for 11 years of follow-up by sex and energy-expenditure tertiles are shown in Figure 1. Survival decreased with time and was higher among women than men in the first and second tertiles of energy expenditure. Sex differences in mortality were not observed among older adults in the highest level of physical activity.
Figure 1.
Cumulative survival probability estimates, adjusted for all confounders, by sex and tertiles of energy expenditure.
Notes: n=1,378; Curves adjusted by age, sex, years of education, interaction term between tertiles of energy expenditure and sex, current smoking, alcohol consumption, body mass index, systolic blood pressure, total cholesterol, fasting glucose, stroke, angina, infarct, Mini-Mental State Examination score and levels of B-type natriuretic peptide. First tertile: 0 to 515.0 MET-minute/week; Second tertile: 515.1 to 1,529.9 MET-minute/week; Third tertile: 1,530.0 to 16,765.5 MET-minute/week.
Abbreviation: MET, metabolic equivalent task.
There was no significant interaction between energy expenditure and age, even after full adjustment (P>0.30). The sensitivity analysis showed that excluding deaths that occurred in the first 2 years of follow-up did not change the significance levels of the associations observed in the three models. However, the effect of physical activity on mortality was stronger after excluding participants who had at least one chronic disease (stroke, angina, infarct, diabetes, and hypertension) at baseline, with a significant association in men (HR 0.41, 95% CI 0.22–0.77 and HR 0.30, 95% CI 0.16–0.58 for the second and third tertiles, respectively). In women, however, this association was not significant (HR 0.81, 95% CI 0.43–1.53 and HR 0.68, 95% CI 0.34–1.39 for the second and third tertiles, respectively).
Discussion
The findings in this study showed an important interaction between sex and energy expenditure due to physical activity on the survival of older Brazilian adults. Higher levels of physical activity were associated with a greater reduction of all-cause mortality risk in men. However, there were no significant associations among older women. It was also found that among the participants in the highest tertile of energy expenditure, the survival rates of men and women were similar. This finding indicates that higher physical activity levels in older men increase their survival rate to a similar rate observed in older women.
The literature on the relationship between physical activity and the reduction of mortality risk in older adults shows conflicting findings, both in terms of the existence of an association11,12,28 and its strength.9,10,29 These differences highlight the importance of investigating sex as a potential effect modifier of the relationship between physical activity and all-cause mortality in older adults. Studies have found similar findings to the results from the Bambuí cohort, which show a protective effect of physical activity only in older men.11,12 However, a German study showed a significant association only in women.28
According to our knowledge, few studies have investigated the interaction between sex and physical activity to predict the risk of mortality in older adults, highlighting the importance of exploring this interaction in various populations.30 Only one study conducted in diabetic participants aged 35 years and older (with a mean age between 56.6 and 58.5 years across categories of physical activity) from ten European countries showed a significant interaction between physical activity and sex in relation to the risk of mortality,31 whereas other studies have not shown a significant interaction.6–8,12 It is important to note that the findings from the Framingham study were similar to those observed in Bambuí, which showed that physical activity did not affect the risk of mortality in women, despite a nonsignificant interaction.12
Regarding the type of physical activity, some studies showed that both leisure physical activities29,30 and household activities29,32 contributed to a reduction in mortality risk in older adults. However, these findings were not replicated by additional studies. For example, a German study found that household activities were associated with mortality risk, but it failed to show a dose response,33 whereas Scottish data showed that household activities did not reduce the risk of cardiovascular events in older adults.34 A study using data from the US Health and Retirement Study found a reduction in mortality risk only in women who practiced leisure physical activity, but not in those who did household or work-related physical activities.30 Among older Chinese adults, household activities had a protective effect in men, but not in women, in relation to all-cause mortality.35 Overall, in Bambuí, walking was the most frequent type of physical activity (72.4%), and among women, household activities were also very common.15 Therefore, it is very important to consider the type of physical activity as a potential explanation for the observed sex differences reported by various studies. The instrument used to assess physical activity in the present study did not allow us to perform separate analyses for each type of activity, which is a limitation of this research.
Sex appears to be a potential effect modifier of the association between physical activity and survival among older adults, and this interaction was also found in Bambuí. The differences in survival rates among men and women disappeared in the highest energy-expenditure group, indicating a significant reduction in mortality risk among men who practiced physical activities. Overall, the observed difference in mortality risk among men and women could be partially attributed to the uneven prevalence of risk factors related to socioeconomic conditions, social networks, health behaviors, and biomarkers.36,37 The findings in the present study highlight the importance of physical activity in maintaining the mortality-risk differences among men and women.
The association between physical activity and mortality could be interpreted as a result of reverse causation, because older adults who report lower levels of physical activity could be in this group because they also presented poorer general health status and therefore higher mortality risk.38 However, sensitivity analyses conducted in this study did not alter the findings, suggesting that the observed effect could be attributed to the physical activity level.
A strength of this study is the fact that it was conducted in an older adult population from Bambuí, with a long follow-up period and a high response rate. Furthermore, the data collected at baseline were obtained by trained professionals using standard techniques with regular quality-control checks. The physical activity data were measured in detail, considering the main physical activities practiced in this local community, and allowed the researchers to calculate energy expenditure. A possible limitation could be that physical activity information was self-reported.38 A study that objectively measured energy expenditure found a stronger association with mortality risk compared to self-reported physical activity measures.39 Another limitation was measuring energy expenditure only at baseline (ie, not assessing physical activity over time). However, these potential limitations could have been partially attenuated by the strength of the associations found in the present study.38
In conclusion, the findings in this study showed that sex is an important effect modifier in the association between energy expenditure and mortality risk among older Brazilian adults. This protective effect of physical activity was only found in men. This study was the first to investigate older adults in Latin America at the population level with a long follow-up period and a very low attrition rate. Finally, it was possible to observe the effect of physical activity on reducing mortality risk, and there was a significant interaction between sex and energy expenditure, which should be considered in the analysis of this association in various populations.
Acknowledgments
The authors thank Financiadora de Estudos e Projetos (FINEP), Centro de Pesquisas René Rachou (CPqRR-Fiocruz Minas), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), which funded this study. CCC, MFLC, JOAF, and SVP are research-productivity scholars of CNPq.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Global Recommendations on Physical Activity for Health. Geneva: WHO; 2010. [Accessed July 11, 2014]. Available from: http://www.who.int/dietphysicalactivity/publications/9789241599979/en. [PubMed] [Google Scholar]
- 3.Malta DC, Barbosa da Silva J. Policies to promote physical activity in Brazil. Lancet. 2012;380(9838):195–196. doi: 10.1016/S0140-6736(12)61041-1. [DOI] [PubMed] [Google Scholar]
- 4.American College of Sports Medicine. Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 5.Woo J, Ho SC, Yu AL. Lifestyle factors and health outcomes in elderly Hong Kong Chinese aged 70 years and over. Gerontology. 2002;48(4):234–240. doi: 10.1159/000058356. [DOI] [PubMed] [Google Scholar]
- 6.Paganini-Hill A, Kawas CH, Corrada MM. Activities and mortality in the elderly: the Leisure World Cohort Study. J Gerontol A Biol Sci Med Sci. 2011;66(5):559–567. doi: 10.1093/gerona/glq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khaw KT, Jakes R, Bingham S, et al. Work and leisure time physical activity assessed using a simple, pragmatic, validated questionnaire and incident cardiovascular disease and all-cause mortality in men and women: the European Prospective Investigation into Cancer in Norfolk prospective population study. Int J Epidemiol. 2006;35(4):1034–1043. doi: 10.1093/ije/dyl079. [DOI] [PubMed] [Google Scholar]
- 8.Leitzmann MF, Park Y, Blair A, et al. Physical activity recommendations and decreased risk of mortality. Arch Intern Med. 2007;167(22):2453–2460. doi: 10.1001/archinte.167.22.2453. [DOI] [PubMed] [Google Scholar]
- 9.Brown WJ, McLaughlin D, Leung J, et al. Physical activity and all-cause mortality in older women and men. Br J Sports Med. 2012;46(9):664–668. doi: 10.1136/bjsports-2011-090529. [DOI] [PubMed] [Google Scholar]
- 10.Ottenbacher AJ, Snih SA, Karmarkar A, et al. Routine physical activity and mortality in Mexican Americans aged 75 and older. J Am Geriatr Soc. 2012;60(6):1085–1091. doi: 10.1111/j.1532-5415.2012.03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayasaka S, Shibata Y, Ishikawa S, et al. Physical activity and all-cause mortality in Japan: the Jichi Medical School (JMS) Cohort Study. J Epidemiol. 2009;19(1):24–27. doi: 10.2188/jea.JE20080043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shortreed SM, Peeters A, Forbes AB. Estimating the effect of long-term physical activity on cardiovascular disease and mortality: evidence from the Framingham Heart Study. Heart. 2013;99(9):649–654. doi: 10.1136/heartjnl-2012-303461. [DOI] [PubMed] [Google Scholar]
- 13.Hallal PC, Victora CG, Wells JC, Lima RC. Physical inactivity: prevalence and associated variables in Brazilian adults. Med Sci Sports Exerc. 2003;35(11):1894–1990. doi: 10.1249/01.MSS.0000093615.33774.0E. [DOI] [PubMed] [Google Scholar]
- 14.Brasil Ministério da Saúde Secretaria de Vigilância em Saúde Secretaria de Gestão Estratégica e Participativa . Vigitel Brasil 2011: Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico. Brasília: Ministério da Saúde; 2012. [Google Scholar]
- 15.Ramalho JR, Lima-Costa MF, Firmo JO, Peixoto SV. Energy expenditure through physical activity in a population of community-dwelling Brazilian elderly: cross-sectional evidences from the Bambuí Cohort Study of Aging. Cad Saude Publica. 2011;27(Suppl 3):S399–S408. doi: 10.1590/s0102-311x2011001500010. [DOI] [PubMed] [Google Scholar]
- 16.Zaitune MP, Barros MB, César CL, Carandina L, Goldbaum M. Variables associated with sedentary leisure time in the elderly in Campinas, São Paulo State, Brazil. Cad Saude Publica. 2007;23(6):1329–1338. doi: 10.1590/s0102-311x2007000600008. Portuguese. [DOI] [PubMed] [Google Scholar]
- 17.Pitanga FJ, Lessa I. Prevalence and variables associated with leisure-time sedentary lifestyle in adults. Cad Saude Publica. 2005;21(3):870–877. doi: 10.1590/s0102-311x2005000300021. Portuguese. [DOI] [PubMed] [Google Scholar]
- 18.Siqueira FV, Facchini LA, Piccini RX, et al. Physical activity in young adults and the elderly in areas covered by primary health care units in municipalities in the south and northeast of Brazil. Cad Saude Publica. 2008;24(1):39–54. doi: 10.1590/s0102-311x2008000100005. Portuguese. [DOI] [PubMed] [Google Scholar]
- 19.Zaitune MP, Barros MB, César CL, Carandina L, Goldbaum M, Alves MC. Factors associated with global and leisure-time physical activity in the elderly: a health survey in São Paulo (ISA-SP), Brazil. Cad Saude Publica. 2010;26(8):1606–1618. doi: 10.1590/s0102-311x2010000800014. Portuguese. [DOI] [PubMed] [Google Scholar]
- 20.Lima-Costa MF, Firmo JO, Uchoa E. Cohort profile: the Bambuí (Brazil) Cohort Study of Ageing. Int J Epidemiol. 2011;40(4):862–867. doi: 10.1093/ije/dyq143. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Herrmann SD, Meckes N, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 22.United States Department of Health and Human Services Physical activity guidelines. 2008. [Accessed January 15, 2010]. Available from: www.health.gov/paguidelines.
- 23.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 24.Lima-Costa MF, Cesar CC, Peixoto SV, Ribeiro AL. Plasma B-type natriuretic peptide as a predictor of mortality in community-dwelling older adults with Chagas disease: 10-year follow-up of the Bambuí Cohort Study of Aging. Am J Epidemiol. 2010;172(2):190–196. doi: 10.1093/aje/kwq106. [DOI] [PubMed] [Google Scholar]
- 25.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;(32):1–407. No authors listed. [PubMed] [Google Scholar]
- 26.Rose G. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 27.Ueshima K, Ishikawa-Takata K, Yorifuji T, et al. Physical activity and mortality risk in the Japanese elderly: a cohort study. Am J Prev Med. 2010;38(4):410–418. doi: 10.1016/j.amepre.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 28.Bucksch J. Physical activity of moderate intensity in leisure time and the risk of all-cause mortality. Br J Sports Med. 2005;39(9):632–638. doi: 10.1136/bjsm.2004.015768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LJ, Fox KR, Ku PW, Sun WJ, Chou P. Prospective associations between household-, work-, and leisure-based physical activity and all-cause mortality among older Taiwanese adults. Asia Pac J Public Health. 2012;24(5):795–805. doi: 10.1177/1010539511404397. [DOI] [PubMed] [Google Scholar]
- 30.Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 31.Sluik D, Buijsse B, Muckelbauer R, et al. Physical activity and mortality in individuals with diabetes mellitus: a prospective study and meta-analysis. Arch Intern Med. 2012;172(17):1285–1295. doi: 10.1001/archinternmed.2012.3130. [DOI] [PubMed] [Google Scholar]
- 32.Park S, Lee J, Kang DY, Rhee CW, Park BJ. Indoor physical activity reduces all-cause and cardiovascular disease mortality among elderly women. J Prev Med Public Health. 2012;45(1):21–28. doi: 10.3961/jpmph.2012.45.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Autenrieth CS, Baumert J, Baumeister SE, et al. Association between domains of physical activity and all-cause, cardiovascular and cancer mortality. Eur J Epidemiol. 2011;26(2):91–99. doi: 10.1007/s10654-010-9517-6. [DOI] [PubMed] [Google Scholar]
- 34.Stamatakis E, Hamer M, Lawlor DA. Physical activity, mortality, and cardiovascular disease: is domestic physical activity beneficial? The Scottish Health Survey – 1995, 1998, and 2003. Am J Epidemiol. 2009;169(10):1191–1200. doi: 10.1093/aje/kwp042. [DOI] [PubMed] [Google Scholar]
- 35.Yu R, Leung J, Woo J. Housework reduces all-cause and cancer mortality in Chinese men. PLoS One. 2013;8(5):e61529. doi: 10.1371/journal.pone.0061529. Erratum in: PLoS One. 2013:8(11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wingard DL. The sex differential in mortality rates: demographic and behavioral factors. Am J Epidemiol. 1982;115(2):205–216. doi: 10.1093/oxfordjournals.aje.a113292. [DOI] [PubMed] [Google Scholar]
- 37.Rogers RG, Everett BG, Onge JM, Krueger PM. Social, behavioral, and biological factors, and sex differences in mortality. Demography. 2010;47(3):555–578. doi: 10.1353/dem.0.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose-response meta-analysis of cohort studies. Int J Epidemiol. 2011;40(5):1382–1400. doi: 10.1093/ije/dyr112. [DOI] [PubMed] [Google Scholar]
- 39.Manini TM, Everhart JE, Patel KV, et al. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296(2):171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]