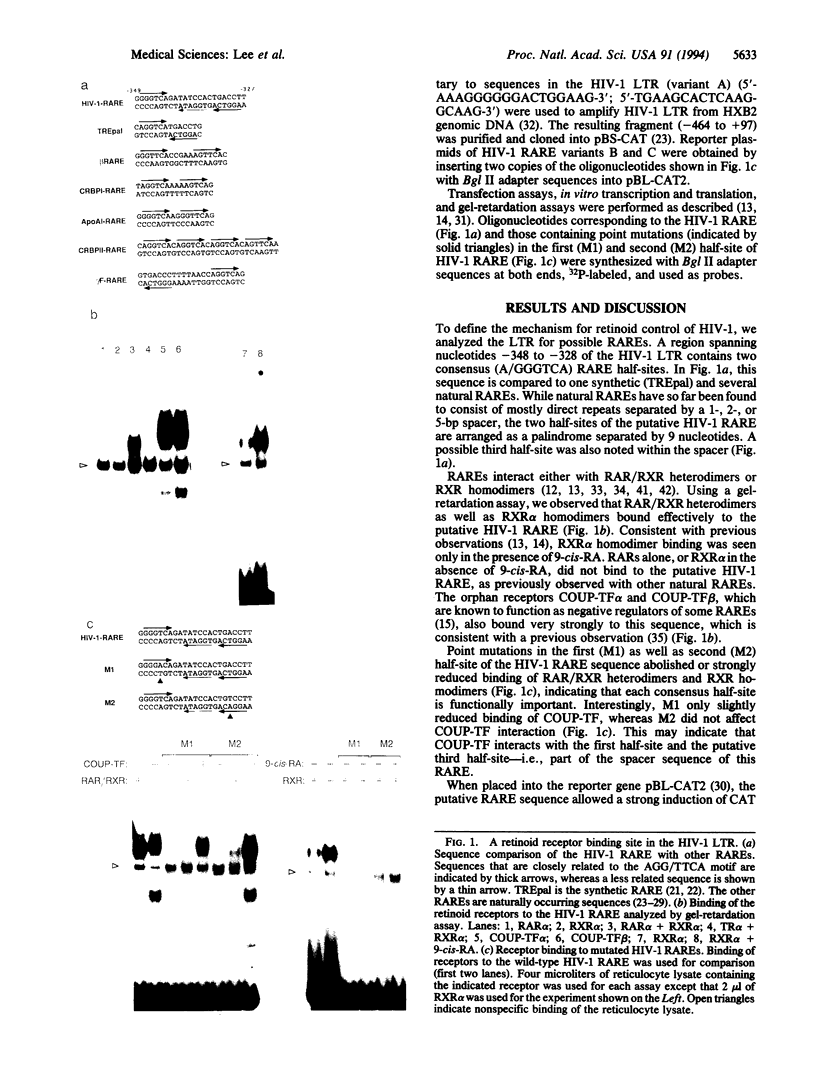
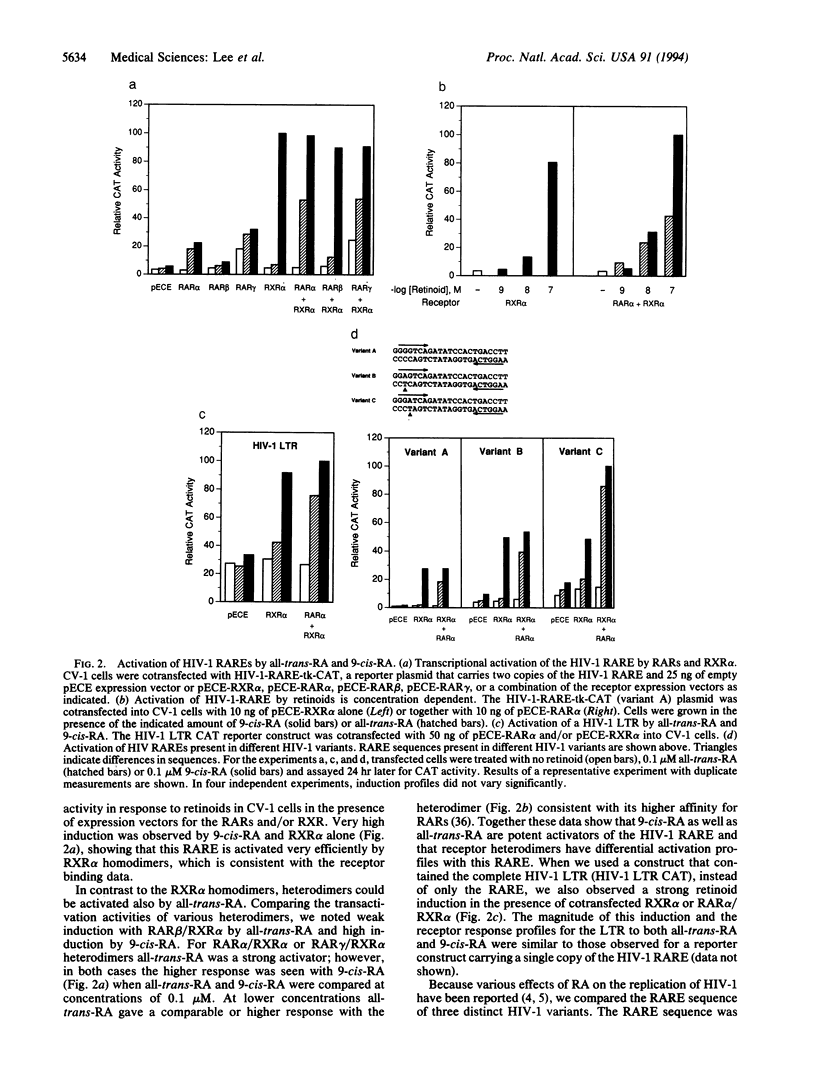
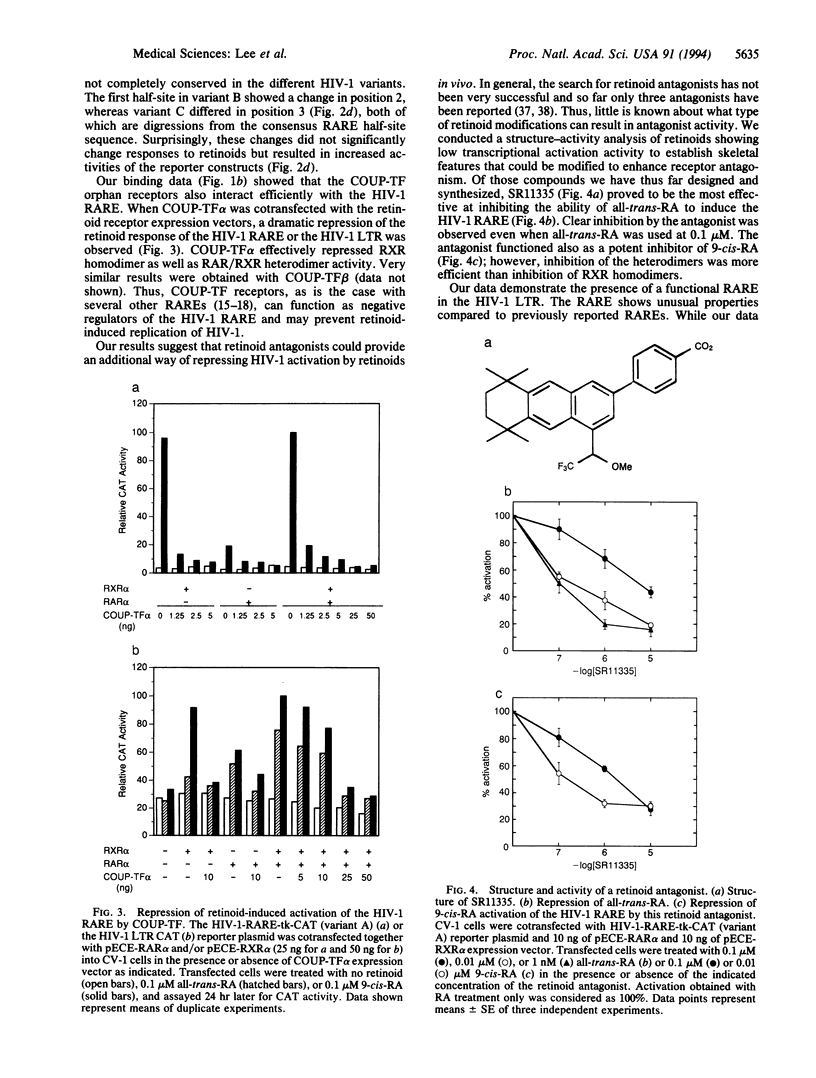
Abstract
Retinoids regulate a broad range of biological processes and affect cell growth and differentiation of many cell types, including the immune system. Recently, it was reported that human immunodeficiency virus type 1 (HIV-1) expression in macrophages is enhanced by retinoic acid (RA). Retinoid signals are mediated by the RA receptors (RARs) and retinoid X receptors (RXRs) that bind to specific RA responsive elements (RAREs) in the promoter region of susceptible genes. Here, we report on a RARE in the long terminal repeat (LTR) region that allows activation of the HIV-1 LTR. The RARE is composed of two consensus RARE half-sites (A/GGGTCA) arranged as a palindrome separated by 9 nucleotides and is activated by both RAR/RXR heterodimers and RXR homodimers. We show that the COUP (chicken ovalbumin upstream promoter) orphan receptors also bind to the HIV-1 RARE and repress the retinoid response of the HIV-1 RARE or the HIV-1 LTR. Furthermore, a newly discovered synthetic retinoid is shown to be a potent inhibitor of retinoid-induced activation of the HIV-1 RARE. These observations suggest additional approaches for the inhibition of HIV replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel C., Bauer F., Crettaz M., Forni L., Kamber M., Kaufmann F., LeMotte P., Pirson W., Klaus M. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7129–7133. doi: 10.1073/pnas.89.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol. 1992 Sep;12(9):4153–4163. doi: 10.1128/mcb.12.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney A. J., Tsai S. Y., O'Malley B. W., Tsai M. J. Chicken ovalbumin upstream promoter transcription factor binds to a negative regulatory region in the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1991 Jun;65(6):2853–2860. doi: 10.1128/jvi.65.6.2853-2860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. I., Hobbs P. D., Derdzinski K. A., Chao W. R., Frenking G., Loew G. H., Jetten A. M., Napoli J. L., Williams J. B., Sani B. P. Effect of structural modifications in the C7-C11 region of the retinoid skeleton on biological activity in a series of aromatic retinoids. J Med Chem. 1989 Jul;32(7):1504–1517. doi: 10.1021/jm00127a018. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazal P., DeMattei C., Giulietti E., Kliewer S. A., Umesono K., Evans R. M. Retinoic acid receptors initiate induction of the cytomegalovirus enhancer in embryonal cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7630–7634. doi: 10.1073/pnas.89.16.7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Graupner G., Wills K. N., Tzukerman M., Zhang X. K., Pfahl M. Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature. 1989 Aug 24;340(6235):653–656. doi: 10.1038/340653a0. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Lehmann J. M., Zhang X. K., Hermann T., Husmann M., Graupner G., Pfahl M. A retinoic acid receptor-specific element controls the retinoic acid receptor-beta promoter. Mol Endocrinol. 1990 Nov;4(11):1727–1736. doi: 10.1210/mend-4-11-1727. [DOI] [PubMed] [Google Scholar]
- Huan B., Siddiqui A. Retinoid X receptor RXR alpha binds to and trans-activates the hepatitis B virus enhancer. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9059–9063. doi: 10.1073/pnas.89.19.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmann M., Hoffmann B., Stump D. G., Chytil F., Pfahl M. A retinoic acid response element from the rat CRBPI promoter is activated by an RAR/RXR heterodimer. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1558–1564. doi: 10.1016/0006-291x(92)90480-9. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J. M., Jong L., Fanjul A., Cameron J. F., Lu X. P., Haefner P., Dawson M. I., Pfahl M. Retinoids selective for retinoid X receptor response pathways. Science. 1992 Dec 18;258(5090):1944–1946. doi: 10.1126/science.1335166. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Zhang X. K., Pfahl M. RAR gamma 2 expression is regulated through a retinoic acid response element embedded in Sp1 sites. Mol Cell Biol. 1992 Jul;12(7):2976–2985. doi: 10.1128/mcb.12.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L. Regulation of expression driven by human immunodeficiency virus type 1 and human T-cell leukemia virus type I long terminal repeats in pluripotential human embryonic cells. J Virol. 1988 Apr;62(4):1398–1407. doi: 10.1128/jvi.62.4.1398-1407.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Orchard K., Lang G., Harris J., Collins M., Latchman D. A palindromic element in the human immunodeficiency virus long terminal repeat binds retinoic acid receptors and can confer retinoic acid responsiveness on a heterologous promoter. J Acquir Immune Defic Syndr. 1993 May;6(5):440–445. [PubMed] [Google Scholar]
- Pfahl M., Tzukerman M., Zhang X. K., Lehmann J. M., Hermann T., Wills K. N., Graupner G. Nuclear retinoic acid receptors: cloning, analysis, and function. Methods Enzymol. 1990;189:256–270. doi: 10.1016/0076-6879(90)89297-u. [DOI] [PubMed] [Google Scholar]
- Poli G., Kinter A. L., Justement J. S., Bressler P., Kehrl J. H., Fauci A. S. Retinoic acid mimics transforming growth factor beta in the regulation of human immunodeficiency virus expression in monocytic cells. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2689–2693. doi: 10.1073/pnas.89.7.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Fisher A., Jagodzinski L. L., Mitsuya H., Liou R. S., Gallo R. C., Wong-Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses. 1987 Spring;3(1):57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- Ross A. C. Cellular metabolism and activation of retinoids: roles of cellular retinoid-binding proteins. FASEB J. 1993 Feb 1;7(2):317–327. doi: 10.1096/fasebj.7.2.8440409. [DOI] [PubMed] [Google Scholar]
- Rottman J. N., Widom R. L., Nadal-Ginard B., Mahdavi V., Karathanasis S. K. A retinoic acid-responsive element in the apolipoprotein AI gene distinguishes between two different retinoic acid response pathways. Mol Cell Biol. 1991 Jul;11(7):3814–3820. doi: 10.1128/mcb.11.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakashita A., Kizaki M., Pakkala S., Schiller G., Tsuruoka N., Tomosaki R., Cameron J. F., Dawson M. I., Koeffler H. P. 9-cis-retinoic acid: effects on normal and leukemic hematopoiesis in vitro. Blood. 1993 Feb 15;81(4):1009–1016. [PubMed] [Google Scholar]
- Tini M., Otulakowski G., Breitman M. L., Tsui L. C., Giguère V. An everted repeat mediates retinoic acid induction of the gamma F-crystallin gene: evidence of a direct role for retinoids in lens development. Genes Dev. 1993 Feb;7(2):295–307. doi: 10.1101/gad.7.2.295. [DOI] [PubMed] [Google Scholar]
- Tran P., Zhang X. K., Salbert G., Hermann T., Lehmann J. M., Pfahl M. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol. 1992 Oct;12(10):4666–4676. doi: 10.1128/mcb.12.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin J. A., Vargo M., Meltzer M. S. Enhanced HIV-1 replication in retinoid-treated monocytes. Retinoid effects mediated through mechanisms related to cell differentiation and to a direct transcriptional action on viral gene expression. J Immunol. 1992 Apr 15;148(8):2539–2546. [PubMed] [Google Scholar]
- Widom R. L., Rhee M., Karathanasis S. K. Repression by ARP-1 sensitizes apolipoprotein AI gene responsiveness to RXR alpha and retinoic acid. Mol Cell Biol. 1992 Aug;12(8):3380–3389. doi: 10.1128/mcb.12.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zeichner S. L., Hirka G., Andrews P. W., Alwine J. C. Differentiation-dependent human immunodeficiency virus long terminal repeat regulatory elements active in human teratocarcinoma cells. J Virol. 1992 Apr;66(4):2268–2273. doi: 10.1128/jvi.66.4.2268-2273.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Lehmann J., Hoffmann B., Dawson M. I., Cameron J., Graupner G., Hermann T., Tran P., Pfahl M. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992 Aug 13;358(6387):587–591. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature. 1990 Jan 11;343(6254):177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]