Abstract
We tested the hypothesis that Ca2+-activated myosin ATPase activity is lower in muscles of aged rats relative to muscles of young rats, independent of changes in myosin isoform expression. Myofibrils were prepared from permeabilized fibers of soleus, plantaris, and semimembranosus muscles of young (8–12 months) and aged (32–38 months) F344 × BN rats and assayed for resting myosin ATPase, Ca2+-activated myosin ATPase, and myosin heavy chain (MHC) and myosin light chain (MLC) isoform compositions. Resting myosin ATPases were not affected by age in any muscle (P ≥ 0.42). Ca2+-activated myosin ATPases of soleus and plantaris myofibrils were not affected by age (P ≥ 0.31) but were 16% lower in semimembranosus myofibrils from aged rats (0.448 ± 0.019 μmol Pi/min/mg) compared to young rats (0.533 ± 0.031 μmol Pi/min/mg; P = 0.03). Correspondingly, maximal unloaded shortening velocity of single semimembranosus fibers from aged rats was slow (4.6 ± 0.2 fiber lengths/s) compared with fibers from young rats (5.8 ± 0.3 fiber lengths/s; P < 0.01). No age-related changes in MHC or regulatory MLC isoforms were detected in any muscle (P ≥ 0.08) but changes in the essential MLC occurred in plantaris and semimembranosus muscles. The data indicate that Ca2+-activated myosin ATPase activity is reduced with age in semimembranosus muscle, independent of age-related changes in MHC isoform expression, and is one mechanism contributing to age-related slowing of contraction in that muscle.
Keywords: Actomyosin adenosinetriphosphatase, Myosin heavy chain, Myosin light chain, Slack test, Aging
1. Introduction
A reduction in the speed with which muscle is able to contract is one of the most pronounced decrements that occurs with age in muscle. At the cellular level, maximal unloaded shortening velocity (V0) is slower in muscle fibers from aged individuals relative to fibers from younger individuals (D’Antona et al., 2003; Degens et al., 1998; Krivickas et al., 2001; Larsson et al., 1997; Li and Larsson, 1996; Thompson, 1999; Thompson and Brown, 1999). It is well known that shortening velocity is directly related to, and dependent upon, Ca2+-activated myosin ATPase activity (Barany, 1967; Reiser et al., 1985). Therefore, the slower V0 of fibers from aged individuals may be caused by reductions in Ca2+-activated myosin ATPase activity (Syrovy and Gutmann, 1970).
Another event that has been reported to occur with age in skeletal muscle is a shift in myosin heavy chain (MHC) isoform expression. In particular, in some rodent muscles there is a shift away from the expression of MHC IIB to MHC IIX (Degens et al., 1998; Larsson et al., 1993; Li and Larsson, 1996). MHC IIB is characterized by a relatively high rate of ATP hydrolysis and fast contractile velocities. The “slowest” isoform of MHC, in terms of both ATP hydrolysis and shortening velocity, is MHC I with MHC IIA and IIX being intermediate [for review see (Moss et al., 1995; Reggiani et al., 2000)]. Thus, age-related shifts are from the faster myosin isoforms to the slower and a shift such as this could explain the age-related myosin ATPase change that was reported previously (Syrovy and Gutmann, 1970). Contractile velocity is also influenced by myosin light chain (MLC) isoform expression (Bottinelli et al., 1991). However, previous studies have not supported the concept that shifts in MLC are related to age-related slowing of V0 (Biral et al., 1999; Larsson et al., 1997; Li and Larsson, 1996). In sum, it is unclear if age-related changes in myosin ATPase activities are simply due to shifts in myosin isoform expression or are due to a direct effect of aging on myosin’s ability to hydrolyze ATP.
To more comprehensively address the issue of the influence of myosin isoform expression on potential age-related changes in myosin ATPase activity, three muscles, each with a unique myosin phenotype, were chosen for this study: (1) the soleus muscle that is composed primarily of type I fibers (i.e., fibers that express MHC I); (2) the plantaris muscle that is considered a “mixed” muscle because it is composed of type I, IIA, IIX, and IIB fibers and (3) the semimembranosus muscle that is composed primarily IIX and IIB fibers (Delp and Duan, 1996). We determined MHC and MLC isoform expression in the same samples from which myosin ATPase activities were measured in order to assess whether or not changes in myosin expression influenced myosin ATPase activity in an age-dependent manner.
Previously, we reported that Ca2+-activated myosin ATPase activity in permeabilized fibers undergoing maximal isometric contractions was not affected by aging (Lowe et al., 2002). However, measuring Ca2+-activated myosin ATPase activity under unloaded shortening conditions, as opposed to isometric conditions, would more directly assess the relationship between age-related changes in myosin ATPase and shortening velocity. A relatively simplistic experimental system for studying muscle contraction during unloaded shortening is the myofibril. Myofibrils are functional muscle units comprised of sarcomeres, such that the thick–thin filament structure is maintained. Myofibrils in solution containing ATP and Ca2+ contract freely corresponding to the condition of an unloaded muscle fiber during shortening (Lionne et al., 2003). We utilized this system in the current study to test the hypothesis that Ca2+-activated myosin ATPase activity is lower in muscles of aged rats relative to muscles of young rats, independent of changes in myosin isoform expression.
2. Materials and methods
2.1. Materials
Sucrose, 3-(N-morpholino) propane-sulfonic acid (MOPS), triton-X-100, sodium citrate, and glycerol were purchased from Fisher Scientific (Pittsburgh, PA). Creatine kinase was purchased from Boehringer Mannheim (Indianapolis, IN). All reagents for sodium dodecyl sulfate poly-acrylamide gel electrophoresis (SDS-PAGE) were supplied by BioRad (Hercules, CA). The bicinchoninic acid (BCA) protein assay kit was obtained from Pierce (Rockford, IL). All other chemical reagents were purchased from Sigma (St. Louis, MO).
2.2. Animal care
An animal care protocol was approved by the Institutional Animal Care and Use Committee of the University of Minnesota and followed the guidelines established by the National Institutes of Health. Male Fischer 344 Brown Norway F1 Hybrid (F344BN) rats were purchased from the National Institute of Aging maintained colony (Harlan; Indianapolis, IN) and housed in individual cages in an animal facility maintained at 20 °C with 12 hr cycles of light and dark. Diet consisted of standard rodent chow and water was provided ad libitum. Young adult rats were 8–12 months old and weighed (mean ± S.D.) 434 ± 57 g (n = 16). Aged rats were 32–38 mo old and weighed 503 ± 60 g (n = 20). 50% mortality of the F344BN rat occurs at ~34 months (Lipman et al., 1996).
2.3. Muscle preparation
Rats were weighed and anesthetized with pentobarbital sodium (35 mg/kg body weight). Soleus, plantaris, and semimembranosus muscles were dissected and then rats were euthanized with an overdose of pentobarbital sodium. Muscles were trimmed of fat and connective tissue and weighed. Approximately 50 mg pieces of each muscle were dissected, placed in cold relaxing solution (7 mM EGTA, 0.016 mM CaCl2, 5.6 mM MgCl2, 80 mM KCl, 20 mM imidazole (pH 7.0), 14.5 mM creatine phosphate, 4.8 mM ATP) and bundles containing ~50 fibers were isolated. The bundles were lengthened to approximately in situ length, tied to glass capillary tubes with 4–0 surgical sutures and stored at −20 °C in a glycerinating solution (50% glycerol and 50% 126 mM K-propionate, 4 mM ATP, 2 mM EGTA, 1 mM MgCl2, 20 mM imidazole (pH, 7.0)). Under these conditions, membranes, including the sarcoplasmic reticulum, are permeabilized within 2d. Thus, most soluble and membrane proteins are released from the muscle, with the myofibrillar complex remaining intact.
2.4. Myosin ATPase activities
Glycerinated muscle bundles were homogenized in 10 mM MOPS (pH, 7.0) and protein concentration was determined using BCA Protein Assay. BSA was used as the protein standard. Myosin ATPase activities of the myofibrils were determined spectrophotometrically at 25 °C by measuring the release of phosphate (Lanzetta et al., 1979) resulting from the hydrolysis of ATP. Myosin ATPase activities were measured under resting conditions (i.e., low Ca2+ which mimics muscle relaxation) and Ca2+-activated conditions which mimics unloaded shortening contractions. The low Ca2+ buffer contained 248 mM K-propionate, 50 mM MOPS (pH, 7.0), 4 mM MgCl2, and 2 mM EGTA. The high Ca2+ buffer contained 242 mM K-propionate, 50 mM MOPS (pH, 7.0), 4 mM MgCl2, and 3 mM CaCl2. Reactions were started by the addition of 5 mM ATP and quenched by 34% citrate. The concentration of phosphate was determined by comparing the sample absorbance at 660 nm with the absorbance of phosphate standards.
2.5. Myosin isoform analysis
MHC and MLC compositions were determined by SDS-PAGE using a 4% (w/v) stacking gel and either an 8% separating gel for MHC or 12% separating gel for the MLC (Laemmli, 1970; Talmadge and Roy, 1993). Proteins from 0.04 μg of muscle homogenate (remaining from the myosin ATPase assays) were solubilized in reducing buffer, separated on the appropriate gel and then silver stained. Gels were imaged using a Fluor-S MultiImaging System (BioRad) and myosin bands were quantified by densito-metric analysis using Quantity One software (BioRad). The relative expression of MHC isoforms was determined from the optical density of the individual MHC isoform relative to the sum of optical densities of all MHC isoforms in each sample. Regulatory and essential MLC isoform expressions were determined by comparing the optical density of the light chain isoforms relative to the sum of the optical densities of the regulatory (MLC2) and essential (MLC1 and MCL3) light chains, respectively. In preliminary experiments we verified that MLC proteins did not co-migrate with troponin I proteins.
2.6. Semimembranosus muscle fiber V0
Contractility of single, glycerinated semimembranosus fibers were determined at 15 °C as described in detail previously (Thompson and Brown, 1999). Fiber segments, ~2 mm long, were submerged in relaxing solution (pH, 7.0) and mounted between a force transducer (model 400A, Cambridge Technology; Cambridge, MA) and a length controller (model 300H, Cambridge Technology). Fibers were lengthened until sarcomere lengths were 2.4 μm and then fiber diameters and lengths were measured. V0 was determined by the slack test. In brief, fibers were activated by exposure to Ca2+ and then at peak isometric force rapidly shortened (slacked) by 10–20% of fiber length such that force dropped to zero. The time between zero force and force redevelopment was measured. This procedure was done five to seven times at different slack distances. The slack distances were then regressed against the corresponding times of force redevelopment and the slope of that line (mm/s) is reported as V0 after normalizing to fiber length (fl/s). Temperature was maintained at 15 ± 0.2 °C. Four to seven semimembranosus muscle fibers from each animal were studied. Each fiber segment was then solubilized in 100 μl of reducing buffer (0.06 mM Tris, 1% SDS, 6 mg/ml EDTA, 5% β-mercaptoethanol, 15% glycerol, 2 mg/ml bromophenol blue), and stored at –80 °C until analyzed by gel electrophoresis for myosin isoform composition as described above.
2.7. Statistical analysis
Data are reported as mean ± S.E. Differences between adult and aged rats were determined using Student’s t-tests with the level of significance set at P < 0.05.
3. Results
3.1. Muscle masses
Age-related muscle atrophy was apparent as muscle wet weights tended to be lower in the aged rats. Semimembranosus muscle wet weight was 49% less in aged rats (0.566 ± 0.063 g (n = 10) versus 1.106 ± 0.041 g (n = 12) for young; P < 0.01). Plantaris muscle wet weight was 17% less in aged rats (0.282 ± 0.014 g (n = 20) versus 0.340 ± 0.020 g (n = 9) for young; P = 0.03). Soleus muscle wet weight was not significantly lower in aged rats (0.167 ± 0.008 g (n = 20) versus 0.183 ± 0.009 g (n = 7) for young; P = 0.28).
3.2. Myosin ATPase activities
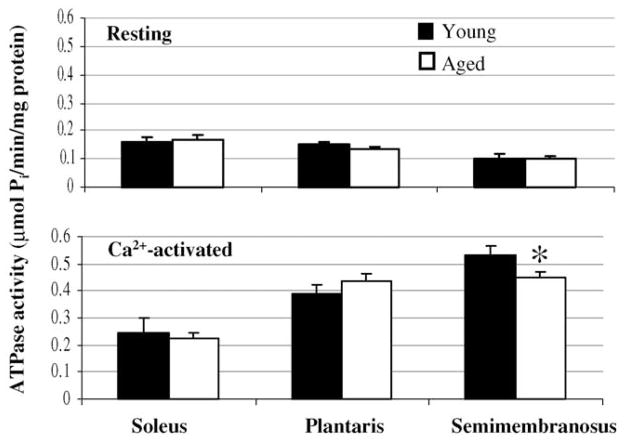
Resting myosin ATPase rates were not different between myofibrils prepared from corresponding muscles of young and aged rats (P ≥ 0.42; Fig. 1). For soleus and plantaris muscles, Ca2+-activated myosin ATPase activities also were not different between the age groups (P ≥ 0.31). Myofibrils prepared from semimembranosus muscles of aged rats had a 16% lower rate of Ca2+-activated myosin ATP hydrolysis compared with myofibrils prepared from semimembranosus muscles of young rats (0.448 ± 0.019 versus 0.533 ± 0.031 μmol Pi/min/mg; P = 0.03; Fig. 1).
Fig. 1.
Resting myosin ATPase activity (i.e., low Ca2+ condition) and Ca2+-activated myosin ATPase activity of freely shortening myofibrils. *Significantly different than young.
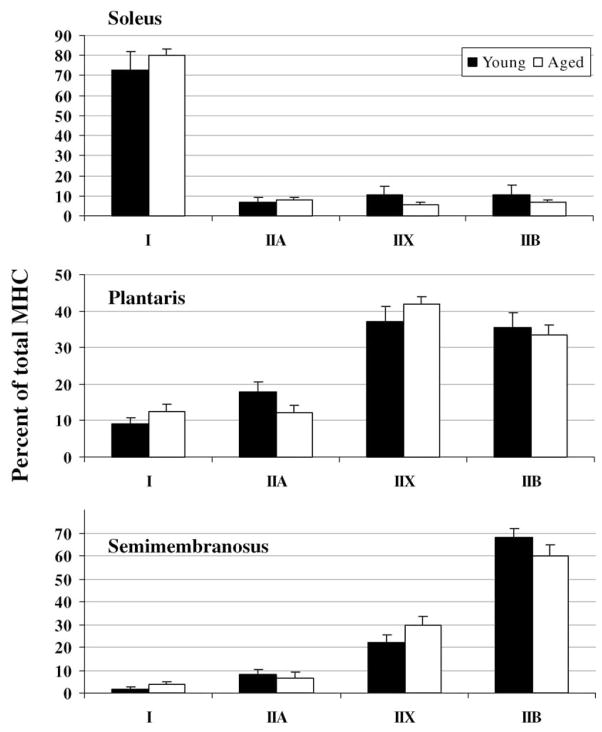
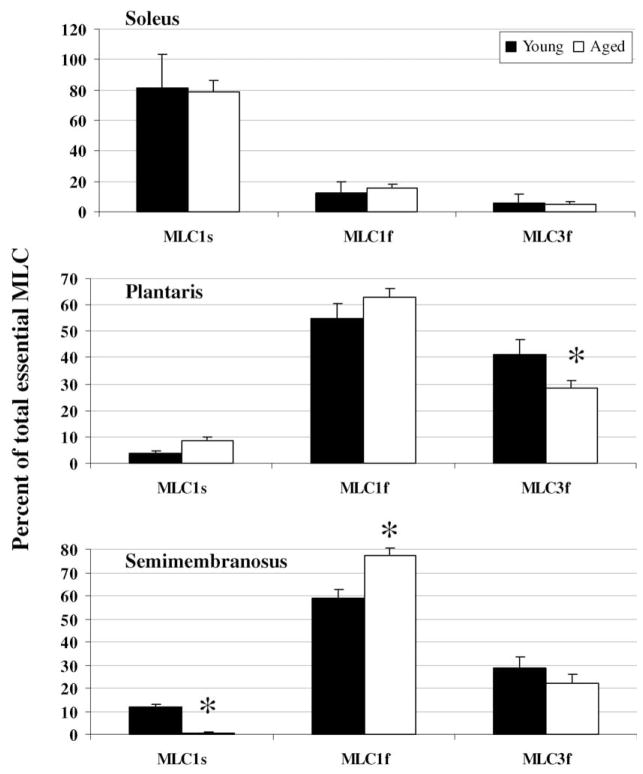
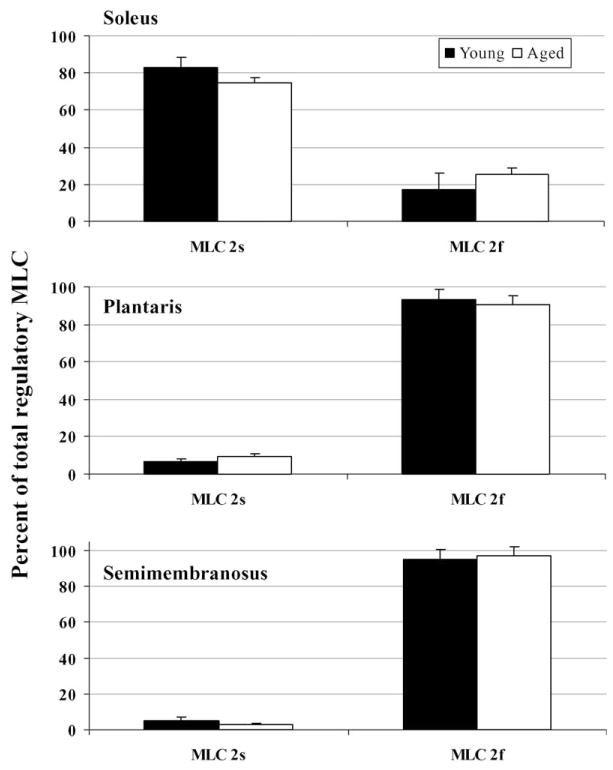
MHC and MLC isoform expression was determined from each myofibril preparation used in the ATPase assays in order to assess possible age-related changes. MHC expression was not different between corresponding muscles from young and aged rats (P ≥ 0.08; Fig. 2). No difference in the essential MLC expression was detected between soleus muscles from young and aged rats (P ≥ 0.59), whereas in the plantaris muscle there was a lower expression of MLC3f in the aged rats and in the semimembranosus muscle there was a lower expression of MLC1s and a higher expression of MLC1f, relative to younger rats (P ≤ 0.04; Fig. 3). Regulatory MLC expression was not different between young and aged rats in any of the muscles studied (P ≥ 0.19; Fig. 4).
Fig. 2.
Myosin heavy chain (MHC) isoform expression in soleus, plantaris, and semimembranosus muscle myofibrils from young and aged rats.
Fig. 3.
Essential myosin light chain isoform expression in soleus, plantaris, and semimembranosus muscle myofibrils from young and aged rats. (*) Significantly different than young.
Fig. 4.
Regulatory myosin light chain isoform expression in soleus, plantaris, and semimembranosus muscle myofibrils from young and aged rats.
3.3. Semimembranosus muscle fiber V0
V0 has not previously been reported for fibers from the semimembranosus muscle of the rat. Additionally, this was the muscle in which we found age-related decrements in myofibrillar ATPases activity so it was important to determine maximal shortening velocity of fibers from this muscle. Overall, V0 was slower in semimembranosus fibers from aged rats relative to fibers from younger rats (P ≤ 0.02; Fig. 5 and Table 1). To determine if the decrement was primarily in fibers expressing IIB or IIX myosin, fibers containing mostly IIB MHC (i.e. ≥ 90% IIB) and those containing mostly IIX MHC were separated (Table 1). IIB fibers from aged rats demonstrated ~40% slower shortening velocity and specific tension compared to IIB MHC-expressing fibers from young rats (P < 0.01). There was no age-related difference in V0 or tension between fibers expressing primarily IIX MHC (P ≤ 0.55). Less than 5% of the fibers studied expressed detectable levels of type I or IIA MHC; those data are not included.
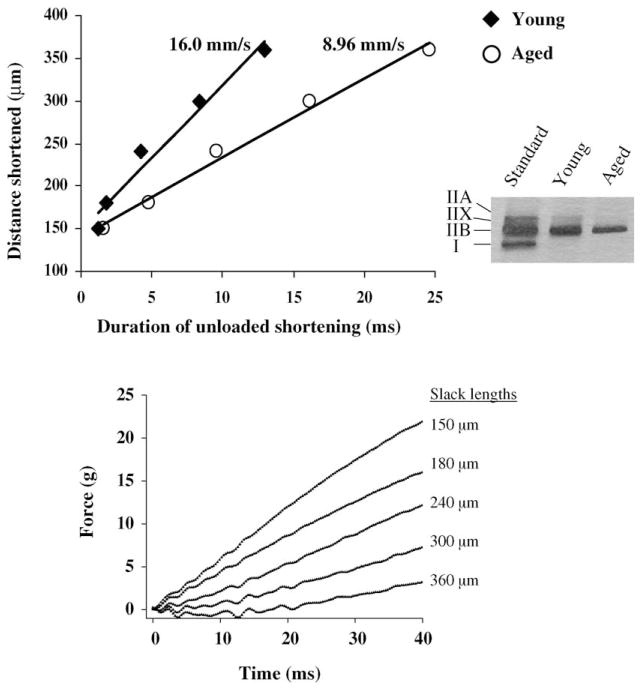
Fig. 5.
Top: representative results of slack tests from two semimembranosus fibers each containing MHC type IIB (gel insert). Each fiber was 1.8 mm long; adult fiber V0 = 8.94 fiber lengths/s, aged fiber V0 = 5.09 fiber lengths/s. Bottom: traces of force redevelopment after five slack lengths for the fiber from the aged rat above. Tests were done at 15 °C.
Table 1.
Characteristics of single, glycerinated fiber segments from semimembranosus muscles of young and aged rats
| Young
|
Aged
|
|||||
|---|---|---|---|---|---|---|
| All fibersa (34) | IIX fibersb (6) | IIB fibersc (18) | All fibersa (41) | IIX fibersb (8) | IIB fibersc (10) | |
| Diameter (μm) | 88 ± 3 | 87 ± 6 | 94 ± 5 | 74 ± 3* | 79 ± 5 | 62 ± 5* |
| Tension (kN/m2) | 151 ± 7 | 162 ± 18 | 154 ± 11 | 124 ± 8* | 167 ± 25 | 87 ± 9* |
| V0 (fl/s) | 5.8 ± 0.3 | 4.6 ± 0.6 | 6.1 ± 0.3 | 4.6 ± 0.2* | 5.0 ± 0.5 | 3.7 ± 0.4* |
| MLC3f (fraction essential MLC) | 0.65 ± 0.08 | 0.52 ± 0.04 | 0.57 ± 0.04 | 0.41 ± 0.03* | 0.55 ± 0.02 | 0.25 ± 0.07* |
Values are means ±S.E. for the number of fibers given in parentheses. V0, maximal unloaded shortening velocity (fl/s), fiber lengths per second. MLC3f, fraction of the essential myosin light chain that was isoform type 3f (remaining essential light chain was type 1f).
All IIX and IIB containing fibers (including fibers listed in the following two columns).
Only fibers containing ≥90% IIX MHC.
Only fibers containing ≥90% IIB MHC.
Significantly different than corresponding young fibers, P < 0.05.
MHC isoform is the primary determinant of shortening velocity but MLC, specifically the essential light chain, also influences this contractile parameter (Bottinelli et al., 1991). Therefore, we assessed MLC expression in each fiber segment studied for V0. In fibers expressing mostly IIB MHC, we found a shift in the essential MLC isoform expression with age (Table 1). IIB fibers from younger rats expressed 57 ± 4% MLC3f and IIB fibers from the aged rats expressed 25 ± 7% MLC3f (P < 0.01). Conversely, MLC1f was higher in fibers from the aged rats. Only the MLC2f regulatory light chain isoform was expressed by the IIB/IIX fibers studied for V0.
4. Discussion
The major finding of the present study is that Ca2+-activated myosin ATPase activity was 16% lower in myofibrils prepared from semimembranosus muscles of aged rats relative to those from younger rats, independent of changes in myosin isoform expression. No age-dependent effect on Ca2+-activated myosin ATPase activity was observed in soleus or plantaris muscles. Thus, we conclude that factors in addition to Ca2+-activated myosin ATPase activity must contribute to age-related reductions in contractile velocities, especially in fibers that express types I and IIA MHC. Attenuated Ca2+-activated myosin ATPase activity is a more predominant factor in the slowing of contractile velocity with age in IIB fibers, such as those from the semimembranosus muscle.
Shortening velocity of individual fiber segments from both human and rodent muscles has been shown to decrease with age. In aged rats, type I fibers from soleus muscle have ~50% slower rates of shortening relative to those fibers from younger rats (Degens et al., 1998; Li and Larsson, 1996; Thompson and Brown, 1999). Our present study and a previous study (Thompson, 1999) show that IIA and IIB fibers from rat muscles also demonstrate a slowing of V0 with age. On the other hand, Larsson and co-workers have reported that IIX and IIB fibers from extensor digitorum longus (EDL) muscles did not differ in V0 between young and aged rats (Degens et al., 1998; Li and Larsson, 1996). Similarly, in the present study we found that IIX fibers did not have age-related contractile decrements. Fiber segments studied from human quadriceps muscle biopsies show consistent slowing of V0 with age (D’Antona et al., 2003; Krivickas et al., 2001; Larsson et al., 1997). Despite some discrepancies, overall there is a consensus that, at the cellular level, muscle shortening velocity is slowed with aging.
Larsson and coworkers have recently extended their investigations to the level of isolated myosin molecules using an in vitro motility assay. They showed that type I MHC extracted from fibers of aged rats, mice, and human moved actin at a rate ~20% slower than myosin extracted from young rats (Hook et al., 1999; Hook et al., 2001). Those data indicate that myosin is involved in the age-related reduction in contractile velocity. Myosin utilizes the energy from the hydrolysis of ATP to move actin, so a logical explanation for the in vitro motility results is that myosin ATPase activity is slower in samples from older animals. Our Ca2+-activated myosin ATPase measurements on myofibrils from soleus muscles, composed predominantly of type I MHC, do not substantiate this explanation. Only in semimembranosus muscle myofibrils, which are composed of IIX and IIB MHC, did we detect a reduction in Ca2+-activated myosin ATPase activity with age. More research is necessary to determine if this age-related decrement is specific to fibers expressing IIX and IIB MHC or if it is specific to the semimembranosus muscle per se.
Recently, we reported that myosin ATPase activity of permeabilized fibers was not different between semimembranosus muscles from young and aged rats, although fibers from the aged rats produced ~20% less specific tension (Lowe et al., 2002). Those measurements were made under maximal isometric conditions, and because the rate-limiting step of the ATPase reaction may be different during isometric and shortening contractions (Lionne et al., 2002), results from that study cannot be extrapolated to elucidate determinants of V0. To more directly assess the possibility of low Ca2+-activated myosin ATPase activity being a mechanism underlying reduced shortening velocity, in the present study, myosin ATPase activity was measured in freely contracting myofibrils corresponding to conditions of maximal unloaded shortening velocity in fibers. We found that Ca2+-activated myosin ATPase activity was 16% lower in semimembranosus muscle from aged rats relative to that in young rats. It has been shown that ADP release is the rate-limiting step during an isometric contraction in fibers (Dantzig et al., 1992) and in myofibrils that are chemically cross-linked such that they do not shorten during contraction (Lionne et al., 2002). On the other hand, both ADP release (Weiss et al., 2001) and Pi release (Lionne et al., 1995; Lionne et al., 2002) have been implicated as the rate-limiting step during shortening contractions. Therefore, it is likely that Pi or ADP release from myosin during a shortening contraction is attenuated with age. We favor the proposal that ADP release is the rate-limiting factor and may be altered with age because we have previously shown that during a maximal isometric contraction there is an increase in the apparent rate constant for myosin detachment from actin with age (Lowe et al., 2002), and ADP release is the critical step controlling detachment. Furthermore, it has been suggested that a structural alteration in the catalytic domain of MHC causes the age-related contractile deficits (Lowe et al., 2001; Lowe et al., 2002; Ramamurthy et al., 2001). A similar proposal has been suggested to explain sarcoplasmic reticulum Ca2+-ATPase functional alterations that occur with age (Chen et al., 1999; Ferrington et al., 1997).
Three previous papers have reported myosin ATPase activity in myofibrils from skeletal muscles of young and aged rats. There was no age-related difference in myosin ATPase activity of soleus, EDL, or diaphragm muscles (Florini and Ewton, 1989) or among “white” or “red” hindlimb muscles (Honorati and Ermini, 1974). Syrovy and Gutmann, on the other hand, reported data showing an age-related trend for low myofibrillar Ca2+-activated ATPase and purified myosin ATPase activities in soleus and EDL muscles (Syrovy and Gutmann, 1970). We have extended those previous observations to include a very “fast” muscle, i.e. the semimembranosus muscle, composed primarily of fibers that express IIX and IIB MHC, and found that myosin ATPase activity was lower by 16% in aged muscle relative to young muscle. In addition, we measured resting myosin ATPase activity of myofibrils, i.e. activity under conditions mimicking muscle relaxation, and detected no age-related differences in any of the muscles studied.
We also extended previous observations by excluding the possibility that shifts in MHC, which sometimes occur with aging (see below), confounded the myosin ATPase results. We did this by verifying that muscle myofibrils from the young and aged rats had similar MHC isoform compositions in each muscle. Determining MHC expression in our semi-membranosus myofibrils was essential because shifts from the faster to the slower isoforms potentially could have explained the age-related 16% reduction in myosin ATPase activity. However, there was no significant difference in MHC isoform expression between muscles from young and aged rats. Age-related shifts in MHC isoform have been reported in some studies of rodent muscles (Degens et al., 1998; Larsson et al., 1993; Li and Larsson, 1996) but not others (Gonzalez et al., 2003; Phillips et al., 1993; Ryall et al., 2004). These discrepancies are likely due to the various species, strains, muscles, ages, and techniques to quantitate MHC isoforms that were utilized in the studies. Shortening velocity is influenced by the essential MLC as well, presumably by altering ATPase hydrolysis (Reggiani et al., 1997). Only small age-related isoform shifts in MLC proteins occurred in the myofibril samples. In fact, there was a small but significant decrease in MLC1s and an increase in MLC1f with age in myofibrils from semimembranosus muscles that would suggest, if anything, an increase in ATPase activity and V0, not a decrease. Among the semimembranosus fiber segments studied for V0, we did detect an age-related decrease in MLC3f, the essential MLC associated with the fastest shortening velocities (Bottinelli et al., 1991), which may have contributed to the age-related reduction in V0.
In summary, contractile velocity is slowed with age but the mechanisms underlying the decrement are not completely known. Our data indicate that a reduction in the ability of myosin to hydrolyze ATP under shortening conditions is one mechanism contributing to the slowing, at least in muscle fibers composed of MHC IIB. Our data also indicate that the slowing of contractile velocity and reduced myosin Ca2+-activated ATPase activity can occur independent of age-related shifts in myosin isoform expression.
Acknowledgments
The authors thank Janice Shoeman, Darin Thom, Carrie Wilson, Andrea Zimmerman, and Dawn Zwakman for technical assistance. This research was supported by a grant from The American Federation for Aging Research (DAF), National Institute on Aging Grants 20990 (DAL), 17768 and 21626 (LVT), and AR32691.
References
- Barany M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967;50 (Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biral D, Ballarin F, Toscano I, Salviati G, Yu F, Larsson L, Betto R. Gender- and thyroid hormone-related transitions of essential myosin light chain isoform expression in rat soleus muscle during ageing. Acta Physiol Scand. 1999;167:317–323. doi: 10.1046/j.1365-201x.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Schiaffino S, Reggiani C. Force–velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Jones TE, Bigelow DJ. The nucleotide-binding site of the sarcoplasmic reticulum Ca-ATPase is conformationally altered in aged skeletal muscle. Biochemistry. 1999;38:14887–14896. doi: 10.1021/bi991125n. [DOI] [PubMed] [Google Scholar]
- D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig JA, Goldman YE, Millar NC, Lacktis J, Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degens H, Yu F, Li X, Larsson L. Effects of age and gender on shortening velocity and myosin isoforms in single rat muscle fibres. Acta Physiol Scand. 1998;163:33–40. doi: 10.1046/j.1365-201x.1998.00329.x. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Jones TE, Qin Z, Miller-Schlyer M, Squier TC, Bigelow DJ. Decreased conformational stability of the sarcoplasmic reticulum Ca-ATPase in aged skeletal muscle. Biochim Bio-phys Acta. 1997;1330:233–247. doi: 10.1016/s0005-2736(97)00158-2. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ. Skeletal muscle fiber types and myosin ATPase activity do not change with age or growth hormone administration. J Gerontol. 1989;44:B110–B117. doi: 10.1093/geronj/44.5.b110. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Messi ML, Zheng Z, Delbono O. Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J Physiol. 2003;552:833–844. doi: 10.1113/jphysiol.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honorati MC, Ermini M. Myofibrillar and mitochondrial ATPase activity of red, white, diaphragmatic and cardiac muscle of young and old rats. Experientia. 1974;30:215–216. [PubMed] [Google Scholar]
- Hook P, Li X, Sleep J, Hughes S, Larsson L. In vitro motility speed of slow myosin extracted from single soleus fibres from young and old rats. J Physiol. 1999;520 (Part 2):463–471. doi: 10.1111/j.1469-7793.1999.00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook P, Sriramoju V, Larsson L. Effects of aging on actin sliding speed on myosin from single skeletal muscle cells of mice, rats, and humans. Am J Phys Cell Physiol. 2001;280:782–788. doi: 10.1152/ajpcell.2001.280.4.C782. [DOI] [PubMed] [Google Scholar]
- Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil. 2001;80:447–455. doi: 10.1097/00002060-200106000-00012. quiz 456–457. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979;100:95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Larsson L, Biral D, Campione M, Schiaffino S. An age-related type IIB to IIX myosin heavy chain switching in rat skeletal muscle. Acta Physiol Scand. 1993;147:227–234. doi: 10.1111/j.1748-1716.1993.tb09493.x. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 1997;272:C638–C649. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- Li X, Larsson L. Maximum shortening velocity and myosin isoforms in single muscle fibers from young and old rats. Am J Physiol. 1996;270:C352–C360. doi: 10.1152/ajpcell.1996.270.1.C352. [DOI] [PubMed] [Google Scholar]
- Lionne C, Brune M, Webb MR, Travers F, Barman T. Time resolved measurements show that phosphate release is the rate limiting step on myofibrillar ATPases. FEBS Lett. 1995;364:59–62. doi: 10.1016/0014-5793(95)00356-e. [DOI] [PubMed] [Google Scholar]
- Lionne C, Iorga B, Candau R, Piroddi N, Webb MR, Belus A, Travers F, Barman T. Evidence that phosphate release is the rate-limiting step on the overall ATPase of psoas myofibrils prevented from shortening by chemical cross-linking. Biochemistry. 2002;41:13297–13308. doi: 10.1021/bi0260278. [DOI] [PubMed] [Google Scholar]
- Lionne C, Iorga B, Candau R, Travers F. Why choose myofibrils to study muscle myosin ATPase? J Muscle Res Cell Motil. 2003;24:139–148. doi: 10.1023/a:1026045328949. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway × Fischer 344, and Fischer 344 × brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280:C540–C547. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- Lowe DA, Thomas DD, Thompson LV. Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers. Am J Physiol Cell Physiol. 2002;283:C187–C192. doi: 10.1152/ajpcell.00008.2002. [DOI] [PubMed] [Google Scholar]
- Moss RL, Diffee GM, Greaser ML. Contractile properties of skeletal muscle fibers in relation to myofibrillar protein isoforms. Rev Physiol Biochem Pharmacol. 1995;126:1–63. doi: 10.1007/BFb0049775. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Wiseman RW, Woledge RC, Kushmerick MJ. Neither changes in phosphorus metabolite levels nor myosin isoforms can explain the weakness in aged mouse muscle. J Physiol. 1993;463:157–167. doi: 10.1113/jphysiol.1993.sp019589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy B, Hook P, Jones AD, Larsson L. Changes in myosin structure and function in response to glycation. FASEB J. 2001;15:2415–2422. doi: 10.1096/fj.01-0183com. [DOI] [PubMed] [Google Scholar]
- Reggiani C, Bottinelli R, Stienen GJ. Sarcomeric myosin isoforms: fine tuning of a molecular motor. News Physiol Sci. 2000;15:26–33. doi: 10.1152/physiologyonline.2000.15.1.26. [DOI] [PubMed] [Google Scholar]
- Reggiani C, Potma EJ, Bottinelli R, Canepari M, Pellegrino MA, Stienen GJ. Chemo-mechanical energy transduction in relation to myosin isoform composition in skeletal muscle fibres of the rat. J Physiol. 1997;502 (Pt 2):449–460. doi: 10.1111/j.1469-7793.1997.449bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser PJ, Moss RL, Giulian GG, Greaser ML. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985;260:9077–9080. [PubMed] [Google Scholar]
- Ryall JG, Plant DR, Gregorevic P, Sillence MN, Lynch GS. Beta2-Agonist administration reverses muscle wasting and improves muscle function in aged rats. J Physiol. 2004;555:175–188. doi: 10.1113/jphysiol.2003.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrovy I, Gutmann E. Changes in speed of contraction and ATPase activity in striated muscle during old age. Exp Gerontol. 1970;5:31–35. doi: 10.1016/0531-5565(70)90026-4. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Thompson LV. Contractile properties and protein isoforms of single skeletal muscle fibers from 12- and 30-month-old Fischer 344 brown Norway F1 hybrid rats. Aging (Milano) 1999;11:109–118. [PubMed] [Google Scholar]
- Thompson LV, Brown M. Age-related changes in contractile properties of single skeletal fibers from the soleus muscle. J Appl Physiol. 1999;86:881–886. doi: 10.1152/jappl.1999.86.3.881. [DOI] [PubMed] [Google Scholar]
- Weiss S, Rossi R, Pellegrino MA, Bottinelli R, Geeves MA. Differing ADP release rates from myosin heavy chain isoforms define the shortening velocity of skeletal muscle fibers. J Biol Chem. 2001;276:45902–45908. doi: 10.1074/jbc.M107434200. [DOI] [PubMed] [Google Scholar]