Abstract
Objective
HIV-infected participants are at a higher risk of cardiovascular disease (CVD). N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a significant predictor of CVD in the general population and is associated with mortality in HIV.
Design and Methods
The 96-week Stopping Atherosclerosis and Treating Unhealthy Bone with Rosuvastatin in HIV (SATURN-HIV) trial randomized 147 patients on stable antiretroviral therapy (ART) with LDL-cholesterol <130mg/dL and without overt heart failure to 10 mg daily rosuvastatin or placebo. We measured NT-proBNP levels by ELISA. Baseline and changes in NT-proBNP were compared between groups. Spearman correlation was used to explore relationships between baseline NT-proBNP, inflammation and CVD risk markers. Multivariable analyses were conducted to assess associations with NT-proBNP levels.
Results
Median age was 46 years, 80% were men, 69% were African American and 46% were on protease inhibitors. At baseline, median (Q1, Q3) NT-proBNP was higher in the rosuvastatin group than placebo [41(20,66.5) vs. 25 pg/mL (11, 56); p=0.012)]. Baseline NT-proBNP correlated with bulb and common carotid artery intima media thickness, coronary calcium score, IL-6 and cystatin C. After 96 weeks, median NT-proBNP decreased significantly in the rosuvastatin group versus placebo (-1.50 vs. +4.50 pg/mL, p=0.041). Within the rosuvastatin group, changes in NT-proBNP were negatively correlated with changes in insulin resistance and total limb fat.
Conclusions
Rosuvastatin reduces plasma NT-proBNP in HIV-infected participants on ART. NT-proBNP correlated with several measures of CVD risk, independent of inflammation markers.
Keywords: inflammation, cardiovascular disease, statin therapy, NT-proBNP
Introduction
B-type natriuretic peptide (BNP) is a 32-amino acid polypeptide secreted by ventricular myocytes during periods of increased ventricular stretch and wall tension. BNP plays an important role in the regulation of volume, osmosis, pressure regulation and sodium balance1. After secretion, the BNP precursor is split into the biologically active peptide and the more stable N-terminal fragment (NT-proBNP). Circulating levels of BNP or NT-proBNP are predictive of left-ventricular dysfunction2-4 and adverse clinical outcomes in patients with acute coronary syndromes5. Because these peptides are directly released from cardiomyocytes during ischemia, it is believed that their levels are also relevant to the vascular events 6.
Many prospective studies have investigated the relationship of lower levels of BNP to CVD events in community-based studies of subjects without overt heart failure. A meta-analysis of 40 long term prospective cohort studies reported on the predictive role of BNP and NT-proBNP on CVD7. Overall, there was an almost 3 fold increase in risk of CVD (any fatal or nonfatal myocardial infarction, stroke, transient ischemic attack or heart failure) for participants with the highest baseline BNP or NT-proBNP.
Data on NT-proBNP in patients with HIV are limited. In the Strategies for Management of Anti-Retroviral Therapy Study (SMART) higher NT-proBNP was associated with greater risk of CVD independently of traditional CVD risk factors and inflammatory markers8. In the Women's Interagency HIV Study (WIHS), women with HIV had higher BNP levels than uninfected controls9, and BNP was independently associated with greater mortality10. HIV-infected patients have been shown to have a higher prevalence of diastolic dysfunction and higher left ventricular mass index when compared to uninfected controls and higher plasma BNP was associated with higher left ventricular mass index but not with diastolic dysfunction11.
In the HIV population, antiretroviral therapy (ART) has significantly decreased morbidity and mortality for patients with HIV12; however when compared to the general population, they remain at a higher risk of cardiovascular disease (CVD) 13-16 The causes are multi-factorial and could include specific antiretroviral agents, HIV viral replication, and enhanced chronic inflammation and immune activation. As the HIV population ages, it is imperative to identify effective therapies to attenuate CVD risk. Beyond their effect of cholesterol lowering, statins, or 3 hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors can reduce inflammation and reactive oxygen species and can improve endothelial function17,18. Data on the effect of statins on BNP levels in the HIV-uninfected populations is sparse and focuses on the therapeutic use in established heart failure. In the setting of heart failure, several studies have shown that plasma NT-proBNP levels are lower in patients who are taking statins19,20. To our knowledge, there are no data on the effect of statins on NT-proBNP levels in HIV infected participants or if NT-proBNP levels are associated with enhanced immune activation and inflammation in this population. In SATURN-HIV, a 96-week statin study in HIV-infected participants on stable ART, we have shown that after 24 weeks of rosuvastatin there was a significant decrease in markers of inflammation, monocyte activation and cystatin C21-23 in HIV-infected participants on antiretroviral therapy. Here, we present the results of an analysis of the statin therapy on NT-proBNP, and the relationship between NT-proBNP, inflammation, and CVD risk measures in HIV-infected adults on stable ART.
Materials and Methods
Study Design
SATURN-HIV is a 96-week randomized, double-blind, placebo controlled study designed to measure the effect of rosuvastatin on markers of cardiovascular risk, skeletal health, and immune activation in HIV disease22. The study was reviewed and approved by the Institutional Review Board of University Hospitals Case Medical Center, Cleveland, Ohio. Written informed consent was provided by all participants. The study is registered on clinicaltrials.gov (NCT01218802). Participants were randomized 1:1 to rosuvastatin 10 mg daily vs. matching placebo. All participants were ≥18 years of age, with HIV-1 infection on stable ART for at least 3 months with cumulative ART duration of at least 6 months, HIV-1 RNA <1000 copies/mL, fasting LDL-cholesterol (LDL-C) ≤130 mg/dL and triglyceride ≤500 mg/dL. Additionally, participants were required to have evidence of either heightened T-cell activation, identified as the proportion of CD8+ T cells that expressed CD8+CD38+HLA-DR+ ≥19%, or levels of high-sensitivity C-reactive protein (hs-CRP) ≥2 mg/L. Participants were excluded if they had a history of coronary disease or diabetes, were pregnant or lactating, or had an active infectious or inflammatory condition,
STUDY EVALUATIONS
At entry, week 48 and 96, fasting (> 12 hours) blood draws were obtained for real time measurements of renal and lipid profiles, glucose and insulin levels. Additionally, blood was processed and plasma, serum and peripheral blood mononuclear cells were stored for measurement of NT-proBNP, soluble and cellular markers of immune activation and markers of systemic inflammation and coagulation. HIV-1 RNA levels and CD4+ cell counts were drawn as part of clinical care and measures closest to study visits were used for analysis. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as described24. Glomerular filtration rate was estimated using the 2009 Chronic Kidney Disease Collaboration creatinine-based equation (eGRFcr)25. NT-proBNP was measured by quantitative sandwich ELISAs (Siemens Healthcare, Newark, New Jersey, USA), coefficient of variation 3-4.4%.
Inflammation and soluble immune activation markers
Soluble plasma biomarkers of monocyte activation (soluble CD14 and soluble CD 163), systemic inflammation [hs-CRP, IL-6, tumor necrosis factor-alpha receptor I (sTNFR-1)], cystatin C and coagulation (D-dimer) were measured as previously described21,22.
Cellular markers of monocyte and T-cell activation
Monocyte and T-cells were phenotyped by flow cytometry as previously described21.
Body composition and cardiovascular measures
Body composition measures, including lean mass, trunk and limb fat were derived from a whole body DEXA scan (Lunar Prodigy Advance (GE Healthcare). Ten-year Framingham risk score was determined using a published risk calculator26.
At entry and week 96, coronary artery calcium score was measured by a non contrast CT scan, as described previously27. B mode ultrasound scan of the carotid arteries was performed using a Philips iU22 ultrasound system with an L9-3 MHz linear array transducer. Mean-mean and mean-max common carotid artery intima media thickness (CCA-IMT) were measured as described previously28. Flow-mediated dilation (FMD) of the brachial artery was calculated as the percentage change in brachial artery diameter from baseline to 60 s post reactive hyperemia28.
Statistical Analysis
The major objectives of this study were to compare changes from baseline to 96 weeks (primary outcome) and baseline to 48 weeks in pro-BNP levels between groups. Secondary objectives were to evaluated changes in pro-BNP within groups over both 48 and 96 weeks, to examine associations between pro-BNP and markers of systemic inflammation, immune activation, coagulation as well as measures of cardiovascular risk and to explore predictors of change in pro-BNP.
Demographics, clinical indices and HIV-related factors are presented overall and by group at baseline. Median and interquartile range (IQR) are reported for continuous variables and frequency and percent for nominal variables. Absolute and percent or relative changes from baseline to week 48 and from baseline to week 96 in pro-BNP levels were determined. All baseline variables as well as endpoints were compared between groups using unpaired t-tests or Wilcoxon Rank Sum tests as warranted by distribution for continuous variables and by Chi-Square tests, Fisher's Exact tests, or Pearson Exact Chi-Square tests as appropriate for categorical variables. Within-group changes were tested using paired t-tests or Wilcoxon Signed Rank tests as appropriate for the distribution.
Spearman correlation analysis was utilized to assess the relationships between baseline and relative change over 96 weeks in pro-BNP , markers of inflammation, immune activation, coagulation and cardiovascular risk as well as clinically relevant demographic and HIV-related factors (continuous variables only). Next, multivariable linear regression was employed to answer three questions (1) what baseline variables are independently associated with pro-BNP; (2) what variables independently predict relative change in pro-BNP over 96 weeks; (3) does pro-BNP independently predict CIMT after adjustment for clinically relevant variables, as well as, markers of inflammation, immune activation and coagulation. For each of these questions, separate multivariable models were constructed. For the first model, baseline pro-BNP was the outcome and all those variables with p<0.1 in the correlation analysis were considered for inclusion. Stepwise selection was utilized to construct the final model keeping only those variables with p<0.05. For the second model where relative change over 96 weeks in pro-BNP was the outcome, randomization group, all those variables with p<0.1 in the correlation analysis and clinically relevant variables regardless of statistical significance were included in the final model. For the final question, we constructed a series of models where baseline CIMT was the outcome. Each model included pro-BNP level and the following clinically relevant variables: age, gender, total lean body mass (g), eGFRcr (ml/min/1.73m2), framingham score and ARV duration (per month). To this model each marker of inflammation and immune activation marker was added in turn to assess whether these variables were independently associated with the outcome or if the parameter estimate for pro-BNP changed or was no longer significant.
All analyses were initially performed using intent-to-treat principles based on randomized treatment assignment which used all available data. Modifications to randomized treatment and missing values were ignored. As-treated analyses did not differ from intent-to-treat analyses; therefore, only the former data are presented. All statistical tests were two-sided and considered significant with p<0.05. Analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics
Overall, 123 out of the 147 SATURN population had stored plasma samples available for BNP measurements and were included in the present analysis. Demographic information and baseline characteristics of the 123 participants are displayed in Table 1; except for prevalence of hepatitis C and HOMA-IR levels which were higher in the placebo arms, all other indices were similar between groups (p>0.05). Overall, the median age of the participants was 46 years; 80% were men, 67% were African American and BMI was 27. Tenofovir and protease inhibitors were used in 87% and 48% of participants, respectively. At baseline, NT-proBNP levels were higher in the statin group [median (Q1, Q3) NT-proBNP level in placebo group was 25 pg/mL (11, 56) and in the statin group was 41pg/mL (20, 66.50) (p= 0.012)].
Table 1.
Baseline Characteristics
| Rosuvastatin | Placebo | |
|---|---|---|
| N=64 | N=59 | |
| Demographics | ||
| Age (years) | 46 (41, 51) | 46 (37, 52) |
| Male sex | 53 (83.00%) | 45 (76.00%) |
| African American | 44 (69%) | 38 (64%) |
| HIV parameters | ||
| HIV duration (months) | 122 (69, 195) | 121 (70, 216) |
| Current CD4+ count (cells/mm3) | 595 (426, 792) | 627 (400, 832) |
| Nadir CD4+ count (cells/mm3) | 177 (85, 314) | 206 (92.5, 292) |
| HIV-1 RNA < 50 copies/ml | 52 (81%) | 49 (83%) |
| ART duration (months) | 56 (30, 115) | 62 (39, 109) |
| Current protease inhibitor use | 26 (48%) | 26 (44%) |
| Current tenofovir use | 56 (87%) | 54 (91%) |
| Metabolic and cardiovascular risk factors | ||
| Body mass index (kg/m2) | 27(23, 30) | 27 (23, 30) |
| Total limb fat (kg) | 8852 (5418, 12546) | 7863 (4870, 14449) |
| Active Hepatitis B | 3 (5%) | 4 (7%) |
| Active Hepatitis C | 3 (5%) | 6 (10%) |
| Systolic blood pressure (mmHg) | 121 (112, 130) | 120 (112, 132) |
| Current anti-hypertensive medication | 17 (27%) | 13 (22%) |
| HDL cholesterol (mg/dL) | 48 | 48 |
| LDL cholesterol (mg/dL) | 94 (75, 105) | 97 (78.5, 119) |
| HOMA-IR ≥2.5 | 20 (31%) | 28 (48%) |
| Current smoking | 37 (58%) | 41 (69%) |
| Framingham risk score | 3 (1, 7) | 4 (1, 7) |
| Measure of subclinical vascular disease | ||
| Mean-Max common carotid artery IMT (mm) | 0.84 (0.73, 0.93) | 0.84 (0.75, 0.95) |
| Carotid bulb IMT (mm) | 0.82 (0.73, 0.93) | 0.83 (0.69, 0.98) |
| FMD (%) | 3.9 (2.1, 6.2) | 3.3 (1.6, 5.1) |
| Coronary artery calcium score | 20 | 50 |
| Inflammation and Immune activation | ||
| hsCRP (μg/mL) | 1.67 (0.77,4.89) | 2.00 (0.72,5.50) |
| D-dimer (μg/mL) | 0.20 (0.13,0.33) | 0.16 (0.09,0.28) |
| Interleukin 6 (pg/mL) | 3 (2, 4.6) | 2.6 (1.9, 5.3) |
| NT-proBNP (pg/mL) | 41 (20, 66) | 25 (11, 55) |
| TNFα- receptor I (pg/mL) | 1619 (1331, 2198) | 1456 (1206, 2418) |
| TNFα- receptor II (pg/mL) | 2477 (1815, 3052) | 2142 (1605, 2582) |
| CD4+CD38+HLADR+ T-cells (%) | 5.34 (3.67,6.84) | 4.60 (3.48,6.13) |
| CD8+CD38+HLADR+ T-cells (%) | 13.30 (8.96,19.10) | 11.35 (8.48,15.66) |
| CD14+CD16+ monocytes (%) | 23.11 (18.20, 33.60) | 22.34 (17.53, 33.93) |
| sCD14 (ng/mL) | 2114 (1787, 2495) | 2195 (1684, 2467) |
| sCD163 (ng/mL) | 645 (533, 804) | 655 (504, 906) |
| Kidney Function | ||
| eGFRcr (ml/min/1.73m2) | 98 | 103 |
| Cystatin C (mg/L) | 0.85 (0.76, 1) | 0.81 (0.71, 0.95) |
Data presented as median (Q1,Q3) for continuous variables and by frequency (column percent) for nominal variables.
P > 0.05 between-groups for all variables listed except for hepatitis C (p= 0.01) and HOMA-IR (p=0.02)
Baseline Associations with NT-proBNP levels
At baseline, baseline NT-proBNP was associated with several demographics and clinical parameters including age, total lean mass, Framingham risk score and creatinine clearance. NT-proBNP was also positively with some markers of systemic inflammation including IL-6 and cystatin C (table 2). HIV-related factors were not associated with NT-proBNP. In multivariate analysis, after adjustment for clinically-relevant variables, factors independently associated with higher baseline NT-proBNP level were lower total lean mass, higher sTNF-RII and higher CD8 activation (% CD8+CD38+HLA-DR+) (table 2).
Table 2.
Univariate and Multivariable relationship of baseline NT- proBNP level
| Spearman r | p- value | Multivariable Analysis estimate | p-value | |
|---|---|---|---|---|
| Demographics and clinical parameter | ||||
| Age (per decade) | 0.317 | 0.0004 | 0.057 | 0.88 |
| Male sex | −0.128 | 0.16 | −7.486 | 0.31 |
| Total lean mass (kg) | −0.209 | 0.02 | −0.0005 | 0.04 |
| Framingham score | 0.191 | 0.03 | −0.400 | 0.62 |
| eGFRcr (ml/min/1.73m2) | −0.302 | 0.0007 | −0.260 | 0.084 |
| Hemoglobin (g/dL) | −0.253 | 0.0048 | ||
| Current anti-hypertensive Medication | 0.225 | 0.01 | ||
| HIV-specific factors and co-infections | ||||
| Nadir CD4 (per 100cells/m3) | −0.094 | 0.29 | ||
| HIV duration (per month) | 0.090 | 0.32 | ||
| ART duration (per month) | 0.160 | 0.09 | 0.092 | 0.052 |
| Hepatitis C co-infection | 0.121 | 0.18 | ||
| Inflammation and Immune activation | ||||
| Interleukin 6 (pg/mL) | 0.178 | 0.049 | ||
| Cystatin C (mg/L) | 0.323 | <0.0001 | ||
| IP 10 | 0.175 | 0.05 | ||
| TNFα- receptor I (pg/mL) | 0.154 | 0.09 | ||
| TNFα- receptor II (pg/mL) | 0.109 | 0.44 | 0.006 | 0.048 |
| CD4+CD38+HLADR+ T-cells (%) | 0.149 | 0.11 | ||
| CD8+CD38+HLADR+ T-cells (%) | 0.172 | 0.06 | 0.591 | 0.049 |
| CD14+CD16+ monocytes (%) | 0.169 | 0.07 | ||
| Cardiovascular measures | ||||
| Carotid bulb IMT (mm) | 0.232 | 0.0137 | ||
| Mean-Max CCA IMT (mm) | 0.265 | 0.0030 | ||
| Coronary artery calcium score | 0.253 | 0.0047 |
Only variables with p <0.1 included; variables tested in Spearman analysis but not included: Demographics and clinical parameters (male sex, Caucasian, African American BMI, trunk fat, HOMA IR, metabolic syndrome and score, HDL, LDL, smoking status, hemoglobin); HIV specific factors (nadir CD4, current CD4, viral load, protease inhibitors, HIV duration, ARV duration); inflammation and immune activation (IP10, CRP, sCD14, sCD163, D-dimer, CD8+CD38+HLADR+ T-cells, CD14+CD16+ monocytes, CD14dimCD16+ monocytes); cardiovascular measures (systolic and diastolic blood pressures, FMD, plaque, hypertension medications)
Relationship between NT-proBNP and markers of cardiovascular disease
At baseline, NT-proBNP levels were positively correlated with markers of cardiovascular, both markers of vascular disease (bulb and CCA-IMT) and atherogenesis (coronary artery calcium score). . After adjustment for factors known to affect CVD risk and markers of immune activation, higher NT-proBNP remained independently associated with larger CCA-IMT (see table 3 for a representative model).
Table 3.
Multivariable analyses of the relationship between baseline CCA-IMT and baseline NT-proBNP and markers of inflammation and immune activation
| Multivariable analysis estimate | p-value | |
|---|---|---|
| NT-proBNP | 0.0004 | 0.0209 |
| Age | 0.0043 | 0.0012 |
| Male sex (vs female) | −0.0190 | 0.4646 |
| Total lean body mass (g) | 0.000 | 0.1490 |
| eGFRcr (ml/min/1.73m2) | 0.0002 | 0.7349 |
| Framingham score | 0.0080 | 0.0076 |
| ARV duration (per month) | −0.0003 | 0.0457 |
| CD4+CD3 8+HLADR+ T-cells (%) | 0.0040 | 0.1607 |
Changes in NT-proBNP after statin
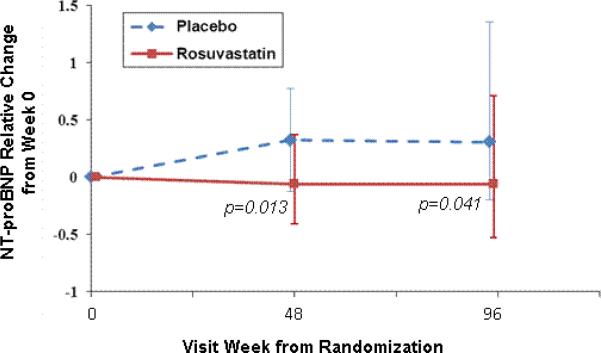
In the rosuvastatin group, NT-proBNP decreased significantly over the 96 weeks of the study. The median (Q1, Q3) percent change in NT-proBNP was -6% (-41%, +37%);p=0.15 after 48 weeks and -6% (-53%, +71%), p=0.04 after 96 weeks], whereas an increase was seen in the placebo group [+32 (-13, +78)%, p=0.006 after 48 weeks; and +30 (-20, +135) %; p=0.005 after 96 weeks]. Further, the changes in NT-proBNP after 48 and 96 weeks were different between the treatment groups (p=0.01 and 0.04, respectively) (Figure 1).
Figure 1. NT-proBNP relative change from 0, 48 and 96 weeks.
Values shown are percent change in NT-proBNP from baseline to 48 and 96 weeks. Error bars shown represent the interquartile ranges. P-values shown are for between group comparisons at each timepoint.
Predictors of change of NT-proBNP levels on study
After adjusting for demographics, renal function, body composition variables (lean, and peripheral and central fat depots), randomization to rosuvastatin remained independently associated with a decrease in NT-proBNP levels. In addition, relative change from baseline to 96 weeks in HOMA-IR and total limb fat were independently associated with relative change of NT-proBNP at week 96 (see table 4).
Table 4.
Multivariable analyses of the relationship between relative change of NT-proBNP at week 96 and statin
| Multivariable analysis estimate | p-value | |
|---|---|---|
| Randomization to statin (vs placebo) | −0.3992 | 0.0168 |
| Age | 0.0248 | 0.0492 |
| Male sex (vs female) | 0.1439 | 0.5537 |
| African American (?vs other) | 0.2409 | 0.1928 |
| Total lean body mass (g) | 0 | 0.5280 |
| Relative change HOMA IR over 96 weeks (%) | −0.1184 | 0.0103 |
| Relative change total limb fat over 96 weeks (%) | −1.0488 | 0.0010 |
| ARV duration (per month) | −0.0018 | 0.2019 |
| eGFRcr (ml/min/1.73m2) | −0.0026 | 0.6009 |
| Framingham score | −0.0331 | 0.1865 |
Discussion
For the first time in HIV infected participants, we investigated the effects of 96 weeks of statin therapy on NT-proBNP levels. This study provides the first evidence that 10 mg of daily rosuvastatin effectively decreases plasma NT-proBNP in treated HIV infection, and that plasma NT-proBNP is associated with carotid IMT.
In HIV-uninfected populations, NT-proBNP is a powerful predictor of many cardiovascular outcomes; however, there is little data on NT-proBNP in HIV. In our study, participants had NT-proBNP levels that are consistent with what is reported in participants without overt heart failure3 where natriuretic peptide concentrations are determined by the balance of low level production (chronic ischemia, left ventricular hypertrophy, and subclinical myocardial dysfunction) and varying levels of clearance (renal disease and adiposity). Additionally, inflammation plays a significant role in the development of cardiovascular complications with HIV infection and this is supported by our finding that NT-proBNP levels are associated with sTNF-RII and CD8+ activation [CD8+CD38+HLADR+].
Statins have been shown to reduce NT-proBNP29 and risk of cardiovascular events in patients with heart failure19,23 and particularly in those who have lower baseline plasma NT-proBNP levels 20,30 . In the CORONA30 (Controlled Rosuvastatin Multinational Trial in Heart Failure) trial, there was no significant differences in primary end- point (cardiovascular mortality, nonfatal myocardial infarction or nonfatal stroke) between patients taking 10 mg of rosuvastatin when compared to placebo, however when restricted to those patients with the lowest tertile of NT-proBNP the hazard ratio favored the patients assigned to rosuvastatin for the primary outcome (hazard ratio 0.65, p=0.005). This effect was seen despite similar effects of statin on lipid profile and hsCRP in each tertile of NT-proBNP. In addition, in the rosuvastatin group, there was a reduction in hospitalizations for cardiovascular reasons and for worsening heart failure in patients with the lowest tertile of NT-proBNP. However, in the Heart Protection Study31, statin therapy reduced the risk of major vascular events regardless of NT-proBNP levels.
In this study, changes in NT-proBNP levels inversely correlated with changes in insulin resistance and limb fat. It has been shown that obese patients have lower levels of NT-proBNP than lean persons32-34. This is despite the fact that obese individuals have higher prevalence of conditions associated with elevated natriuretic peptides such as hypertension and left ventricular hypertrophy32. This is believed to be secondary to a “natriuretic handicap” with a reduced response to cardiac wall stress35 impairing blood pressure regulation and one of the potential links between hypertension and obesity36. Weight loss in obese patients is associated with an increase and sustained response in NT-proBNP without increase in systolic blood pressure or left ventricular pressure 37 suggesting that obesity directly causes lower levels of NT-proBNP through adipocyte receptor mediated clearance32. This is likely largely mediated by insulin resistance as NT-proBNP in the lower range (<120pg/mL) is inversely and independently associated with insulin resistance36,38,39. Although, statins are known to cause insulin resistance, the statin effect on NT-proBNP persisted in our model even after adjustment for changes in HOMA-IR.
NT-proBNP undergoes renal clearance and so a lower GFR is expected to be associated with a higher NT-proBNP; therefore, the use of NT-proBNP as a marker of CVD in patients with poor renal function has been debated40. In the PREVEND (Prevention of Renal and Vascular End-stage Disease) study, NT-proBNP remained associated with cardiovascular events after adjusting for eGFR, albuminuria, age and gender. In our study, baseline eGFR was associated with baseline NT-proBNP levels, but this was no longer true when markers of inflammation were added to the models. Thus, non-GFR determinants such as inflammation may play a larger role in influencing NT-proBNP, as suggested in studies outside of HIV41,42. Our findings that circulating marker of generalized inflammation (sTNF-RII) and marker of T-cell activation (%CD8+CD38+HLADR+ T-cells) were independently associated with baseline NT-proBNP further support this hypothesis. In addition, inflammation itself may be linked to renal function and glomerular filtration 43.
Subjects included in this study were excluded if they had a history of coronary disease or diabetes but not if they were on antihypertensive medications as 25% of the subjects were on antihypertensive medications. At baseline, there was no statistical difference between the statin and placebo groups for hypertensive medications (p=0.4). When comparing patients on hypertensive medications compared to no medications, NT-proBNP values differed between the groups at baseline (p=0.013). However, by week 96 the percent change in NT-proBNP between these groups were similar (13% vs 15% respectively, p=0.9).
NT-proBNP is associated with co-morbidities9 but there is little data on how it relates to markers of cardiovascular disease in HIV. Data recently presented on the WIHS44 study examined the relationship of NT-proBNP with mortality during two different time periods (1994-1997 and 2008) in HIV-infected women. During both time periods, an elevated NT-proBNP level was associated with worse overall survival in with an adjusted hazard ratio of 1.78 and 2.81 during early and late periods respectively. This relationship was not seen in HIV uninfected persons. The causes of deaths were not described but many are likely due to cardiopulmonary disease. In our present study, NT-proBNP levels were associated with multiple measures of subclinical vascular disease. Further, even after adjustment for markers of general inflammation, and monocyte and T-cell activation, NT-proBNP remained independently associated with CCA IMT. Therefore, this study enhances the understanding of NT-proBNP as a predictor of cardiovascular risk in patients with treated HIV infection.
Our study has several strengths, including the double-blind, placebo controlled randomized trial design, and the comprehensive evaluations of inflammation, immune activation and cardiovascular disease risk. For the cross-sectional analyses, we cannot prove causal relationships or exclude the possibility of residual confounding. In addition, the lack of echocardiograms precludes us from making any observations regarding the structural heart indices and their relationship to NT-proBNP levels. Finally, we investigated a specific population of interest-HIV-infected persons with heightened inflammation but normal LDL cholesterol level. Our population was also mostly men and blacks, so our findings may not be applicable to other HIV-infected populations.
In conclusion, we show that when compared to placebo, rosuvastatin 10 mg daily reduces NT-proBNP, a marker correlated with several measures of CVD risk, in HIV-infected patients on ART. Vascular disease and myocardial dysfunction are prominent in patients with HIV. HIV infected subjects on ART with elevated levels of inflammation but overall low levels of NT-proBNP may gain a protective cardiovascular benefit from statins. The effect of statins in HIV-infected subjects with higher levels of proBNP, underlying CVD, and/or myocardial injury deserves further investigations.
Acknowledgements
The authors would like to thank the patients who participated in this research.
Source of support: The work was supported by the National Institutes of Health R01 NR012642 to GAM. Technical support was provided by the Center for AIDS Research, Case Western Reserve University (P30 AI36219).
Conflicts of Interest and Sources of Funding:
GAM served as a consultant, speaker, and has received research funding from Bristol-Myers Squibb, GlaxoSmithKline, Gilead, and Merck. GAM also chaired a DSMB for a Pfizer-funded study. CTL is supported by the National Institutes of Health (K23 HL123341), a Wolf Family Foundation Scholars Grant, and Medtronic Philanthropy. He has received research grants from Bristol-Myers Squibb. COH is supported by the National Institutes of Health (K23HL116209).
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Study drugs and matching placebo were donated by Astra Zeneca. Parts of this dataset have been presented at the ICAAC Conference 2014 with details.
Author Contributions:
GM designed the study and obtained funding. SD and Y.J. provided statistical support. All authors contributed to data analysis and writing of the manuscript.
References
- 1.Martinez-Rumayor A, Richards AM, Burnett JC, Januzzi JL., Jr. Biology of the natriuretic peptides. The American journal of cardiology. 2008;101:3–8. doi: 10.1016/j.amjcard.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Benjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA : the journal of the American Medical Association. 2002;288:1252–9. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 3.Galasko GI, Lahiri A, Barnes SC, Collinson P, Senior R. What is the normal range for N-terminal pro-brain natriuretic peptide? How well does this normal range screen for cardiovascular disease? European heart journal. 2005;26:2269–76. doi: 10.1093/eurheartj/ehi410. [DOI] [PubMed] [Google Scholar]
- 4.Zethelius B, Berglund L, Sundstrom J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. The New England journal of medicine. 2008;358:2107–16. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 5.Ang DS, Kong CF, Kao MP, Struthers AD. Serial bedside B-type natriuretic peptide strongly predicts prognosis in acute coronary syndrome independent of echocardiographic abnormalities. American heart journal. 2009;158:133–40. doi: 10.1016/j.ahj.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Laukkanen JA, Kurl S, Ala-Kopsala M, et al. Plasma N-terminal fragments of natriuretic propeptides predict the risk of cardiovascular events and mortality in middle-aged men. European heart journal. 2006;27:1230–7. doi: 10.1093/eurheartj/ehi878. [DOI] [PubMed] [Google Scholar]
- 7.Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–87. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 8.Duprez DA, Neuhaus J, Tracy R, et al. N-terminal-proB-type natriuretic peptide predicts cardiovascular disease events in HIV-infected patients. AIDS (London, England) 2011;25:651–7. doi: 10.1097/QAD.0b013e32834404a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansoor A, Althoff K, Gange S, et al. Elevated NT-pro-BNP levels are associated with comorbidities among HIV-infected women. AIDS research and human retroviruses. 2009;25:997–1004. doi: 10.1089/aid.2009.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996-2006: collaborative analysis of 13 HIV cohort studies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50:1387–96. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsue PY, Hunt PW, Ho JE, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circulation Heart failure. 2010;3:132–9. doi: 10.1161/CIRCHEARTFAILURE.109.854943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS one. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus JL, Leyden WA, Chao CR, et al. HIV infection and incidence of ischemic stroke. AIDS (London, England) 2014 doi: 10.1097/QAD.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 14.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of clinical endocrinology and metabolism. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. Journal of acquired immune deficiency syndromes (1999) 2012;60:351–8. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173:614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tousoulis D, Oikonomou E, Siasos G, et al. Dose-dependent effects of short term atorvastatin treatment on arterial wall properties and on indices of left ventricular remodeling in ischemic heart failure. Atherosclerosis. 2013;227:367–72. doi: 10.1016/j.atherosclerosis.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. The New England journal of medicine. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 19.Stypmann J, Schubert A, Welp H, et al. Atorvastatin therapy is associated with reduced levels of N-terminal prohormone brain natriuretic peptide and improved cardiac function in patients with heart failure. Clinical cardiology. 2008;31:478–81. doi: 10.1002/clc.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Node K, Fujita M, Kitakaze M, Hori M, Liao JK. Short-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation. 2003;108:839–43. doi: 10.1161/01.CIR.0000084539.58092.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58:588–95. doi: 10.1093/cid/cit748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. The Journal of infectious diseases. 2014;209:1156–64. doi: 10.1093/infdis/jiu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longenecker CT, Hileman CO, Funderburg NT, McComsey GA. Rosuvastatin Preserves Renal Function and Lowers Cystatin C in HIV-infected Subjects on Antiretroviral Therapy: the SATURN-HIV Trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 doi: 10.1093/cid/ciu523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 27.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS (London, England) 2014;28:969–77. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longenecker CT, Funderburg NT, Jiang Y, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV medicine. 2013;14:385–90. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bielecka-Dabrowa A, Goch JH, Mikhailidis DP, Rysz J, Maciejewski M, Banach M. The influence of atorvastatin on parameters of inflammation and function of the left ventricle in patients with dilated cardiomyopathy. Medical science monitor : international medical journal of experimental and clinical research. 2009;15:MS12–23. [PubMed] [Google Scholar]
- 30.Cleland JG, McMurray JJ, Kjekshus J, et al. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure). Journal of the American College of Cardiology. 2009;54:1850–9. doi: 10.1016/j.jacc.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 31.Emberson JR, Ng LL, Armitage J, Bowman L, Parish S, Collins R. N-terminal Pro-B-type natriuretic peptide, vascular disease risk, and cholesterol reduction among 20,536 patients in the MRC/BHF heart protection study. Journal of the American College of Cardiology. 2007;49:311–9. doi: 10.1016/j.jacc.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 32.Taylor JA, Christenson RH, Rao K, Jorge M, Gottlieb SS. B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide are depressed in obesity despite higher left ventricular end diastolic pressures. American heart journal. 2006;152:1071–6. doi: 10.1016/j.ahj.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. The American journal of cardiology. 2002;90:254–8. doi: 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- 34.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 35.Dessi-Fulgheri P, Sarzani R, Tamburrini P, et al. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. Journal of hypertension. 1997;15:1695–9. doi: 10.1097/00004872-199715120-00074. [DOI] [PubMed] [Google Scholar]
- 36.Olsen MH, Hansen TW, Christensen MK, et al. N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension. 2005;46:660–6. doi: 10.1161/01.HYP.0000179575.13739.72. [DOI] [PubMed] [Google Scholar]
- 37.Chen-Tournoux A, Khan AM, Baggish AL, et al. Effect of weight loss after weight loss surgery on plasma N-terminal pro-B-type natriuretic peptide levels. The American journal of cardiology. 2010;106:1450–5. doi: 10.1016/j.amjcard.2010.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez OA, Duprez DA, Bahrami H, et al. The associations between metabolic variables and NT-proBNP are blunted at pathological ranges: the Multi-Ethnic Study of Atherosclerosis. Metabolism: clinical and experimental. 2014;63:475–83. doi: 10.1016/j.metabol.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan AM, Cheng S, Magnusson M, et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. The Journal of clinical endocrinology and metabolism. 2011;96:3242–9. doi: 10.1210/jc.2011-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheven L, de Jong PE, Hillege HL, et al. High-sensitive troponin T and N-terminal pro-B type natriuretic peptide are associated with cardiovascular events despite the cross-sectional association with albuminuria and glomerular filtration rate. European heart journal. 2012;33:2272–81. doi: 10.1093/eurheartj/ehs163. [DOI] [PubMed] [Google Scholar]
- 41.van Diepen S, Roe MT, Lopes RD, et al. Baseline NT-proBNP and biomarkers of inflammation and necrosis in patients with ST-segment elevation myocardial infarction: insights from the APEX-AMI trial. Journal of thrombosis and thrombolysis. 2012;34:106–13. doi: 10.1007/s11239-012-0691-0. [DOI] [PubMed] [Google Scholar]
- 42.Jensen J, Ma LP, Fu ML, Svaninger D, Lundberg PA, Hammarsten O. Inflammation increases NT-proBNP and the NT-proBNP/BNP ratio. Clinical research in cardiology : official journal of the German Cardiac Society. 2010;99:445–52. doi: 10.1007/s00392-010-0140-z. [DOI] [PubMed] [Google Scholar]
- 43.Roy MS, Janal MN, Crosby J, Donnelly R. Markers of endothelial dysfunction and inflammation predict progression of diabetic nephropathy in African Americans with type 1 diabetes. Kidney international. 2014 doi: 10.1038/ki.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthew R, Gingo YZ, Ghebrehawariat Kidane, et al. Elevated NT-pro-BNP levels predict mortality in HIV-infected women [Abstract 723]. Topics in Antiviral Medicine. 2014:368. [Google Scholar]