Abstract
Background
Metamizole is used to treat pain in many parts of the world. Information on the safety profile of metamizole is scarce; no conclusive summary of the literature exists.
Objective
To determine whether metamizole is clinically safe compared to placebo and other analgesics.
Methods
We searched CENTRAL, MEDLINE, EMBASE, CINAHL, and several clinical trial registries. We screened the reference lists of included trials and previous systematic reviews. We included randomized controlled trials that compared the effects of metamizole, administered to adults in any form and for any indication, to other analgesics or to placebo. Two authors extracted data regarding trial design and size, indications for pain medication, patient characteristics, treatment regimens, and methodological characteristics. Adverse events (AEs), serious adverse events (SAEs), and dropouts were assessed. We conducted separate meta-analyses for each metamizole comparator, using standard inverse-variance random effects meta-analysis to pool the estimates across trials, reported as risk ratios (RRs). We calculated the DerSimonian and Laird variance estimate T2 to measure heterogeneity between trials. The pre-specified primary end point was any AE during the trial period.
Results
Of the 696 potentially eligible trials, 79 trials including almost 4000 patients with short-term metamizole use of less than two weeks met our inclusion criteria. Fewer AEs were reported for metamizole compared to opioids, RR = 0.79 (confidence interval 0.79 to 0.96). We found no differences between metamizole and placebo, paracetamol and NSAIDs. Only a few SAEs were reported, with no difference between metamizole and other analgesics. No agranulocytosis or deaths were reported. Our results were limited by the mediocre overall quality of the reports.
Conclusion
For short-term use in the hospital setting, metamizole seems to be a safe choice when compared to other widely used analgesics. High-quality, adequately sized trials assessing the intermediate- and long-term safety of metamizole are needed.
Introduction
Metamizole, or dipyrone, is a pyrazolone derivate whose structure is closely related to that of amidopyrine. It was launched commercially as an analgesic and antipyretic by Hoechst AG in 1922 [1], and is commonly used to treat postoperative pain, colic pain, cancer pain and migraine [2–4]. In many parts of the world, including most countries in the European Union and Latin America, it is the most popular non-opioid first-line analgesic and is sometimes even available over-the-counter. A few countries however, including the United States, the United Kingdom, Sweden, and most recently India [5], have banned metamizole because health authorities judged the risk of agranulocytosis to outweigh the benefits [6–8].
Although used for more than 90 years, the risks and harms of metamizole are not well documented, and information on adverse events related to metamizole is scarce. There are no large randomized controlled trials or conclusive summaries of the existing literature [9]. Three current Cochrane reviews on the effectiveness and safety of metamizole for acute postoperative pain [2], acute renal colic pain [3], and acute primary headaches [4] concluded that metamizole offers good short-term pain relief. In each of these systematic reviews, however, the number of included participants was too small and the authors did not conduct a meta-analysis of safety issues, but they did associate metamizole with somnolence, gastric discomfort and nausea.
Given the still ongoing controversies about the gastrointestinal and cardiovascular safety profiles of non-steroidal anti-inflammatory drugs (NSAIDs) [10–12], which are among the alternatives to metamizole, a comprehensive systematic review of randomized controlled trials investigating metamizole use for all indications is needed. We therefore determined whether metamizole was clinically safe compared to placebo and other commonly used analgesics.
Methods
We used a standard review protocol that was submitted to the funding body prior to the start of the study.
Literature Search
We searched electronic databases from inception to February 2013, without language restriction, including the Cochrane Controlled Trials Register (CENTRAL) through The Cochrane Library (http://mrw.interscience.wiley.com/cochrane/), MEDLINE and EMBASE through the Ovid platform (http://www.ovid.com/) and CINAHL through EBSCOhost. The search algorithm for EMBASE is displayed in S1 Table (it was slightly modified for the other databases). We searched several clinical trial registries (http://www.controlled-trials.com/;http://www.clinicaltrials.gov/, http://www.actr.org.au/, http://www.umin.ac.jp/ctr/) to identify unpublished trials. We also screened the reference lists of included trials and previous systematic reviews.
Trial selection
We included randomized controlled trials that compared the effects of metamizole in adult patients, administered in any form and for any indication, to other analgesics or to placebo. Quasi-randomized trials were not eligible. We selected for inclusion trials that reported any adverse events. We excluded trials in which metamizole was a co-treatment in more than one arm.
Two review authors independently evaluated all titles and abstracts for eligibility. We resolved disagreements by consensus or discussion with a third reviewer. We included studies regardless of length of follow-up or language of publication. If multiple reports described the same trial, we chose the most recent full-text publication in a peer-reviewed journal as the main report.
Outcome measures
The pre-specified primary end point was any adverse event during the trial period. Secondary end points were serious adverse events, overall dropouts and dropouts due to adverse events and serious adverse events. We defined serious adverse events as those that resulted in inpatient hospitalization, persistent or significant disability, congenital abnormality of offspring, life-threatening events or death [13]. We extracted the number of patients per group who experienced at least one event before the end of the trial, and categorized and reported them using the International Classification of Primary Care (ICPC; 2nd edition), which allows for reporting of both complaints and diagnosis [14].
Data Collection and Quality Assessment
Two authors independently extracted data from the full-text articles. They used a standardized, piloted extraction form accompanied by a codebook created for this review [15]. Reviewers resolved disagreements by consensus or through discussion with a third author.
We extracted data regarding trial size, trial design, trial duration (defined as time from first application until end of follow-up), indication for pain medication, patient characteristics (sex, age), treatment regimens (application form, duration and/or frequency of treatment), adverse events and funding source. We extracted data only from the first period of crossover trials, because carry-over effects might be present in the later periods. We attempted to contact studies’ corresponding authors to obtain missing information.
Two review authors independently assessed concealment of treatment allocation, blinding of patients, adequate adverse event assessment and adequacy of analyses. We considered allocation concealment to be adequate if the investigators who selected patients were unable to guess which treatment patients would be allocated to. We considered patients to be blinded if the trial was reported as “double blind”. Adverse event assessment was considered adequate if it was conducted systematically and prospectively. Analyses were considered adequate if all recruited patients were analyzed in the group to which they were originally allocated, regardless of the treatment received (intention-to-treat principle). See S2 Table for definitions used to classify trials according to components of methodological quality. Disagreements were resolved by discussion with a third reviewer and subsequent consensus.
Data Synthesis and Analysis
We conducted separate meta-analyses for each metamizole comparator. We first calculated the log risk ratio and its standard error for each trial, and then used standard inverse-variance random effects meta-analysis to pool these estimates across trials [16]. The pooled estimate was then exponentiated to report treatment effect estimates as risk ratios (RRs). A RR below 1 indicates that metamizole is a safer intervention than its comparator. We calculated the DerSimonian and Laird variance estimate T2 to measure heterogeneity between trials [16]. A T2 of 0.04 was pre-specified to represent low heterogeneity, 0.09 moderate heterogeneity and 0.16 high heterogeneity between trials [17]. We performed stratified analyses of primary and secondary outcomes according to dose and route of administration. Uni-variable random-effects meta-regression models were used for tests of interaction between outcomes and these characteristics. We distinguished between single dose and multiple doses, determined the association of the log risk ratio with daily dose and cumulative dose on a continuous scale, and classified route of administration as i.m., i.v., or p.o. for this purpose. All p-values are two-sided. Analyses were conducted using STATA, release 11 (Stata-Corp, College Station, Texas).
Results
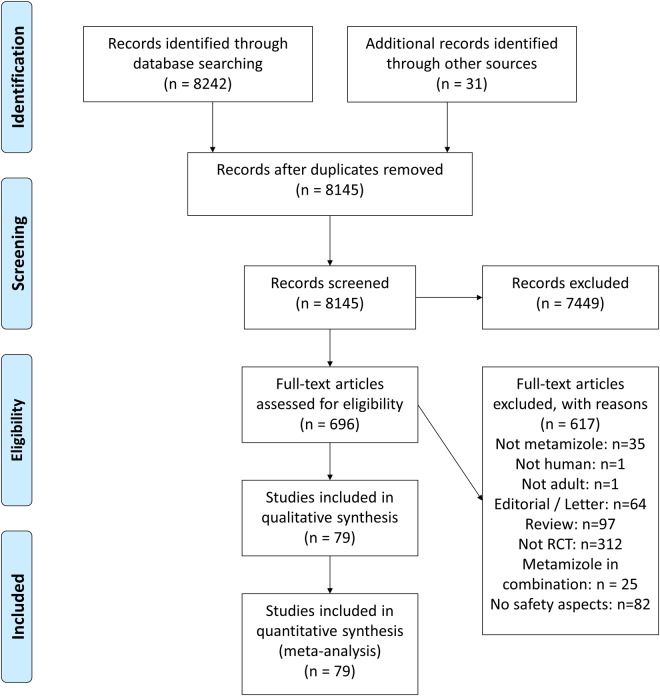
We identified 8242 references in our literature search, of which 696 were potentially eligible. Seventy-nine reports describing 79 randomized controlled trials met our inclusion criteria and were included in the meta-analysis (see PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart [Fig 1] and checklist [S1 PRISMA Checklist]). Forty-nine trials were published in English, 12 in Spanish, six in Portuguese, six in German, four in Italian, and two in Turkish. Trials were conducted in several European and Latin American countries where metamizole is widely available. All trials were published as full-text journal articles. The median year of publication was 1995 (range: 1974–2011). A search of trial registries yielded no ongoing trials.
Fig 1. PRISMA flowchart.
The 79 randomized controlled trials [18–95] assessed mainly short-term use of common analgesics. Forty-two trials (53%) investigated the use of only a single dose of metamizole. In addition, 18 trials (23%) had a maximum treatment duration of only one day, while in 17 trials treatment lasted from two days up to one week (22%) and only two trials (2%) had a treatment duration of two weeks. Average length of follow-up ranged from 0.33 to 366 hours (median: 24 hours). Four trials (5%) had sample sizes of at least 100 patients per randomized group (median: 30 patients).
The average age of participants ranged from 22–64 years (median: 45 years). Women made up 45.8% of the subjects. A total of 3716 patients received metamizole, 1077 received placebo, 303 received aspirin, 1983 received NSAIDs, 829 received opioids, 362 received paracetamol, and 156 patients received other pain medications. Very few of the 79 included randomized controlled trials were conducted in the ambulatory setting. Table 1 describes the clinical characteristics of the trials.
Table 1. Clinical characteristics of the trials.
| Trial | year | n 1 | Indication | Comparison | form of application 2 | Treatment duration (days) |
|---|---|---|---|---|---|---|
| Ajgaonkar | 1985 | 42/46 | Fever | Placebo | p.o. | single dose |
| Arnau | 1991 | 217/ 116 | Renal colic | Diclofenac, Pethidine | i.m. | single dose |
| Atalay | 1995 | 14/38 | Post-OP | Fentanyl, Morphine | i.m. / epidural catheter | single dose |
| Babej-Dölle | 1994 | 88/ 172 | Diclofenac, Placebo | i.m. | 2 | |
| Bagan | 1998 | 40/80 | Post-OP | Dexketoprofen | p.o. | 3 |
| Bigal | 2002 | 74/60 | Migraine | Placebo | i.v. | single dose |
| Bilgin | 2003 | 25/25 | Post-OP | Bupivacaine | extrapleural catheter | 3 |
| Blendinger | 1980 | 7/10 | Post-OP | ASS | i.v. | single dose |
| Bloch | 1985 | 68/65 | Post-OP | Pethidine | i.m. | 2 |
| Boraks | 1987 | 39/ 120 | Post-OP | Flurbiprofen, Placebo | p.o. | single dose |
| Braun | 1999 | 76/79 | Post-OP | Placebo | i.v. | 0.042 |
| Brodner | 2011 | 49/ 147 | Post-OP | Parecoxib, PCM, Placebo | i.v. | 2 |
| Castro | 2000 | 30/68 | Post-OP | Ketorolac, Tramadol | i.v. | 1 |
| Castro Gonzales | 1986 | 28/58 | Post-OP | Dexketoprofen, Ibuprofen | p.o. | 1.667 |
| Cruz | 2002 | 30/30 | Fever | Propacetamol | i.v. | single dose |
| De Miguel Rivero | 1997 | 35/72 | Post-OP | Ibuprofen, Placebo | i.m. / p.o. | single dose |
| Diaz-Chavez | 2009 | 47/48 | Post-OP | Ketorolac | i.v. / p.o. | 1 |
| Dos Santos Pereira | 1986 | 28/57 | Post-OP | Acetaminophen, Placebo | p.o. | single dose |
| Duarte Souza | 2007 | 18/16 | Cancer | Placebo | p.o. | 2 |
| Fernandes Filho | 2006 | 12/15 | Migraine | MCP | i.v. | single dose |
| Ferrario | 1984 | 14/14 | Post-OP | ASS | i.m. / i.v. | single dose |
| Gomes-Marquez | 2004 | 25/25 | Post-OP | Parecoxib | i.v. | single dose |
| Gonzalez-Garcia | 1994 | unclear | Post-OP | Ketorolac | p.o. | single dose |
| Grundmann | 2006 | 20/60 | Post-OP | Parecoxib, PCM, Placebo | i.v. | single dose |
| Guberti | 1982 | 14/14 | Post-OP | Nefopam | i.v. | 4 |
| Hernandez Llenas | 1997 | 15/15 | Post-OP | Diclofenac | i.v. | 0.336 |
| Herrera Barroso | 1982 | 30/60 | Post-OP | Zomepirac, Placebo | p.o. | single dose |
| Ibarra-Ibarra | 1993 | 48/49 | Post-OP | Ketorolac | i.m. | single dose |
| Jage | 1990 | 40/40 | Post-OP | Placebo | i.v. | 1 |
| Jovic | 2008 | 30/30 | Post-OP | Ketoprofen | i.v. | 5 |
| Kampe | 2006 | 20/20 | Post-OP | PCM | i.v. | 1 |
| Karaman | 2010 | 30/60 | Post-OP | Dexketoprofen, PCM | i.v. | 1 |
| Kemal | 2007 | 20/20 | Post-OP | Lornoxicam | i.v. | 1 |
| Knüsel | 1982 | 40/40 | Osteoarthritis | Zomepirac | p.o. | 14 |
| Krymchantowski | 2008 | 15/15 | Migraine | Lysin Clonixinate | i.v. | single dose |
| Landwehr | 2005 | 13/12 | Post-OP | PCM | i.v. | 1.021 |
| Lehmann | 2001 | 40/40 | Post-OP | Placebo | i.v. | 1 |
| Lehtonen | 1983 | 45/124 | Renal colic | Indomethacin, Pethidine | i.v. | single dose |
| Marin-Bertolin | 1997 | 46/46 | Post-OP | Ketorolac | i.m. | 2 |
| Martin-Duce | 1997 | 187/48 | Post-OP | Diclofenac | i.m. / i.v. | single dose |
| Martinez-Martin | 2001 | 204/207 | Migraine | ASS | p.o. | single dose |
| Mateu | 1992 | 38/80 | Post-Trauma | ASS, PCM | p.o. | single dose |
| Mehta | 1986 | 91/163 | Post-OP | ASS, Placebo | p.o. | single dose |
| Monso | 1996 | 37/67 | Shivering | Pethidine, Placebo | i.v. | single dose |
| Muriel | 1993 | 87/41 | Renal colic | Diclofenac | i.v. | single dose |
| Muriel-Villoria | 1995 | 239/54 | Renal colic | Diclofenac | i.m. | single dose |
| Ocampo Flores | 1986 | 15/30 | Post-OP | Ibuprofen, Dextropropoxifene | p.o. | 1.667 |
| Pardo | 1984 | 30/60 | Renal colic | Ceruletide, Placebo | i.v. | single dose |
| Patel | 1980 | 51/49 | Post-OP | Pethidine | i.m. | single dose |
| Pavlik | 2004 | 32/32 | Renal colic | Cizolirtin | i.v. | single dose |
| Peiró | 2008 | 8/8 | Pancreatitis | Morphine | i.v. / s.c. | 2 |
| Pernia | 2000 | 30/37 | Post-OP | Propacetamol | i.v. | 1 |
| Pinto | 1984 | 27/29 | Post-OP | Acetaminophen | p.o. | single dose |
| Planas | 1998 | 147/106 | Post-OP | Ibuprofen, Placebo | p.o. | single dose |
| Prada | 1974 | 20/40 | Post-OP | Pentazocine | supp. | single dose |
| Primus | 1989 | 30/30 | Renal colic | Tramadol | i.v. | single dose |
| Rawal | 2001 | 40/80 | Post-OP | Tramadol | p.o. | 2 |
| Rejman | 1984 | 25/25 | Biliary colic | Indomethacin | i.v. | single dose |
| Reyes | 1988 | 25/25 | Post-OP | Diclofenac | i.v. | single dose |
| Reyes-Armijo | 1974 | 15/15 | Migraine | Vitamin B1, B6 | p.o. | 14 |
| Rodriguez | 1994 | 79/42 | Cancer | Morphine | p.o. | 7 |
| Rubinstein | 1986 | 30/60 | Post-OP | Acetaminophen | p.o. | single dose |
| Sanchez-Carpena | 2003 | 108/225 | Renal colic | Dexketoprofen | i.m. | single dose |
| Sanchez-Carpena | 2007 | 103/205 | Renal colic | Dexketoprofen | i.v. | single dose |
| Saray | 2001 | 80/80 | Post-OP | Diclofenac | i.m. | 2 |
| Savoca | 1985 | 15/15 | Post-OP | Imidazol-2-Hydroxybenzoat | p.o. | 1 |
| Schmieder | 1993 | 25/49 | Biliary colic | Butylscopolamin | i.v. | single dose |
| Sener | 2008 | 40/160 | Post-OP | Diclofenac, Ketoprofen, Lornoxicam, Placebo | i.m. | 0.667 |
| Spacek | 2003 | 30/30 | Post-OP | Placebo | i.v. | 1 |
| Stankov | 1994 | 36/68 | Renal colic | Butylscopolamin | i.v. | single dose |
| Stankov | 1995 | 51/49 | Post-OP | Tramadol | i.v. | single dose |
| Steffen | 1997 | 20/20 | Post-OP | Tramadol | i.v. | 0.5 |
| Striebel | 1992 | 30/30 | Post-OP | Placebo | i.v. | 0.167 |
| Tempel | 1996 | 54/52 | Post-OP | Placebo | i.v. | 0.667 |
| Tonolli Jacob | 1986 | 33/66 | Post-Trauma | Diclofenac, Placebo | i.m. | single dose |
| Torres | 1993 | 50/100 | Post-OP | Buprenorphin, Morphine | i.v. | 0.072 |
| Torres | 2001 | 73/78 | Post-OP | Tramadol | i.v. | 1 |
| Uzun | 2010 | 23/20 | Post-OP | Placebo | i.v. | single dose |
| Vargha von Szeged | 1986 | 30/30 | Migraine | Suprofen | p.o. | 5 |
1: n (patients) Metamizole / n (patients) comparisons.
2: i.m.: intramuscular, i.v.: intravenous, p.o.: per os, supp: suppository, s.c.: subcutaneous.
Twenty-four studies (31%) used adequate randomization methods, fourteen studies (18%) adequately concealed allocation, fifty-five studies (70%) adequately blinded patients and 13 studies (17%) adequately assessed adverse events. Forty-two studies (53%) analyzed all patients according to the intention-to-treat principle. S3 Table describes the trials’ methodological characteristics. The heterogeneity between the studies was low for most adverse events (T2 <0.01). The data extracted from single studies can be found in S4 Table and S1 Dataset.
Metamizole versus Placebo
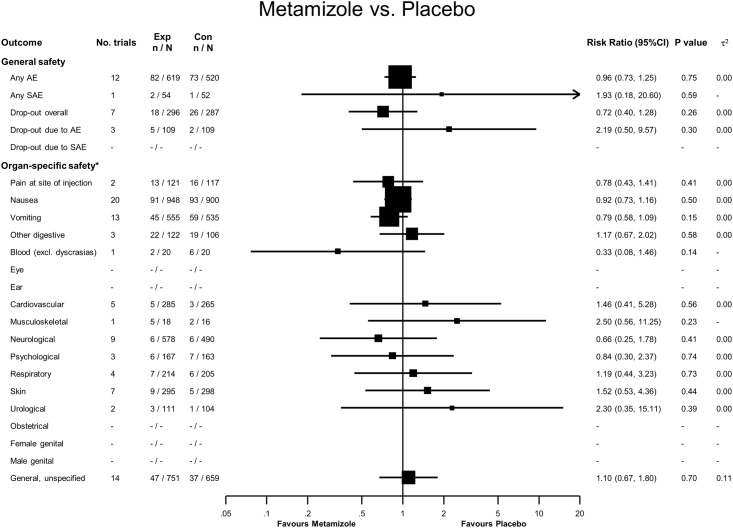
Eighty-two adverse events were reported in 619 patients treated with metamizole, compared to 73 adverse events in 520 patients treated with placebo, yielding a RR of 0.96 (95% confidence interval (CI) 0.73 to 1.25, see Fig 2). Two serious adverse events were reported in the metamizole group: one case of leucopenia due to septicemia following aspiration and one case of post-operative hemorrhage after prostatectomy. In comparison, one serious adverse event was reported in the placebo group, a case of leucopenia due to anastomosis insufficiency [90], yielding a RR of 1.93 (95% CI 0.18 to 20.6). For overall dropouts and dropouts due to adverse events, 95% CI overlapped the line of no difference. We found no statistically significant difference in organ-specific safety.
Fig 2. Forest plot—Metamizole versus Placebo.
Categories according to the International Classification of Primary Care (see Methods section and [13]). Results from single studies are of limited interpretability but are displayed for the sake of completeness. RR = risk ratio. AE = adverse events. SAE = serious adverse events.
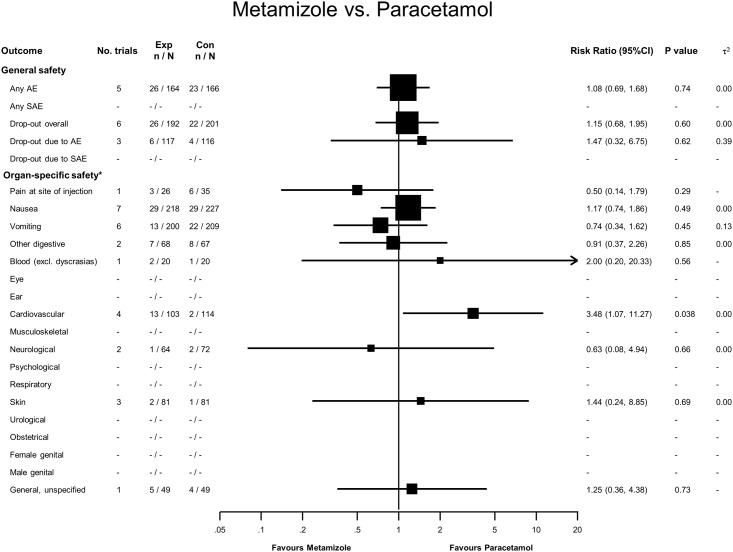
Metamizole versus Paracetamol
Twenty-six adverse events were reported in 164 patients treated with metamizole, and 23 in 166 treated with paracetamol, with an RR of 1.08 (95% CI 0.69 to 1.68, see Fig 3). No serious adverse events were reported, and for overall dropouts and dropouts due to adverse events, 95% CI overlapped the line of no difference. Thirteen patients treated with metamizole had cardiovascular adverse events, compared to two patients treated with paracetamol (RR = 3.48, 95% CI 1.07 to 11.27). All of these patients had hypotension due to intravenous injection of metamizole.
Fig 3. Forest plot—Metamizole versus Paracetamol.
Categories according to the International Classification of Primary Care (see Methods section and [13]). Results from single studies are of limited interpretability but are displayed for the sake of completeness. RR = risk ratio. AE = adverse events. SAE = serious adverse events.
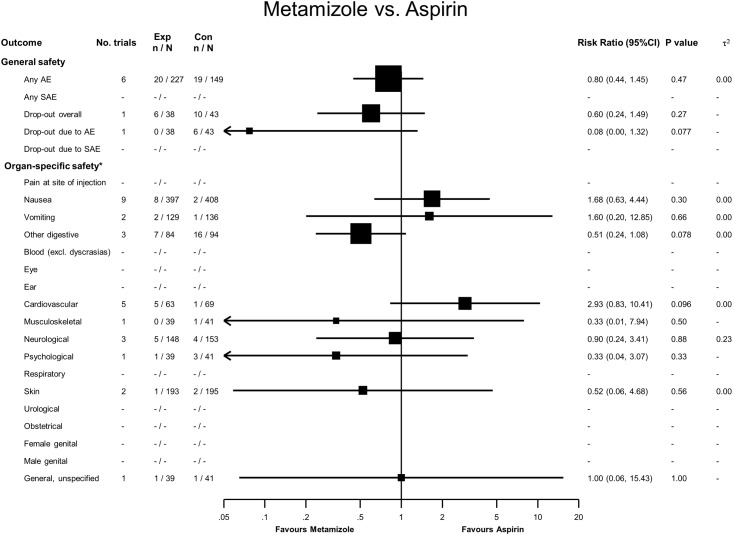
Metamizole versus Aspirin
Twenty adverse events were reported in 227 patients treated with metamizole, and 19 in 149 treated with aspirin, with a RR of 0.80 (95% CI 0.44 to 1.45, see Fig 4). No serious adverse events were reported, and for overall dropouts and dropouts due to adverse events, 95% CI overlapped the line of no difference. We found no statistically significant difference in organ-specific safety.
Fig 4. Forest plot—Metamizole versus Aspirin.
Categories according to the International Classification of Primary Care (see Methods section and [13]). Results from single studies are of limited interpretability but are displayed for the sake of completeness. RR = risk ratio. AE = adverse events. SAE = serious adverse events.
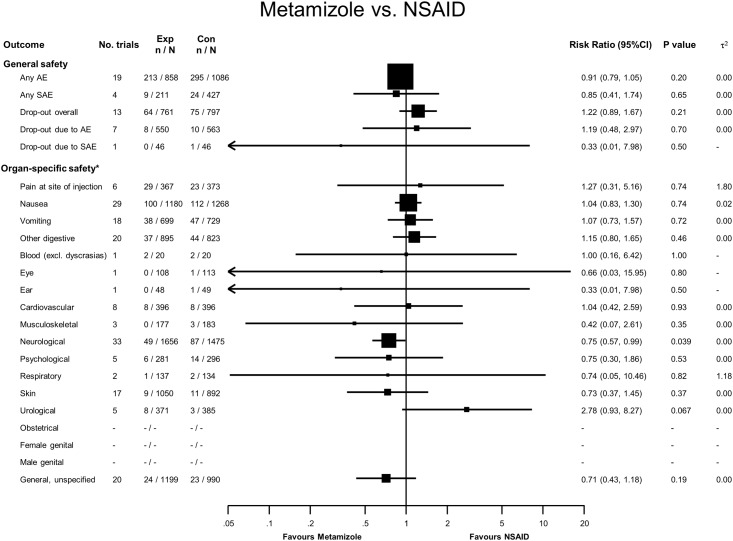
Metamizole versus NSAIDs
Two hundred and thirteen adverse events were reported in 858 patients treated with metamizole, compared to 295 adverse events in 1086 treated with NSAIDs, yielding a RR of 0.91 (95% CI 0.79 to 1.05, see Fig 5). Nine serious adverse events were reported in the metamizole group and 24 serious adverse events were reported in the NSAID group. With the exception of two postoperative hemorrhages after abdominoplasty described in participants allocated to an NSAID in one trial [56], the majority of the remaining 22 serious adverse events in two trials by the same research group were described as ‘cases of recurrence of renal pain that led to hospitalization [80,81]. For overall dropouts, dropouts due to adverse events and drop-outs due to serious adverse events, CI overlapped the line of no difference. One dropout due to serious adverse events was a post-operative hemorrhage in the NSAID group [56]. Forty-nine patients treated with metamizole had neurological adverse events compared to 87 treated with NSAIDs (RR 0.75, 95% CI 0.57 to 0.99). The most common reason was headache or unspecific vertigo and dizziness.
Fig 5. Forest plot—Metamizole versus NSAIDs.
Categories according to the International Classification of Primary Care (see Methods section and [13]). Results from single studies are of limited interpretability but are displayed for the sake of completeness. RR = risk ratio. AE = adverse events. SAE = serious adverse events.
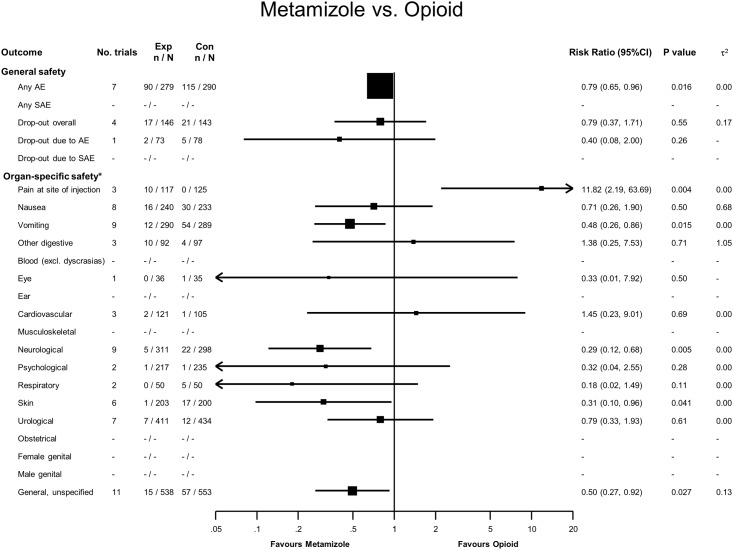
Metamizole versus Opioids
Ninety adverse events were reported in 279 patients treated with metamizole, compared to 115 adverse events in 290 treated with opioids, yielding a RR of 0.79 (95% CI 0.79 to 0.96, see Fig 6). No serious adverse events were reported, and for overall dropouts and dropouts due to adverse events, CI overlapped the line of no difference. Ten patients in the metamizole group reported pain at the injection site, compared to none in the opioid group (RR = 11.8, 95% CI 2.2 to 63.7). Twelve patients in the metamizole group reported vomiting compared to 54 in the opioid group (RR = 0.48, 95% CI 0.26 to 0.86), while five metamizole patients compared to 22 taking opioids reported neurological signs, most commonly vertigo, dizziness or headache (RR = 0.29, 95% CI 0.12 to 0.68). Unspecific and general complaints such as sweating, tiredness, somnolence, shaking and fever were less common in the metamizole group than in the opioid group (15 versus 57 events, RR = 0.50, 95% CI 0.27 to 0.92).
Fig 6. Forest plot—Metamizole versus Opioids.
Categories according to the International Classification of Primary Care (see Methods section and [13]). Results from single studies are of limited interpretability but are displayed for the sake of completeness. RR = risk ratio. AE = adverse events. SAE = serious adverse events.
Tests of interaction indicated no evidence of an association between outcomes and dose or route of administration. Results of these interaction tests must be carefully interpreted given their low power, however.
Serious adverse events, agranulocytosis and death
None of the trials reported agranulocytosis or withdrawals due to death. In addition, the trialists did not attribute any of the above-mentioned serious adverse events to metamizole.
Discussion
In our meta-analysis of randomized controlled trials that compared the safety of metamizole to placebo and other analgesics, there was no difference in adverse events between metamizole and placebo, paracetamol, aspirin, or NSAIDs, and fewer adverse events compared to opioids. These 79 trials, which included almost 4000 patients with short-term metamizole use of less than two weeks, reported few serious adverse events, with no difference between metamizole and the comparators, and no cases of agranulocytosis.
Our approach to studying organ-specific differences identified several statistically significant differences in the frequency of adverse events, most of which seemed clinically plausible. First, compared to paracetamol, patients treated with metamizole had a significantly higher rate of adverse cardiovascular events, most commonly hypotension. Most of the trials in which these adverse events were reported used intravenously administered metamizole, which can decrease systolic blood pressure [96]. Second, compared with NSAIDs, metamizole was less likely to be associated with neurological adverse events such as headache, vertigo or dizziness. This finding is consistent with a standard textbook on drug side effects1, which describes neurological adverse events as “never” being caused by metamizole. Third, compared to opioids, metamizole was less frequently associated with neurological and unspecific adverse events, most commonly vertigo, dizziness, tiredness, sedation and CNS depression. Opioids and metamizole have different modes of action, and the described adverse events are well documented for opioids but not for metamizole [1]. However, it is not clear why metamizole caused more pain at the injection site compared to opioids.
Our systematic review was strengthened by the highly sensitive search strategy employed. We captured as many relevant studies as possible by including trials published in all languages, contacting the authors of studies published in the last 12 years and performing the additional reference list search. Because we included studies that examined metamizole for all indications, we were able to analyze 79 studies, while the three Cochrane reviews, which assessed clinical effectiveness only in postoperative pain, renal colic pain and acute primary headaches, analyzed 15 [2], 11 [3] and four [4] studies, respectively. We also included more patients; our study evaluated 8426 patients, while the Cochrane reviews evaluated 1436 [2], 1053 [3] and 636 [4]. Our search strategy enabled us to conduct a safety analysis and identify serious adverse events, which the Cochrane reviews were not able to do.
The generalizability of our results is limited by the methodological characteristics of the studies we included. Studies did not consistently assess adverse events, and only 17% of studies had a low risk of bias. Adverse events were also reported differently between trials, which further limits the validity of our results [97]. The trials we included were underpowered to demonstrate differences in drug safety. Few trials were conducted in ambulatory settings, limiting the generalizability of our results for community usage. The included trials also had very short follow-up periods, so it is impossible to draw conclusions about intermediate- and long-term adverse events from the available evidence. The small sample size and short-term follow-up of included trials means that the number of accumulated events (see Figs 2–6) is small for nearly all safety outcomes, which renders our results imprecise and our meta-analyses unreliable as previously discussed by Flather et al [98]. Because many of the included studies were conducted before mandatory trial registers were introduced, we cannot rule out publication bias; we did, however, search different trial registers to minimize the probability of bias.
We only considered the safety aspects of metamizole and did not evaluate efficacy, which the above-mentioned Cochrane reviews found was similar to that of other widely used analgesics for three different indications: postoperative pain, renal colic pain and acute primary headaches [2–4]. Given these findings, an analgesic should be chosen based on its safety profile. Metamizole was associated with fewer adverse events than opioids and had a better neurological side effect profile than NSAIDs. However, we found no differences regarding the most recently discussed end points of gastrointestinal and cardiovascular safety [10–12]. This contrasts with a comparative overall safety analysis of four different analgesics (aspirin, diclofenac, acetaminophen and metamizole) based on epidemiological studies published between 1970 and 1995 [7]. In this analysis, Andrade et al included data on short-term (one-week) use of the analgesics and estimated the excess mortality due to agranulocytosis, aplastic anemia, anaphylaxis and serious upper gastrointestinal complications. The authors found an excess mortality for metamizole of 25 per 100 million compared to 592 per million for diclofenac, 185 for aspirin and 20 for paracetamol. Serious upper gastrointestinal complications largely accounted for the excess mortality associated with diclofenac and aspirin [7].
None of the included randomized trials reported agranulocytosis, which is a rare but very harmful adverse event associated with metamizole [8]. There are huge variations in its estimated incidence [99], ranging from 1 case per 1431 prescriptions in a Swedish study [100] to 9 cases per million per year in the International Agranulocytosis and Aplastic Anemia Study [101]. Even if we assume the Swedish estimate to be correct, the number and size of the trials included in our review would be too small to yield more than only a few agranulocytosis cases. A systematic review of observational studies investigating the frequency of metamizole-associated agranulocytosis is currently under preparation by our working group.
Conclusion
For short-term use in the hospital setting, such as to treat renal colic or postoperative pain, metamizole seems to be a safe choice when compared to other widely used analgesics. There is very limited information available on the intermediate- and long-term safety of metamizole. High-quality, adequately sized trials assessing metamizole-associated adverse events in the ambulatory setting are needed.
Supporting Information
(XML)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
The authors thank Dalila Boulkhemair for her valuable help during literature retrieval. We thank Kali Tal for her editorial contribution.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded by the German Federal Ministry of Research and Education (Grant-No. 01KG1017). The funding source did not play a role in the design, conduct or reporting of the study, or the decision to submit the manuscript for publication.
References
- 1. Aronson JK (Editor). Meyler’s Side Effects of Analgesics and Antiinflammatory Drugs. 1st ed Amsterdam: Elsevier; 2010. [Google Scholar]
- 2. Derry S, Faura C, Edwards J, McQuay HJ, Moore RA. Single-dose dipyrone for acute postoperative pain. Cochrane Database Syst Rev. 2013;CD003227 10.1002/14651858.CD003227.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edwards J, Meseguer F, Faura C, Moore RA, McQuay HJ. Single dose dipyrone for acute renal colic pain. Cochrane Database Syst Rev. 2011;CD003867 10.1002/14651858.CD003867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramacciotti AS, Soares BGO, Atallah AN. Dipyrone for acute primary headaches. Cochrane Database Syst Rev. 2007;CD004842 10.1002/14651858.CD004842.pub3 [DOI] [PubMed] [Google Scholar]
- 5. Bhaumik S. India’s health ministry bans pioglitazone, metamizole, and flupentixol-melitracen. BMJ. 2013;347: f4366 10.1136/bmj.f4366 [DOI] [PubMed] [Google Scholar]
- 6. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146: 657–665. [DOI] [PubMed] [Google Scholar]
- 7. Andrade SE, Martinez C, Walker AM. Comparative safety evaluation of non-narcotic analgesics. J Clin Epidemiol 1998;51: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 8. Heimpel H. Drug-induced agranulocytosis. Med Toxicol Adverse Drug Exp. 1988;3: 449–462. [DOI] [PubMed] [Google Scholar]
- 9. Benseñor IM. To use or not to use dipyrone? Or maybe, Central Station versus ER? That is the question. São Paulo Med J Rev Paul Med. 2001;119: 190–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. (2011) Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342: c7086 10.1136/bmj.c7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coxib and traditional NSAID Trialists’ (CNT) Collaboration, Bhala N, Emberson J, Merhi A, Abramson S, Arber N, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382: 769–779. 10.1016/S0140-6736(13)60900-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Abajo FJ, Gil MJ, García Poza P, Bryant V, Oliva B, Timoner J, et al. Risk of nonfatal acute myocardial infarction associated with non-steroidal antiinflammatory drugs, non-narcotic analgesics and other drugs used in osteoarthritis: a nested case-control study. Pharmacoepidemiol Drug Saf. 2014;23: 1128–38. 10.1002/pds.3617 [DOI] [PubMed] [Google Scholar]
- 13.European Commission Directorate General for Health and Consumers. Guidelines on Medical Devices. Clinical Investigations: Serious Adverse Event Reporting Under Directives 90/385/EEC and 93/ 42/EEC. 2010. Available: http://ec. europa.eu/health/medical-devices/files/meddev/2_7_3_en.pdf.
- 14.Wonca International Classification Committee. An Introduction to the International Classification of Primary Care—Version 2. Available: http://www.ph3c.org/PH3C/docs/27/000098/0000054.pdf.
- 15. Rutjes AWS, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157: 180–191. 10.7326/0003-4819-157-3-201208070-00473 [DOI] [PubMed] [Google Scholar]
- 16. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7: 177–188. [DOI] [PubMed] [Google Scholar]
- 17. Spiegelhalter D, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. John Wiley & Sons; 2004. 10.1002/0470092602 [DOI] [Google Scholar]
- 18. Ajgaonkar VS, Pinto Pereira LM. Patient study of antipyretic drug efficacy. Curr Ther Res Clin Exp. 1985;37: 440–445. [Google Scholar]
- 19. Arnau JM, Cami J, Garcia-Alonso F, Laporte JR, Palop R. Comparative study of the efficacy of dipyrone, diclofenac sodium and pethidine in acute renal colic. Eur J Clin Pharmacol. 1991;40: 543–546. [DOI] [PubMed] [Google Scholar]
- 20. Atalay H, Tanriverdi B. Evaluation of extradural morphine, fentanyl and i.m. dipyron in the postoperative analgesia. Agri Derg. 1995;7: 22–27. [Google Scholar]
- 21. Babej-Dölle R, Freytag S, Eckmeyer J, Zerle G, Schinzel S, Schmeider G, et al. Parenteral dipyrone versus diclofenac and placebo in patients with acute lumbago or sciatic pain: Randomized observer-blind multicenter study. Int J Clin Pharmacol Ther. 1994;32: 204–209. [PubMed] [Google Scholar]
- 22. Bagan JV, Lopez Arranz JS, Valencia E, Santamaria J, Eguidazu I, Horas M, et al. Clinical comparison of dexketoprofen trometamol and dipyrone in postoperative dental pain. J Clin Pharmacol. 1998;38(12 Suppl): 55S–64S. [PubMed] [Google Scholar]
- 23. Bigal ME, Bordini CA, Tepper SJ, Speciali JG. Intravenous dipyrone in the acute treatment of migraine without aura and migraine with aura: A randomized, double blind, placebo controlled study. Headache. 2002;42: 862–871. [DOI] [PubMed] [Google Scholar]
- 24. Bilgin M, Akcali Y, Oguzkaya F. Extrapleural regional versus systemic analgesia for relieving postthoracotomy pain: a clinical study of bupivacaine compared with metamizol. J Thorac Cardiovasc Surg. 2003;126: 1580–1583. [DOI] [PubMed] [Google Scholar]
- 25. Blendinger I, Eberlein HJ. Comparison of intravenous acetylsalicylic acid and dipyrone in postoperative pain: an interim report. Br J Clin Pharmacol. 1980;10(Suppl 2): 339S–341S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bloch B, Smythe E, Weeks R. Analgescis for pain relief after gynaecological surgery: A two-phase study. S Afr Med J. 1985;67: 325–329. [PubMed] [Google Scholar]
- 27. Boraks S [Flurbiprofen in low dosage compared with metamizole sodium (dipyrone), acetylsalicylic acid and placebo in treatment of pain following dental extractions.]. Arq Bras Med. 1987;61: 424–430. [Google Scholar]
- 28. Braun R, Buche I, Maier P, Thiele H. Perioperative analgesia with intraoperatively started infusion of high- dose dipyrone in orthopaedic and trauma surgery. Acute Pain: 1999;2: 167–171. [Google Scholar]
- 29. Brodner G, Gogarten W, Van Aken H, Hahnenkamp K, Wempe C, Freise H, et al. Efficacy of intravenous paracetamol compared to dipyrone and parecoxib for postoperative pain management after minor-to-intermediate surgery: a randomised, double-blind trial. Eur J Anaesthesiol. 2011;28: 125–132. 10.1097/EJA.0b013e32833fedfa [DOI] [PubMed] [Google Scholar]
- 30. Castro F, Pardo D, Mosquera G, Peleteiro R, Camba MA. [Management of postoperative pain with PCA in upper abdominal surgery. Comparative study: Tramadol versus metamizol and kerotolac.]. Rev Soc Espanola Dolor. 2000;7: 12–16. [Google Scholar]
- 31. Castro Gonzalez F, Martinez Garza A. [Comparative study of analgesic anti-inflammatory effect of ibuprofen, dipyrone and dextropropoxyphene in buco-dento-maxillar surgery.]. Compend Investig Clin Latinoam. 1986;6: 61–67. [Google Scholar]
- 32. Cruz P, Garutti I, Diaz S, Fernandez-Quero L. Metamizol versus propacetamol: comparative study of the hemodynamic and antipyretic effects in critically ill patients. Rev Esp Anestesiol Reanim. 2002;49: 391–396. [PubMed] [Google Scholar]
- 33. De Miguel Rivero C, Garcia Araujo C, Mella Sousa M, Lopez De Rueda FS, Luna Gonzalez F, Padilla Márquez A, et al. Comparative efficacy of oral ibuprofen-arginine, intramuscular magnesic dipyrone and placebo in patients with postoperative pain following total hip replacement. Clin Drug Investig. 1997;14: 276–285. [Google Scholar]
- 34. Díaz-Chávez EP, Medina-Chávez JL, Avalos-González J, Hernández-Moreno JJ, Cabrera-Mendoza AU, Trujillo-Hernández B. [Comparison of sublingual ketorolac vs. IV metamizole in the management of pain after same-day surgery.]. Cir Ciruj. 2009;77: 45–49. [PubMed] [Google Scholar]
- 35. Dos Santos Pereira E. [Comparative study of paracetamol, dipyrone, and placebo for the treatment of postoperative orthopaedic pain.]. Folha Med. 1986;92: 99–105. [Google Scholar]
- 36. Duarte Souza JF, Lajolo PP, Pinczowski H, del Giglio A. Adjunct dipyrone in association with oral morphine for cancer-related pain: the sooner the better. Support Care Cancer. 2007;15: 1319–1323. [DOI] [PubMed] [Google Scholar]
- 37. Fernandes Filho SMM, Costa MS, Fernandes MT, Foerster MV. Comparison of intravenous dipyrone to intravenous metoclopramide in the treatment of acute crisis of migraine: Randomized clinical trial. Arq Neuropsiquiatr. 2006;64: 1005–1008. [DOI] [PubMed] [Google Scholar]
- 38. Ferrario F, Frassineti L. [IV ASA for post-surgical pain of the spine.]. Eur Rev Med Pharmacol Sci 1984;6: 147–152. [Google Scholar]
- 39. Gomez-Marquez JDJ, Polendo-Villarreal JA, Luna-Cruz B, Ruiz-Valverde P, Osornio-Rodriguez M. Analgesic effectiveness of parecoxib in immediate postoperative in open abdominal surgery. Rev Mex Anestesiol. 2004;27: 152–156. [Google Scholar]
- 40. Gonzalez-Garcia CA, Ibarra-Ibarra LG, Barbosa-Vivanco S. Comparative study of ketorolac and dipyrone administered orally in the treatment of postoperative pain. Proc West Pharmacol Soc. 1994;37: 121–122. [PubMed] [Google Scholar]
- 41. Grundmann U, Wornle C, Biedler A, Kreuer S, Wrobel M, Wilhelm W. The efficacy of the non-opioid analgesics parecoxib, paracetamol and metamizol for postoperative pain relief after lumbar microdiscectomy. Anesth Analg. 2006;103: 217–222. [DOI] [PubMed] [Google Scholar]
- 42. Guberti A, Gini M, Zanetta R. Comparison between analgesic power of nefopam and glafenine administered by os, and of nefopam and novalgine given by i.v. Acta Anaesthesiol Ital 1992;33: 697–704. [Google Scholar]
- 43. Hernandez Llenas G, Barrientos Baez G, Lopez DF. Comparison of analgesia provided by diclofenac and dipirone in postoperated patients with abdominal surgery. Anest En Mex 1997;9: 137–142. [Google Scholar]
- 44. Herrera Barroso M, Urias Carranza JA, Gutierrez Garcia JL, Gonzalez Rodriguez L. Double-blind evaluation of the analgesic efficacy and tolerance of sodium zomepirac and dipyrone, with a single dose in post-operatory pain. Investig Medica Int. 1982;9: 164–170. [Google Scholar]
- 45. Ibarra-Ibarra LG, Cubillo MA, Silva-Adaya A, Gonzalez-Garcia CA. Comparative study of ketorolac and dipyrone in the treatment of postoperative pain. Proc West Pharmacol Soc. 1993;36: 133–135. [PubMed] [Google Scholar]
- 46. Jage J, Göb J, Wagner W, Henneberg T, Lehmann KA. Postoperative Schmerztherapie mit Piritramid und Metamizol. Schmerz. 1990;4: 29–36. [DOI] [PubMed] [Google Scholar]
- 47. Jovic R, Dragicevic D, Komazec Z, Sabo A. Ketoprofen is superior to metamizole in relieving postoperative pain after head and neck tumor operation. J B U On. 2008;13: 519–523. [PubMed] [Google Scholar]
- 48. Kampe S, Warm M, Landwehr S, Dagtekin O, Haussmann S, Paul M, et al. Clinical equivalence of IV paracetamol compared to IV dipyrone for postoperative analgesia after surgery for breast cancer. Curr Med Res Opin. 2006;22: 1949–1954. [DOI] [PubMed] [Google Scholar]
- 49. Karaman Y, Cukurova I, Demirhan E, Gonullu M, Altunbas S. Efficacy of dexketoprofen trometamol for acute postoperative pain relief after ENT surgery: A comparison with paracetamol and metamizole. Nobel Med. 2010;6: 47–52. [Google Scholar]
- 50. Kemal SO, Sahin S, Apan A. [Comparison of tramadol, tramadol-metamizol and tramadol-lornoxicam administered by intravenous PCA in management of postoperative pain]. Agri. 2007;19: 24–31. [PubMed] [Google Scholar]
- 51. Knüsel O. Zomepirac compared with dipyrone (metamizole) as analgesic for patients with osteoarthritis. Therapiewoche. 1982;32: 4933–4938. [Google Scholar]
- 52. Krymchantowski AV, Carneiro H, Barbosa J, Jevoux C. Lysine clonixinate versus dipyrone (metamizole) for the acute treatment of severe migraine attacks: a single-blind, randomized study. Arq Neuropsiquiatr. 2008;66: 216–220. [DOI] [PubMed] [Google Scholar]
- 53. Landwehr S, Kiencke P, Giesecke T, Eggert D, Thumann G, Kampe S. A comparison between IV paracetamol and IV metamizol for postoperative analgesia after retinal surgery. Curr Med Res Opin. 2005;21: 1569–1575. [DOI] [PubMed] [Google Scholar]
- 54. Lehmann KA, Paral F, Sabatowski R. [Postoperative intravenous patient-controlled analgesia using hydromorphone metamizole (dipyrone). A prospective randomised study. Anaesthesist.]. 2001;50: 750–756. [DOI] [PubMed] [Google Scholar]
- 55. Lehtonen T, Kellokumpu I, Permi J, Sarsila O. Intravenous indomethacin in the treatment of ureteric colic. A clinical multicentre study with pethidine and metamizol as the control preparations. Ann Clin Res. 1983;15: 197–199. [PubMed] [Google Scholar]
- 56. Marin-Bertolin S, De Andres J, Gonzalez-Martinez R, Valia Vera JC, Amorrortu-Velayos J. A controlled, randomized, double-blind study of ketorolac for postoperative analgesia after plastic surgery. Ann Plast Surg. 1997;38: 478–484. [PubMed] [Google Scholar]
- 57. Martin-Duce A, Moreno J, Puerta J, Ortiz P. Effectiveness of metamizol in the management of pain after abdominal surgery: Comparison of 1 or 2 g by the intramuscular or intravenous route. Pain Clin. 1997;10: 27–34. 8986385 [Google Scholar]
- 58. Martínez-Martín P, Raffaelli E, Titus F, Despuig J, Fragoso YD, Díez-Tejedor, et al. Efficacy and safety of metamizol vs. acetylsalicylic acid in patients with moderate episodic tension-type headache: a randomized, double-blind, placebo- and active-controlled, multicentre study. Cephalalgia. 2001;21: 604–610. [DOI] [PubMed] [Google Scholar]
- 59. Mateu M, Ortiz P, Bellod P. The efficacy and tolerance of minor analgesics in slight thoracic trauma. Curr Ther Res Clin Exp. 1992;51: 626–633. [Google Scholar]
- 60. Mehta SD. A randomized double-blind placebo-controlled study of dipyrone and aspirin in post-operative orthopaedic patients. J Int Med Res. 1986;14: 63–66. [DOI] [PubMed] [Google Scholar]
- 61. Monso A, Riudeubas J, Barbal F, Laporte JR, Arnau JM. A randomized, double-blind, placebo-controlled trial comparing pethidine to metamizol for treatment of post-anaesthetic shivering. Br J Clin Pharmacol. 1996;42: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Muriel C, Ortiz P, the Cooperative Study Group. Efficacy of two different intramuscular doses of dipyrone in acute renal colic. Methods Find Exp Clin Pharmacol. 1993;15: 465–469. [PubMed] [Google Scholar]
- 63. Muriel-Villoria C, Zungri-Telo E, Diaz-Curiel M, Fernandez-Guerrero M, Moreno J, Puerta J, et al. Comparison of the onset and duration of the analgesic effect of dipyrone, 1 or 2 g, by the intramuscular or intravenous route, in acute renal colic. Eur J Clin Pharmacol. 1995;48: 103–107. [DOI] [PubMed] [Google Scholar]
- 64. Ocampo Flores P, Cortes Flores A, Orozco Vazquez C. Comparative study of 3 analgesics (ibuprofen, dipyrone, and dextro-propoxyphene) in oral surgery. ADM Asoc Dent Mex. 1986;43: 132–138. [PubMed] [Google Scholar]
- 65. Pardo A, Iriarte JL, de Paolis C. [Comparison of the effects of ceruletide with respect to dipyrone and to a single infusion of physiologic serum in patients with pain due to nephritic colic.]. Arch Esp Urol. 1984;37: 94–100. [PubMed] [Google Scholar]
- 66. Patel CV, Koppikar MG, Patel MS, Parulkar GB, Pinto Pereira LM. Management of pain after abdominal surgery: dipyrone compared with pethidine. Br J Clin Pharmacol. 1980;10(Suppl 2): 351S–354S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pavlik I, Suchy J, Pacik D, Bokr R, Sust M, Abad M, et al. Comparison of cizolirtine citrate and metamizol sodium in the treatment of adult acute renal colic: A randomized, double-blind, clinical pilot study. Clin Ther. 2004;26: 1061–1072. [DOI] [PubMed] [Google Scholar]
- 68. Peiro AM, Martinez J, Martinez E, De Madaria E, Llorens P, Horga JF, et al. Efficacy and tolerance of metamizole versus morphine for acute pancreatitis pain. Pancreatology. 2008;8: 25–29. 10.1159/000114852 [DOI] [PubMed] [Google Scholar]
- 69. Pernia A, Torres LM, Calderon E. Management of postoperative pain through intravenous patient-controlled analgesia (i.v. PCA) versus propacetamol and metamizol. Rev Soc Espanola Dolor. 2000;7: 354–360. [Google Scholar]
- 70. Pinto J, Pinheiro J, Mekhitarian N, Andrade N. [Evaluation of acetaminophen, dipyrone and placebo in the treatment from post-tonsillectomy pain.]. Rev Bras Cir. 1984;74: 185–189. [Google Scholar]
- 71. Planas ME, Gay-Escoda C, Bagán JV, Santamaría J, Peñarrocha M, Donado M, et al. Oral metamizol (1 g and 2 g) versus ibuprofen and placebo in the treatment of lower third molar surgery pain: randomised double-blind multi-centre study. Cooperative Study Group. Eur J Clin Pharmacol. 1998;53: 405–409. [DOI] [PubMed] [Google Scholar]
- 72. Prada A. Analgesic effect of pentazocine suppositories. Minerva Anestesiol. 1974;40: 527–536. [PubMed] [Google Scholar]
- 73. Primus G, Pummer K, Vucsina F, Meindl N. Tramadol versus metimazole in ureteral colic. Urol—Ausg A. 1989;28: 103–105. [PubMed] [Google Scholar]
- 74. Rawal N, Allvin R, Amilon A, Ohlsson T, Hallen J. Postoperative analgesia at home after ambulatory hand surgery: A controlled comparison of tramadol, metamizol, and paracetamol. Anesth Analg. 2001:92: 347–351. [DOI] [PubMed] [Google Scholar]
- 75. Rejman F. Intravenous indomethacin in biliary pain: A clinical investigation with metamizole as the control. IRCS Med Sci. 1984;12: 399–400. [PubMed] [Google Scholar]
- 76. Reyes Armijo E. A double blind comparative study with Dolo Neurobion and its separate constituents in the treatment of polyneuritis. J Int Med Res. 1974;2: 32–35. [Google Scholar]
- 77. Reyes F. [Randomized comparative double-blind study of diclofenac sodium and dipyrone in postoperative pain.]. Comp Inv Lat Am Mex. 1988;8: 65–73. [Google Scholar]
- 78. Rodriguez M, Barutell C, Rull M, Galvez R, Pallares J, Vidal L, et al. Efficacy and tolerance of oral dipyrone versus oral morphine for cancer pain. Eur J Cancer. 1994;30: 584–587. [DOI] [PubMed] [Google Scholar]
- 79. Rubinstein I, Canalini AF. [Double-blind comparative trial of acetaminophen, dipyrone, and placebo in the treatment of postoperative pain in urology.]. F Med BR. 1986;92: 201–206. [Google Scholar]
- 80. Sanchez-Carpena J, Sesma-Sanchez J, Sanchez-Juan C, Tomas-Vecina S, Garcia-Alonso D, Rico-Salvado J, et al. Comparison of dexketoprofen trometamol and dipyrone in the treatment of renal colic. Clin Drug Investig. 2003;23: 139–152. 10.2165/00044011-200323030-00001 [DOI] [PubMed] [Google Scholar]
- 81. Sanchez-Carpena J, Dominguez-Hervella F, Garcia I, Gene E, Bugarin R, Martín A, et al. Comparison of intravenous dexketoprofen and dipyrone in acute renal colic. Eur J Clin Pharmacol. 2007;63: 751–760. [DOI] [PubMed] [Google Scholar]
- 82. Saray A, Buyukkocak U, Cinel I, Tellioglu AT, Oral U. Diclofenac and metamizol in postoperative analgesia in plastic surgery. Acta Chir Plast. 2001;43: 71–76. [PubMed] [Google Scholar]
- 83. Savoca G, Libra C, Mollica Q, Chinea B. [Comparison between imidazole-2-hydroxybenzoate and noramidopyrine in the treatment of postoperative pain in orthopedic patients]. Boll Chim Farm. 1985;124: 113S–115S. [PubMed] [Google Scholar]
- 84. Schmieder G, Stankow G, Zerle G, Schinzel G, Brune K. Observer-blind study with metamizole versus tramadol and butylscopolamine in acute biliary colic pain. Arzneim Forschung. 1993;43: 1216–1221. [PubMed] [Google Scholar]
- 85. Sener M, Yilmazer C, Yilmaz I, Bozdogan N, Ozer C, Donmez A, et al. Efficacy of lornoxicam for acute postoperative pain relief after septoplasty: a comparison with diclofenac, ketoprofen, and dipyrone. J Clin Anesth. 2008;20: 103–108. 10.1016/j.jclinane.2007.09.009 [DOI] [PubMed] [Google Scholar]
- 86. Spacek A, Goraj E, Neiger FX, Jarosz J, Kress HG. Superior postoperative analgesic efficacy of a continuous infusion of tramadol and dipyrone (metamizol) versus tramadol alone. Acute Pain. 2003;5: 3–9. [Google Scholar]
- 87. Stankov G, Schmieder G, Lechner FJ, Schinzel S. Observer-blind multicentre study with dipyrone versus tramadol in postoperative pain. Eur J Pain. 1995;16: 56–63. [Google Scholar]
- 88. Steffen P, Seeling W, Kunz R, Schuhmacher I, Georgieff M. [Postoperative analgesia after endoscopic abdominal operations. A randomized double-blind study of perioperative effectiveness of metamizole.]. Chirurg. 1997;68: 806–810. [DOI] [PubMed] [Google Scholar]
- 89. Striebel HW, Hackenberger J. Comparison of tramadol/metamizole infusion versus combined tramadol infusion and ibuprofen suppositories for postoperative pain management after hysterectomy. Anaesthesist. 1992;41: 354–360. [PubMed] [Google Scholar]
- 90. Tempel G, von Hundelshausen B, Reeker W. The opiate-sparing effect of dipyrone in post-operative pain therapy with morphine using a patient-controlled analgesic system. Intensive Care Med. 1996;22: 1043–1047. [DOI] [PubMed] [Google Scholar]
- 91. Tonolli Jacob B, Manoel Dos Santos Povoa R, Albuquerque Roncada PG. Clinical evaluation of injectable diclofenac potassium, dipyrone sodium and placebo in painful post-traumatic conditions. Arq Bras Med. 1986;60: 337–341. [Google Scholar]
- 92. Torres LM, Collado F, Almarcha JM, Huertas VG, De Antonio P, Rodríguez M. Intravenous PCA for postoperative pain: A comparison of morphine, metamizole and buprenorphine. Rev Esp Anestesiol Reanim. 1993;40: 181–184. [PubMed] [Google Scholar]
- 93. Torres LM, Rodríguez MJ, Montero A, Herrera J, Calderón E, Cabrera J, et al. Efficacy and safety of dipyrone versus tramadol in the management of pain after hysterectomy: a randomized, double-blind, multicenter study. Reg Anesth Pain Med. 2001;26: 118–124. [DOI] [PubMed] [Google Scholar]
- 94. Uzun S, Onguc Aycan I, Erden IA, Sahin A, Aypar U. The Addition of Metamizole to Morphine and Paracetamol Improves Early Postoperative Analgesia and Patient Satisfaction after Lumbar Disc Surgery. Turk Neurosurg. 2010;20: 341–347. 10.5137/1019-5149.JTN.3081-10.3 [DOI] [PubMed] [Google Scholar]
- 95. Vargha von Szeged A, Michos N. Controlled single-blind clinical study of suprofen syrup versus metamizole syrup. Arzneimittelforschung. 1986;36: 1110–1112. [PubMed] [Google Scholar]
- 96. Hoigné R, Zoppi M, Sollberger J, Hess T, Fritschy D. Fall in systolic blood pressure due to metamizol (dipyrone, noramidopyrine, novaminsulfone). Results from the Comprehensive Hospital Drug Monitoring Berne (CHDMB). Agents Actions Suppl. 1986;19: 189–195. [PubMed] [Google Scholar]
- 97. Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. J Pain Symptom Manage. 1999;18: 427–437. [DOI] [PubMed] [Google Scholar]
- 98. Flather MD, Farkouh ME, Pogue JM, Yusuf S. Strengths and limitations of meta-analysis: larger studies may be more reliable.Control Clin Trial. 1997;18: 568–579. [DOI] [PubMed] [Google Scholar]
- 99. Edwards JE, McQuay HJ. Dipyrone and agranulocytosis: what is the risk? Lancet. 2002;360: 1438 10.1016/S0140-6736(02)11489-9 [DOI] [PubMed] [Google Scholar]
- 100. Hedenmalm K, Spigset O. Agranulocytosis and other blood dyscrasias associated with dipyrone (metamizole). Eur J Clin Pharmacol. 2002;58: 265–274. [DOI] [PubMed] [Google Scholar]
- 101. Kramer MS, Lane DA, Hutchinson TA. The International Agranulocytosis and Aplastic Anemia Study (IAAAS). J Clin Epidemiol. 1988;41: 613–616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XML)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.