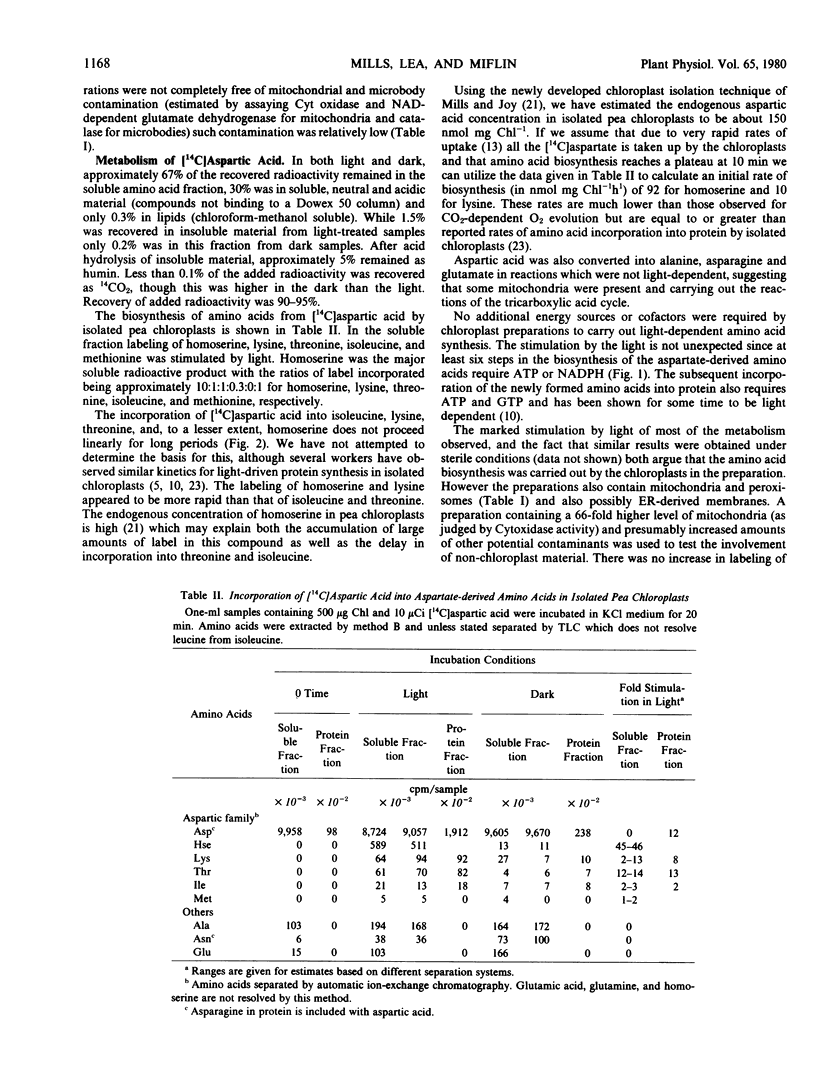
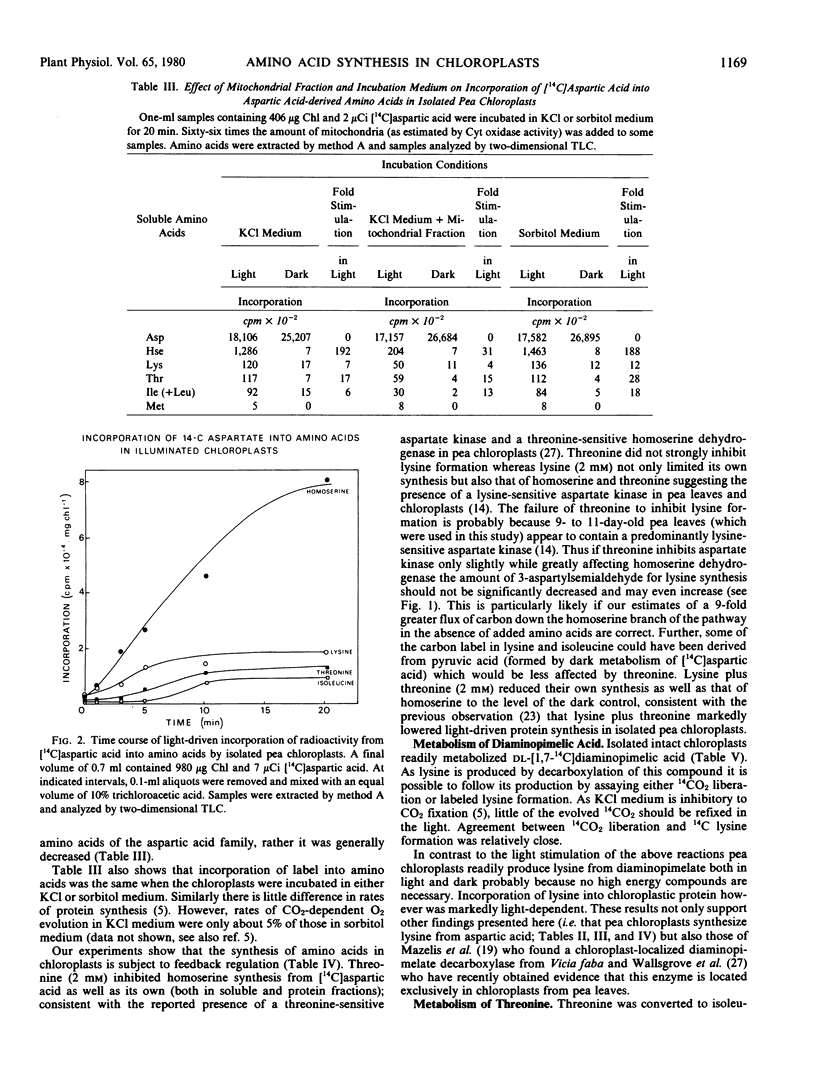
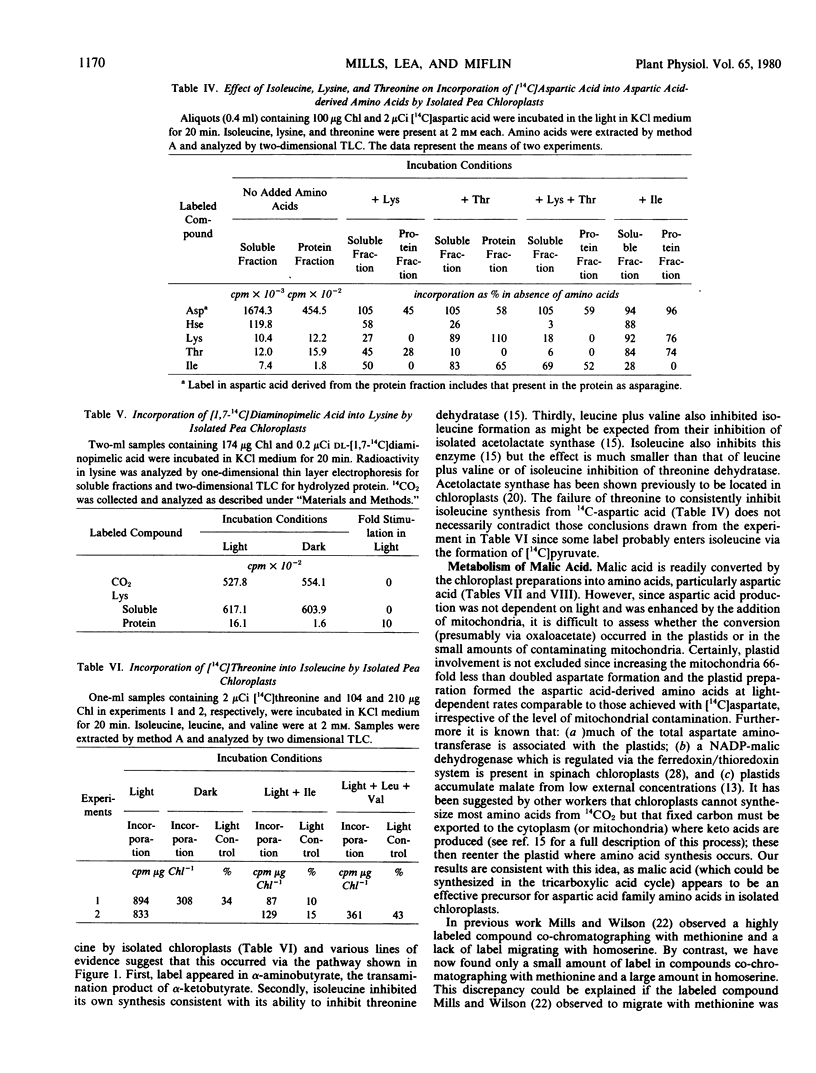
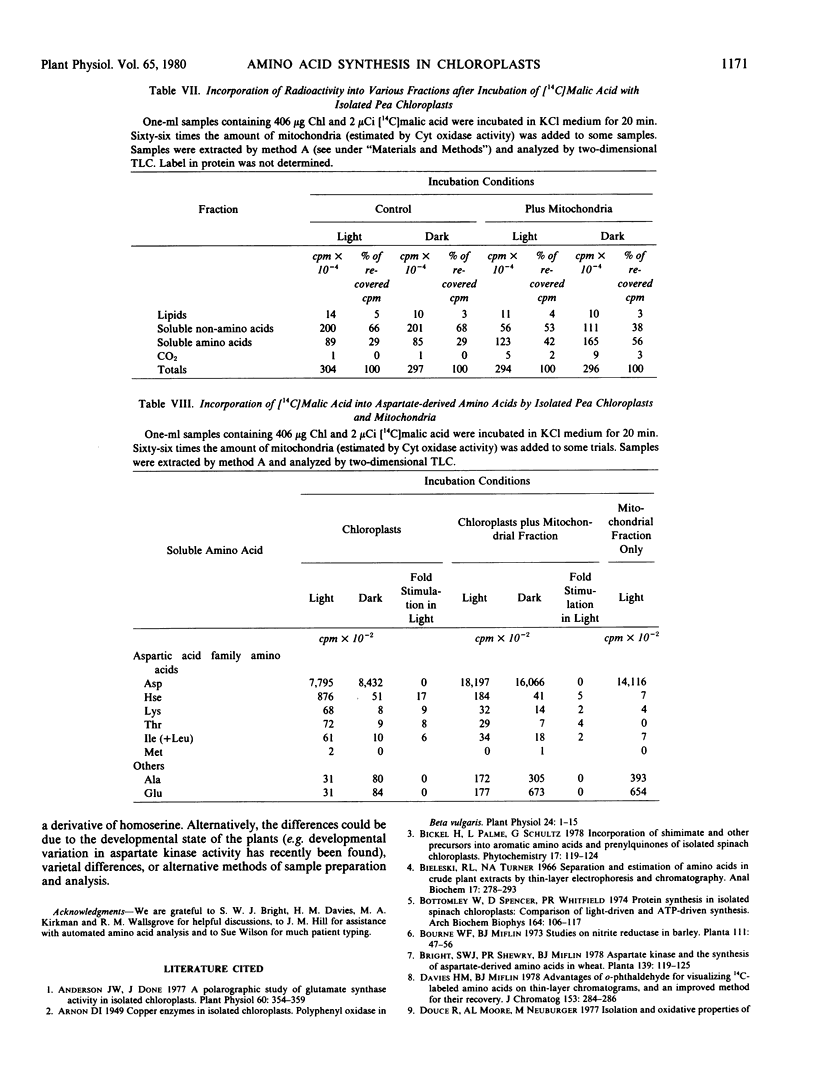
Abstract
The metabolism of 14C-labeled aspartic acid, diaminopimelic acid, malic acid and threonine by isolated pea (Pisum sativum L.) chloroplasts was examined. Light enhanced the incorporation of [14C] aspartic acid into soluble homoserine, isoleucine, lysine, methionine and threonine and protein-bound aspartic acid plus asparagine, isoleucine, lysine, and threonine. Lysine (2 millimolar) inhibited its own formation as well as that of homoserine, isoleucine and threonine. Threonine (2 millimolar) inhibited its own synthesis and that of homoserine but had only a small effect on isoleucine and lysine formation. Lysine and threonine (2 millimolar each) in combination strongly inhibited their own synthesis as well as that of homoserine. Radioactive [1,7-14C]diaminopimelic acid was readily converted into [14C]threonine in the light and its labeling was reduced by exogenous isoleucine (2 millimolar) or a combination of leucine and valine (2 millimolar each). The strong light stimulation of amino acid formation illustrates the point that photosynthetic energy is used in situ for amino acid and protein biosynthesis, not solely for CO2 fixation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Done J. A polarographic study of glutamate synthase activity in isolated chloroplasts. Plant Physiol. 1977 Sep;60(3):354–359. doi: 10.1104/pp.60.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski R. L., Turner N. A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal Biochem. 1966 Nov;17(2):278–293. doi: 10.1016/0003-2697(66)90206-5. [DOI] [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Whitfeld P. R. Protein synthesis in isolated spinach chloroplasts: comparison of light-driven and ATP-driven synthesis. Arch Biochem Biophys. 1974 Sep;164(1):106–117. doi: 10.1016/0003-9861(74)90012-5. [DOI] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Lea P. J., Mills W. R., Miflin B. J. The isolation of a lysine-sensitive aspartate kinase from pea leaves and its involvement in homoserine biosynthesis in isolated chloroplasts. FEBS Lett. 1979 Feb 1;98(1):165–168. doi: 10.1016/0014-5793(79)80175-1. [DOI] [PubMed] [Google Scholar]
- Mazelis M., Miflin B. J., Pratt H. M. A chloroplast-localized diaminopimelate decarboxylase in higher plants. FEBS Lett. 1976 Apr 15;64(1):197–200. doi: 10.1016/0014-5793(76)80282-7. [DOI] [PubMed] [Google Scholar]
- Miflin B. J. The location of nitrite reductase and other enzymes related to amino Acid biosynthesis in the plastids of root and leaves. Plant Physiol. 1974 Oct;54(4):550–555. doi: 10.1104/pp.54.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega A., Nunn P. B. A lithium buffer system for single-column amino acid analysis. Anal Biochem. 1969 Dec;32(3):446–453. doi: 10.1016/s0003-2697(69)80012-6. [DOI] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]



