Abstract
1. Moths (Lepidoptera) are the major nocturnal pollinators of flowers. However, their importance and contribution to the provision of pollination ecosystem services may have been under-appreciated. Evidence was identified that moths are important pollinators of a diverse range of plant species in diverse ecosystems across the world.
2. Moth populations are known to be undergoing significant declines in several European countries. Among the potential drivers of this decline is increasing light pollution. The known and possible effects of artificial night lighting upon moths were reviewed, and suggest how artificial night lighting might in turn affect the provision of pollination by moths. The need for studies of the effects of artificial night lighting upon whole communities of moths was highlighted.
3. An ecological network approach is one valuable method to consider the effects of artificial night lighting upon the provision of pollination by moths, as it provides useful insights into ecosystem functioning and stability, and may help elucidate the indirect effects of artificial light upon communities of moths and the plants they pollinate.
4. It was concluded that nocturnal pollination is an ecosystem process that may potentially be disrupted by increasing light pollution, although the nature of this disruption remains to be tested.
Keywords: Agro-ecosystems, artificial night lighting, ecological networks, ecosystem services, flowering plants, food-webs, moths, population declines
Introduction
Pollinating insects have been undergoing significant declines for several decades in many parts of the world (Williams, 1982; Potts et al., 2010; Carvalheiro et al., 2013). This is of concern because pollination represents a critical ecosystem service (Costanza et al., 1997; Ollerton et al., 2011; Garibaldi et al., 2013), and declines in pollinators have been linked with declines in the plants that they interact with (Biesmeijer et al., 2006; Pauw, 2007; Potts et al., 2010). However, most studies to date have focused on diurnal pollinating insects, largely ignoring nocturnal insects, many of which have also undergone significant declines. In Great Britain, two-thirds of widespread larger moth species populations declined over a 40-year period (Fox et al., 2013), with probable detrimental cascading effects on ecosystem functioning: the nature of these is considered a priority, policy-relevant question (Sutherland et al., 2006). Recent work suggests that nocturnal moths (Lepidoptera) may perform an important, although often overlooked, functional role as plant pollinators (Philipp et al., 2006; Devoto et al., 2011; LeCroy et al., 2013), but little is known about the scale and importance of nocturnal pollination services. Here, we review the scientific literature for evidence of the importance of nocturnal Lepidoptera (moths) as plant pollinators.
Nocturnal insect pollinators, including moths, face many of the same threats as diurnal pollinators, including habitat fragmentation, climate change, and agrochemical use (Fox et al., 2014). They may also be affected by increasing light pollution (Hölker et al., 2010a), but the effects of artificial night lighting on nocturnal pollinator communities have not yet been established. We examine how the known effects of artificial light upon moths may potentially affect pollination processes. We also consider how recent advances in network ecology can be used to examine the impacts of light pollution on moth communities and their interactions with plants.
Nocturnal pollination
The experimental methods used in the majority of field studies of plant–pollinator interactions involve observations of insect visitors to flowers. Such observations almost always take place during daylight hours (e.g. Forup et al., 2008; Bosch et al., 2009; Popic et al., 2013), because conducting surveys in the dark is difficult (Martinell et al., 2010). However, to fully understand plant–pollinator networks, we must also understand the role played by nocturnal pollinators (Fig. 1). In addition to some bats (Chiroptera), beetles (Coleoptera), and flies (Diptera), moths are important nocturnal pollinators (Willmer, 2011); in particular, nectarivorous species from the families Sphingidae, Noctuidae, and Geometridae (Winfree et al., 2011) and probably also the newly defined Erebidae (LeCroy et al., 2013).
Fig 1.
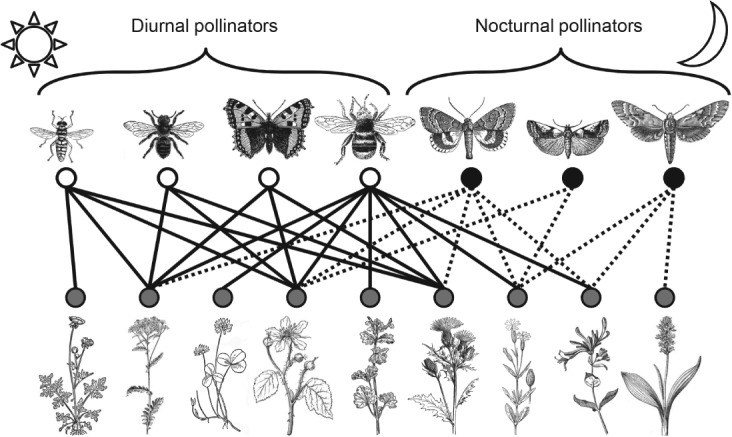
An illustrative temperate grassland network combining diurnal and nocturnal pollination. Combined networks may reveal the extent of redundancy and complementarity of pollination interactions in ecosystems. Some apparently specialist plants in diurnal networks may be generalist with nocturnal visitors included. Thus, nocturnal visitors may provide redundancy to plants pollinated by diurnal visitors, and vice versa. Nocturnal interactions are derived from Table S1.2, Appendix S2 and diurnal interactions from Pocock et al. (2012). Nodes represent species: white = diurnal insects, black = nocturnal insects, grey = plants. Pollinators (from left): hoverfly (Diptera), leaf-cutter bee (Hymenoptera), butterfly (Lepidoptera), bumblebee (Hymenoptera), noctuid moth, pyralid moth, sphingid moth (all Lepidoptera); plants (from left): Ranunculus sp. (Ranunculaceae), Jacobaea vulgaris (Asteraceae), Trifolium sp. (Fabaceae), Rubus sp. (Rosaceae), Lamium sp. (Lamiaceae), Cirsium sp. (Asteraceae), Silene latifolia (Caryophyllaceae), Lonicera sp. (Caprifoliaceae), Gymnadenia conopsea (Orchidaceae). Links represent hypothetical pollination interactions: solid = diurnal, dashed = nocturnal. Drawings of pollinators and plants are for illustration only and may not precisely represent the named plant or animal. Drawings are used under license from ClipArt ETC (see Appendix S1 for full acknowledgements).
To determine the importance of moths as providers of nocturnal pollination services, and which plants are pollinated, we searched ISI Web of Knowledge for papers containing the terms ‘moth’ and ‘pollinat*’ (30 January 2014) and searched the bibliography of each relevant publication for further citations. Any paper demonstrating the existence of a moth–plant pollination interaction or providing evidence for such an interaction was considered relevant and included in the review. Levels of evidence supporting pollination interactions varied from observed flower visitation alone to proven dependence of the flower on moths for pollination (Table 1). Eight studies only inferred moth pollination from floral characteristics and did not present further evidence. While a high proportion of flower visitors at any particular flower species may not be effective pollinators (King et al., 2013), flower visitation or pollen transfer by insects is frequently used as a proxy for insect-pollination. Therefore, for simplicity, we hereafter use the terms ‘pollination’ and ‘pollinator’ where there was reasonable evidence that moths acted as pollinators, although we note that in many cases pollination was not strictly proven. Using this method, we identified 168 studies from between 1971 and 2013 detailing examples of nocturnal moths involved in pollination (this search was comprehensive, but we recognise that some additional published examples may exist).
Table 1.
Types of evidence for moth pollination given by studies reviewed (see Table S1.2, Appendix S2)
| Evidence | Types of evidence | No. studies |
|---|---|---|
| Only flower visitation recorded | VF, VO, VR, VT | 52 |
| Flower visitation and moths observed making contact with floral reproductive organs | C + (VF, VO, VR, VT) | 11 |
| Only pollen found on moths | P | 15 |
| Flower visitation recorded and pollen found on moths | P + (VF, VO, VR, VT) | 49 |
| Flower visitation recorded with other additional evidence | (VF, VO, VR, VT) + X | 9 |
| Pollen found on moths with other additional evidence | P + X | 2 |
| Flower visitation and pollen found on moths with other additional evidence | P + (VF, VO, VR, VT) + X | 8 |
| Other | X | 4 |
| Only inferred from floral syndrome | I | 8 |
| Unspecified/unknown | U | 5 |
In column 2: C = contact with anthers and/or stigmas observed, D = pollen deposited on stigmas and/or removed from anthers, E = plants pollinated when experimentally exposed only to visits by moths, I = inferred from pollination syndrome, P = pollen present on captured moths, S = moth scales or hairs present on stigmas, VF = flower visitation determined by fluorescent markers transferred by visiting moths, VO = flower visitation determined by observations, VR = flower visitation determined by video recordings, VT = flower visitation determined by flower-visitor trapping, U = unspecified/unknown; X = any combination of C, D, E, and S.
Fourteen of these studies examined complete pollinator communities, finding moths to be of general importance to pollination in a variety of ecosystems (Table S1.1, Appendix S2), including tropical rainforest and savannah, temperate coniferous forest and meadow, and oceanic islands, and including examples from all continents except Antarctica. In several studies, moths were considered to be second in importance only to bees, in terms of pollination provision (Bawa et al., 1985; Kato & Kawakita, 2004; Ramirez, 2004; Chamorro et al., 2012).
Moth pollination was important for a wide range of plant species. We found representatives of 75 different plant families (Table 2), including 289 species and some wider taxa, reported to be partially or exclusively pollinated by moths (Table S1.2, Appendix S2) of 21 families (Table S3, Appendix S2). The majority of plants were angiosperms; the one exception was the gymnosperm Gnetum gnemon Linne var. tenerum Markgraf (Gnetaceae), reportedly pollinated by moths of Geometridae and Pyralidae (Kato et al., 1995). Many species within the angiosperms were dicotyledons, especially from the orders Caryophyllales, Ericales, Gentianales, and Lamiales, but moth-pollinated plants in the monocotyledons included many in the order Asparagales (including Orchidaceae, Amaryllidaceae, Asparagaceae, and others), and the economically important species Elaeis guineensis Jacq. oil palm (Arecaceae), visited by large numbers of moths in the genus Pyroderces (Cosmopterigidae; Syed, 1979). These observed patterns may be a function of both real effects and bias in recorder effort, so we treat them with caution.
Table 2.
Studies of moth-pollinated plants by family (see Table S1.2, Appendix S2)
| Plant family | No. known moth-pollinated species or wider taxa | Known pollinating moth families | Plant family | No. known moth-pollinated species or wider taxa | Known pollinating moth families |
|---|---|---|---|---|---|
| Adoxaceae | 1 | N | Liliaceae | 4 | G, N, P, S |
| Amaranthaceae | 1 | — | Linaceae | 1 | — |
| Amaryllidaceae | 10 | E, N, S | Loasaceae | 1 | S |
| Anacardiaceae | 1 | — | Loganiaceae | 2 | — |
| Apiaceae | 1 | — | Malvaceae | 2 | Ct, E, G, N, P, Se, S, U |
| Apocynaceae | 20 | E, G, N, P, S, T | Meliaceae | 1 | S |
| Arecaceae | 1 | C | Myrtaceae | 2 | Ct, S |
| Asparagaceae | 7 | N, Pr, S | Nepenthaceae | 1 | — |
| Asteraceae | 13 | G, N, P, S | Nyctaginaceae | 5 | N, S |
| Balsaminaceae | 2 | S | Oleaceae | 3 | S |
| Bignoniaceae | 3 | E, G, L, N, S | Onagraceae | 8 | E, G, N, P, S |
| Boraginaceae | 4 | N, P, S | Orchidaceae | 45 | G, N, Pr, Pt, P, Se, S, T |
| Brassicaceae | 3 | S | Orobanchaceae | 2 | S |
| Cactaceae | 7 | G, N, P, Sa, S | Passifloraceae | 2 | S |
| Capparaceae | 1 | P | Phrymaceae | 1 | S |
| Caprifoliaceae | 3 | N, S | Phyllanthaceae | 10 | Ge, Gr |
| Caricaceae | 1 | — | Plantaginaceae | 1 | — |
| Caryocaraceae | 1 | S | Polemoniaceae | 1 | S |
| Caryophyllaceae | 12 | Cr, G, N, P, S | Polygonaceae | 1 | — |
| Cleomaceae | 1 | S | Primulaceae | 2 | — |
| Convulvulaceae | 4 | S | Proteaceae | 2 | S |
| Crassulaceae | 1 | G | Ranunculaceae | 5 | S |
| Cucurbitaceae | 1 | N, S | Rhamnaceae | 1 | — |
| Dipterocarpaceae | 2 | G, N, S | Rosaceae | 2 | — |
| Ebenaceae | 1 | — | Rubiaceae | 16 | Ct, N, S |
| Ericaceae | 4 | G, N, P, S | Rutaceae | 1 | G |
| Escalloniaceae | 1 | G | Santalaceae | 2 | — |
| Euphorbiaceae | 4 | S | Sapotaceae | 2 | — |
| Fabaceae | 12 | E, G, N, P, S, U | Saxifragaceae | 3 | Pr |
| Geraniaceae | 1 | — | Scrophulariaceae | 2 | G, N, P, T |
| Gesneriaceae | 1 | — | Solanaceae | 6 | S |
| Gnetaceae | 1 | G, P | Thymelaeaceae | 8 | E, G, L, N, No, P, Th |
| Hyacinthaceae | 1 | N | Urticaceae | 1 | — |
| Hypericaceae | 1 | N | Verbenaceae | 3 | P, S |
| Iridaceae | 3 | G, N, S | Violaceae | 1 | S |
| Lamiaceae | 2 | S | Vochysiaceae | 5 | S |
| Lecythidaceae | 1 | Gl | Winteraceae | 2 | M |
| Lentibulariaceae | 1 | N, P, S, U | — | — | — |
In column 2, ‘known’ moth-pollinated taxa are those identified in this review as having evidence of being moth-pollinated; ‘wider taxa’ includes any named group at a hierarchical level above species and below family. In column 3: C = Cosmopterigidae, Cr = Crambidae, Ct = Ctenuchidae, E = Erebidae, Ge = Gelechiidae, G = Geometridae, Gl = Glyphipterigidae, Gr = Gracillariidae, L = Lasiocampidae, M = Micropterigidae, N = Noctuidae, No = Nolidae, Pr = Prodoxidae, Pt = Pterophoridae, P = Pyralidae, Sa = Saturniidae, Se = Sesiidae, S = Sphingidae, Th = Thyrididae, T = Tortricidae, U = Uranidae.
Traditionally, pollination by moths has been subdivided into two ‘pollination syndromes’ (Willmer, 2011): sphingophily (pollination by hovering moths of the Sphingidae) and phalaenophily (pollination by settling moths of other families). The best-known examples of moth pollination are of sphingophilous plants (e.g. Wasserthal, 1997). To examine if this has led to a bias towards sphingophily in studies of moth pollination, we categorised all studies in Table S1.2, Appendix S2 according to whether they made any explicit or implicit prediction of sphingophily. In general, we did not find evidence of bias towards sphingophily leading to other pollination interactions being overlooked. Fifty-six studies (35% of those reviewed) made a prediction of sphingophily. Of these, 53 (95%) found Sphingidae and 18 (32%) found non-sphingid moths to be pollinators, even although the experimental methods in all but two studies were sufficient to detect both sphingid and non-sphingid pollinators. From the 103 studies not predicting sphingophily, 82 (80%) found non-sphingid moths and 50 (49%) found Sphingidae to be pollinators; the experimental methods in all but nine were sufficient to detect both sphingid and non-sphingid pollinators (Table S2, Appendix S2).
Moths primarily visit flowers to obtain nectar, which is an energy-rich food source and the main adult food source in the majority of moth species that feed as adults (Willmer, 2011). Several studies have also documented moths acting as pollinating seed parasites (Table S1.3, Appendix S2). In these specialised interactions, moths both pollinate and lay eggs in flowers, so providing a food supply for their larvae, which feed on developing seedheads.
Pollination by moths may be an advantageous strategy for plants in some examples. Several studies evaluate aspects of pollination in generalist plants pollinated both by moths (both Sphingidae and other families) and diurnal pollinators; for example, Lonicera japonica Thunb. (Caprifoliaceae; Miyake & Yahara, 1998), Asclepias spp. (Apocynaceae; Bertin & Willson, 1980; Morse & Fritz, 1983; Jennersten & Morse, 1991) and Silene spp. (Caryophyllaceae; Young, 2002; Barthelmess et al., 2006). Compared with diurnal pollinators, the moths in these examples provided benefits including: greater interpopulation gene flow, shown by movement of genetic markers between experimental populations of plants (Barthelmess et al., 2006); longer-distance dispersal of dye-marked pollen (Miyake & Yahara, 1998; Young, 2002); higher quality pollination, causing equal or greater seed set in spite of transferring fewer pollinia (Bertin & Willson, 1980; Jennersten & Morse, 1991; but see Morse & Fritz, 1983); and more efficient pollination, having a lower ratio of pollen removed to pollen deposited after visits by single pollinators (Miyake & Yahara, 1998). In the latter example, moths visiting L. japonica were thought to be more efficient pollinators than bees because the latter actively collect pollen to provision their larvae, and so must remove substantially more pollen than moths for the same level of pollen deposition to occur (Miyake & Yahara, 1999). As a result, moth-pollinated plants could perhaps invest fewer resources into producing pollen without compromising reproductive success (Cruden, 1973); however, analysis of pollen–ovule ratios for diurnally and nocturnally pollinated members of Caryophyllaceae does not support this (Jürgens et al., 2002).
The literature, therefore, contains numerous examples of moths serving as pollinators which, in many cases, are of considerable importance to individual species and to communities. A diverse selection of plant taxa in an equally wide range of ecosystems benefit from pollination by moths. It is important to consider how environmental change may threaten this ecosystem service.
Artificial light as a driver of environmental change
There are many drivers of environmental change, but artificial night lighting is one which is uniquely important for nocturnal organisms, through direct interaction with a light source such as a streetlamp, increased background illumination at night, and altered perception of photoperiod (Hölker et al., 2010b; Lyytimäki, 2013; Lewanzik & Voigt, 2014). Light pollution has increased considerably and continues to increase worldwide, often associated with urban development (Cinzano et al., 2001; Bruce-White & Shardlow, 2011), although levels may be declining in some economically developed regions (Bennie et al., 2014). The predominant types of artificial lighting in use are also changing; lights emitting a broader spectrum of wavelengths are increasingly favoured because they facilitate human discernment of colours at night and, in the case of light-emitting diodes (LEDs), are more energy-efficient (Bruce-White & Shardlow, 2011; Gaston et al., 2012).
Artificial night lighting, even at low levels, exerts an influence at every level of biological organisation (Gaston et al., 2013), from cell (Navara & Nelson, 2007) to organism (Longcore & Rich, 2004) and community (Davies et al., 2012). However, little is currently known about the effects of light pollution on species population dynamics, whole communities, and networks of interacting species, or ecosystem functioning.
Long-term declines in populations and distributions of many moth species have been found in Great Britain (Conrad et al., 2004, 2006; Fox et al., 2011, 2013), the Netherlands (Groenendijk & Ellis, 2011), and Finland (Mattila et al., 2006, 2008). Habitat degradation and climate change are likely drivers of these declines (Fox et al., 2014), as with diurnal pollinators (Potts et al., 2010); however, artificial night lighting has also been proposed as a potential contributing factor (Fox, 2013; Fox et al., 2013). Conrad et al. (2006) found no significant correlation between a change in light pollution and a change in light-trap catches from 1992 and 2000, but short-term trends in moth (and other insect) populations can be difficult to detect, as large inter-annual fluctuations are normal (Conrad et al., 2004).
Below, we describe a range of mechanisms by which artificial night lighting could impact negatively upon moths. Many such impacts are not empirically proven. Therefore, we describe first the well-established mechanisms, followed by those unproven, but for which some evidence exists. Even where negative impacts have been demonstrated, their effects at the population level are mostly unknown.
Established effects of artificial light on moths
Individual moths are certainly affected by artificial night lighting, famously appearing to be attracted to artificial lights, sometimes in huge numbers (Howe, 1959). Numerous theories have been put forward to explain flight-to-light behaviour (Robinson & Robinson, 1950; Mazokhin-Porshnyakov, 1961; Callahan, 1965; Hsiao, 1973; Sotthibandhu & Baker, 1979; Hamdorf & Höglund, 1981), although the debate is inconclusive. Nevertheless, this observation has led to the popularity of using light-baited traps to survey many families of moths.
The extent to which moths are attracted to light varies according to a number of factors. It has been recognised for many years that shorter wavelengths are, in general, more attractive to moths (Frank, 2006, and references therein); attractiveness appears to peak around wavelengths of 400 nm (violet light; Cowan & Gries, 2009). The degree of attraction and preferred wavelengths both vary between moth taxa (Merckx & Slade, 2014); typically, larger-bodied moths with larger eyes are more likely to be attracted to light dominated by smaller wavelengths (van Langevelde et al., 2011; Somers-Yeates et al., 2013). Variation also appears to exist between sexes; males of some species are significantly more likely to be recorded at light traps than females (Garris & Snyder, 2010), but it is not clear if this is due to stronger male attraction to lights, or males being more active and therefore more likely to move into the zone of influence of a given light (Altermatt et al., 2009).
Aside from flight-to-light behaviour, moths may be further affected by artificial night lighting through other mechanisms, related to direct interaction with lights, increased ambient light at night, and locally altered perception of photoperiods in the vicinity of artificial lights. Contact with hot components of lamps or radiant energy from bright lights can kill insects or damage their wings, legs, and antennae (Eisenbeis, 2006; Frank, 2006). Insects killed by light-baited electric traps, primarily targeting biting Diptera, contain a high proportion of nocturnal Lepidoptera (Frick & Tallamy, 1996).
Reproduction
Reproductive success of moths could also be negatively affected by artificial night lighting. Low levels of artificial light inhibited the release of sex pheromones by female moths of a Geometridae species (Sower et al., 1970). Artificial light can suppress oviposition (Nemec, 1969) or act as an ecological trap, causing females to lay eggs at an unusually high density and/or in unsuitable locations near to lights (Pfrimmer et al., 1955; Brown, 1984), either of which could increase larval competition for limited food resources.
Artificial light may also have an effect on larvae, which are nocturnal in many Lepidopteran species, including some that are diurnal as adults (butterflies and day-flying moths). Even at a low intensity, light caused reductions in age and mass at pupation in males and inhibited diapause in both sexes of a Noctuidae species in the laboratory (van Geffen et al., 2014). However, few studies have investigated the effects of artificial night lighting on Lepidopteran larvae.
Predation
Predators of moths have been observed to hunt at artificial lights, exploiting above-average prey densities caused by flight-to-light behaviour (Frank, 2006). This includes both active hunters, such as bats (Rydell, 1992) and predatory insects (Warren, 1990), and sit-and-wait predators, such as spiders (Heiling, 1999), reptiles, and amphibians (Henderson & Powell, 2001). Artificial light also interferes with the anti-bat defensive behaviour of moths, increasing their vulnerability to predation (Svensson & Rydell, 1998; Acharya & Fenton, 1999).
Possible further effects of artificial light on moths
In addition to the known mechanisms described above, a number of other mechanisms have the potential to affect moths but have not yet been conclusively demonstrated.
Reproduction
Changes in photoperiod disrupted the pheromone release behaviour of females of a Pyralidae species (Fatzinger, 1973), which could disrupt mating. Competition in male moths between light traps and pheromone traps (Delisle et al., 1998) suggests that artificial lighting could distract males from female pheromone signals and thus reduce mating frequency. More severely, radiant energy from bright lights can sterilise other insects in the laboratory (Riordan, 1964; Eisenbeis, 2006); this could occur with moths in the wild. Artificial lights have been observed to divert dispersing or migrating moths to locations that are unsuitable for breeding (Frank, 2006, and references therein), potentially creating an ecological trap.
A reduction of the dark scotophase of the photoperiod prevented diapause in the larval stage of a Tortricidae species in the laboratory (Berlinger & Ankersmit, 1976); however, this result could not be replicated in field trials. In addition, moth larvae may be attracted to artificial lights in much the same way as adults (Gillett & Gardner, 2009).
Predation
Artificial light may also increase the risk of predation by disrupting crypsis, both by causing moths to rest in unsuitable locations where their wing patterns are an ineffective disguise, and by concentrating moths in a small area, assisting predators in establishing a search image of cryptic wing patterns (Frank, 2006). Similarly, repeat exposure can habituate predators to stimuli that elicit startle reactions, such as patterned hindwings or bodies (Schlenoff, 1985; Ingalls, 1993); highly visible aggregations of moths around lights could accelerate the habituation process (Frank, 2006).
Vision
Artificial light affects the sensitivity of the compound eyes of moths (Frank, 2006). Screening pigment reduces ocular sensitivity within 23 min of exposure to light (Hamdorf & Höglund, 1981); the return to full ocular sensitivity is far slower, taking around 30 min (Bernhard & Ottoson, 1960). To what extent these effects may be exerted by exposure to artificial lights in natural settings is unclear. However, moths attracted to a light will often rest on vegetation or the ground for a period of time, sometimes before even reaching the light (Hartstack et al., 1968; Hsiao, 1973); this behaviour could represent a period of readjustment to full ocular sensitivity.
In addition to compound eyes, most insects (including moths) have simple eyes (dorsal ocelli) that are sensitive to changes in light intensity (Mizunami, 1995), and appear to have a role in timing flight initiation at dusk in moths (Eaton et al., 1983). It is possible that artificial night lighting could delay or even prevent the onset of nocturnal activity. While this effect is likely to be localised to the immediate vicinity of light sources, it could negatively affect moth fitness (and hence population growth) and nocturnal pollination.
The visual capacity of moths could also be indirectly affected by artificial night lighting altering the spectrum of background illumination. Ultraviolet (UV) radiation (10–400 nm), predominantly at longer wavelengths close to visible light (Eguchi et al., 1982), is particularly important to pollinating moths, as moths orient themselves to flowers by a combination of olfactory and visual cues (Raguso & Willis, 2005) including UV-reflecting markers on flowers (Barth, 1985). The spectral content of artificial night lighting will therefore determine its effect upon flower-visiting moths (Davies et al., 2013): UV-rich lighting (e.g. from mercury vapour lights) could accentuate these nectar guides, whereas UV-poor lighting (e.g. from low-pressure sodium lights), by illuminating other parts of the nocturnal environment relatively more brightly, could cause nectar guides to stand out less clearly (Frank, 2006).
Moths and pollination: an ecological network approach
The studies above considered the direct effects of artificial light upon moths, mostly at the level of the individual. Whether artificial night lighting, through these effects, is a contributing factor in declines in moth populations remains a key research question. It is also necessary to consider the indirect effects of artificial light mediated by moth pollination, as can be demonstrated with an ecological network approach. Ecological networks describe the structure of communities as the occurrence (and frequency) of interactions between species, such as plants and pollinators (Montoya et al., 2006; Bascompte, 2007). From descriptions of the network's structure, its function can be inferred (Tylianakis et al., 2010); for example, its robustness to perturbations such as species extinction and their cascading effects (Bascompte, 2009; Ings et al., 2009; Evans et al., 2013). It has been demonstrated that drivers of environmental change, such as climate change, can alter the composition and balance of networks (Tylianakis et al., 2008), including plant–pollinator networks (Rathke & Jules, 1993; Memmott et al., 2007). Removal of pollinator species can cause plant species diversity to suffer (Memmott et al., 2004; Fontaine et al., 2006), while loss of plants can likewise affect pollinators (Wallis De Vries et al., 2012).
Two attributes of networks are particularly important. First, many pollinator networks have a nested structure, in which specialist species (with few connections in the network) tend to interact with generalists (with many connections) more frequently than with other specialists (Dicks et al., 2002; Bascompte et al., 2003). Nested systems have high tolerance to the random loss of species from the community but are sensitive to the removal of certain highly connected species (Solé & Montoya, 2001; Memmott et al., 2004). Second, these systems are also modular, in which sets of species within modules interact strongly with each other; these modules are akin to pollination syndromes (Olesen et al., 2007), and increase overall robustness because impacts cascade less quickly between modules and through the whole system. Some modules are as a result of close co-evolutionary relationships; in extreme examples, plants are entirely reliant on a single or few species of moth [eg. Oxyanthus pyriformis (Hochst.) Skeels (Johnson et al., 2004)]. In such cases, minor disruption of the pollinator will directly impact the reproductive success of the plant (Pauw, 2007). The modules themselves may be nested within the whole system, and species will often be nested within modules.
Most studies of plant–pollinator networks to date have focused on diurnal interactions. Two exceptions considering nocturnal plant–pollinator networks are Devoto et al. (2011) and Banza (2011); these authors identified nocturnal moth–flower interactions by sampling pollen on captured moths. Combining nocturnal pollination networks with diurnal ones could lead to increased modularity (if there are distinct sets of flowers visited by diurnal and nocturnal pollinators), such that the effects of environmental change (e.g. artificial night lighting) could be substantial in one part of the network but not cascade through the whole network. It could also lead to increased redundancy (if flowers share diurnal and nocturnal pollinators), such that the plants in the network may be robust to the disruption of one set of pollinators (e.g. moths). Testing for differences in the structure of plant–moth pollinator networks between unlit and artificially lit sites will begin to empirically reveal the functional impact of artificial night lighting on wider communities through indirect, as well as direct, effects.
Potential effects of artificial light on moth pollination
A variety of changes in moth abundance, composition of moth assemblages, and moth behaviour are all possible results of artificial lighting at night, but the overall effect on the whole community via disruption of pollination remains to be tested (Fig. 2). Moths may be drawn in towards a light from several metres away (Baker & Sadovy, 1978; Truxa & Fiedler, 2012; van Grunsven et al., 2014); this might alter local moth abundance and the composition of moth assemblages both in the vicinity of lights, and in the source habitats from which attracted moths are drawn (Fig. 2: concentration and ecological trap effects). Interactions could also be weakened or lost through behavioural changes in moths, even if their abundance is unchanged (Fig. 2: disruption effect). The level and nature of disruption might vary between moth species (van Langevelde et al., 2011; Somers-Yeates et al., 2013), leading to some interactions being more strongly affected than others (Fig. 2: preferential disruption effect). If reproduction is affected, some moth species may decline in abundance or go extinct, leading to further loss of interactions. Therefore, the effects of increasing artificial light may be positive for some moth or plant species and negative for others in any given community, leading to cascading changes in the system that are difficult to predict prior to empirical, experimental research.
Fig 2.
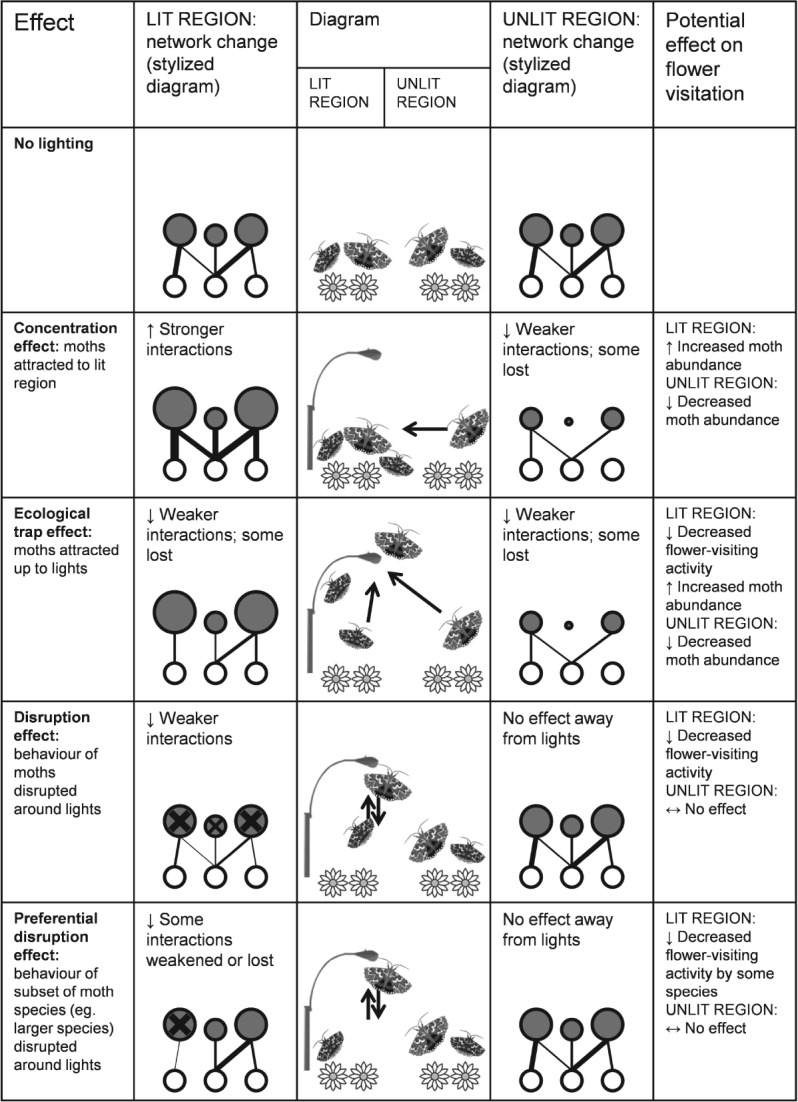
Possible scenarios for change in plant–moth pollination networks as a result of artificial night lighting, with predictions for effects on local flower-visitation activity by moths. In network representations, nodes represent species (lower = flowering plants, upper = moths) and links represent pollination interactions. Node width represents relative species abundance and link thickness represents interaction strength. Crosses indicate disruption of behaviour.
Discussion
Future research directions
We believe that our findings in this review highlight a number of key priorities for future research (Fig. 3). While we have described evidence that moths are pollinators of a diverse range of plant species, the extent of their role as pollinators in maintaining botanical diversity, in agro-ecosystems, and especially of commercially valuable crops demands attention.
Fig 3.
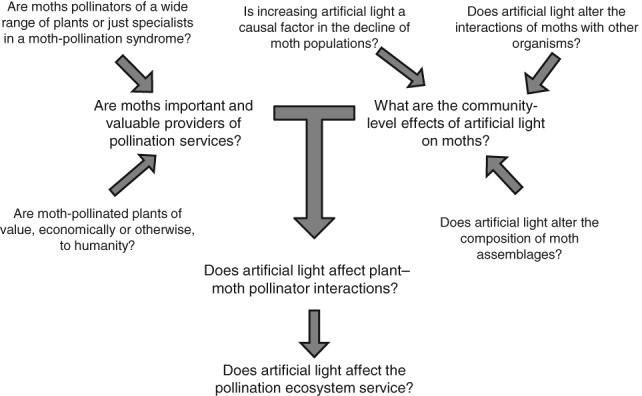
Future research directions raised in this review.
The effects of artificial night lighting on moths, too, should be investigated further. Many of the individual-level effects summarised above have not been empirically demonstrated to occur under natural conditions. Moreover, there are no published studies into the community-level effects of artificial night lighting on moths; this is a major research gap (Fox, 2013; Gaston et al., 2013). The impacts of lighting on plant–moth pollination networks are difficult to predict (Fig. 2) and also require empirical testing. It is worth noting that moths are a food source for many other organisms including birds and bats (Fox, 2013); therefore, a similar approach with trophic networks may also be worthwhile.
Conclusion
In this review, we show the importance of moths as pollinators for a diverse range of plant species in ecosystems worldwide and, hence, their role in ecosystem functioning. We discuss the many ways in which moths are known to be affected by artificial night lighting, and suggest how these effects may, in turn, impact pollination interactions between moths and plants.
The effects of artificial night lighting may go beyond simple declines in moth populations, with potential changes in the composition of moth assemblages and in the nature and frequency of interspecies interactions between moths and other taxa; this justifies an ecological network approach to the problem (Fig. 2).
Artificial night lighting may negatively affect a range of ecosystem services (Lyytimäki, 2013; Lewanzik & Voigt, 2014). Based on the evidence summarised in this review, we consider pollination to be one such ecosystem service that may be disrupted by increasing ecological light pollution. The research directions outlined will help develop an understanding of what form that disruption may take, and may direct ways to mitigate the negative effects of artificial night lighting upon moths and the ecosystem processes that rely upon them.
Acknowledgments
This work was supported by the Natural Environment Research Council and Butterfly Conservation (Industrial CASE studentship awarded to C.J.M., Project Reference: NE/K007394/1). We thank Mark Parsons, Herbert Macgregor, and three anonymous reviewers for their helpful comments on the manuscript. All authors contributed to the literature review and manuscript writing.
Supporting Information
Additional Supporting Information may be found in the online version of this article under the DOI reference: 10.1111/een.12174
Appendix S1. References for Fig. 1.
Appendix S2. Further tables summarising results of the moth-pollination review.
References
- Acharya L, Fenton MB. Bat attacks and moth defensive behaviour around street lights. Canadian Journal of Zoology. 1999;77:27–33. [Google Scholar]
- Altermatt F, Baumeyer A, Ebert D. Experimental evidence for male biased flight-to-light behaviour in two moth species. Entomologia Experimentalis et Applicata. 2009;130:259–265. [Google Scholar]
- Baker RR, Sadovy Y. The distance and nature of the light-trap response of moths. Nature. 1978;276:818–821. [Google Scholar]
- Banza P. 2011. Investigating the importance of nocturnal Lepidoptera as pollinators: a network approach. MSc thesis, Universidade de Évora, Portugal.
- Barth FG. Insects and Flowers: The Biology of a Partnership. Princeton, New Jersey: Princeton University Press; 1985. [Google Scholar]
- Barthelmess EL, Richards CM, McCauley DE. Relative effects of nocturnal vs diurnal pollinators and distance on gene flow in small Silene alba populations. New Phytologist. 2006;169:689–698. doi: 10.1111/j.1469-8137.2005.01580.x. [DOI] [PubMed] [Google Scholar]
- Bascompte J. Networks in ecology. Basic and Applied Ecology. 2007;8:485–490. [Google Scholar]
- Bascompte J. Disentangling the web of life. Science. 2009;325:416–419. doi: 10.1126/science.1170749. [DOI] [PubMed] [Google Scholar]
- Bascompte J, Jordano P, Melián CJ, Olesen JM. The nested assembly of plant–animal mutualistic networks. Proceedings of the National Academy of Sciences. 2003;100:9383–9387. doi: 10.1073/pnas.1633576100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa KS, Bullock SH, Perry DR, Coville RE, Grayum MH. Reproductive biology of tropical lowland rain forest trees. II. Pollination systems. American Journal of Botany. 1985;72:346–356. [Google Scholar]
- Bennie J, Davies TW, Duffy JP, Inger R, Gaston KJ. Contrasting trends in light pollution across Europe based on satellite observed night time lights. Scientific Reports. 2014;4:3789. doi: 10.1038/srep03789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlinger MJ, Ankersmit GW. Manipulation with the photoperiod as a method of control of Adoxophyes orana (Lepidoptera, Torticidae) Entomologia Experimentalis et Applicata. 1976;19:96–107. [Google Scholar]
- Bernhard CG, Ottoson D. Studies on the relation between the pigment migration and the sensitivity changes during dark adaptation in diurnal and nocturnal Lepidoptera. Journal of General Physiology. 1960;44:205–215. doi: 10.1085/jgp.44.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin RI, Willson MF. Effectiveness of diurnal and nocturnal pollination of two milkweeds. Canadian Journal of Botany. 1980;58:1744–1746. [Google Scholar]
- Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, Peeters T, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- Bosch J, Gonzalez AMM, Rodrigo A, Navarro D. Plant–pollinator networks: adding the pollinator's perspective. Ecology Letters. 2009;12:409–419. doi: 10.1111/j.1461-0248.2009.01296.x. [DOI] [PubMed] [Google Scholar]
- Brown LN. Population outbreak of Pandora moths (Coloradia pandora Blake) on the Kaibab plateau, Arizona (Saturniidae) Journal of the Lepidopterists' Society. 1984;38:65. [Google Scholar]
- Bruce-White C, Shardlow M. A Review of the Impact of Artificial Light on Invertebrates. U.K: Buglife; 2011. [Google Scholar]
- Callahan PS. Intermediate and far infrared sensing of nocturnal insects. Part I. Evidences for a far infrared (FIR) electromagnetic theory of communication and sensing in moths and its relationship to the limiting biosphere of the corn earworm. Annals of the Entomological Society of America. 1965;58:727–745. [Google Scholar]
- Carvalheiro LG, Kunin WE, Keil P, Aguirre-Gutiérrez J, Ellis WN, Fox R, et al. Species richness declines and biotic homogenisation have slowed down for NW-European pollinators and plants. Ecology Letters. 2013;16:870–878. doi: 10.1111/ele.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro S, Heleno R, Olesen JM, McMullen CK, Traveset A. Pollination patterns and plant breeding systems in the Galápagos: a review. Annals of Botany. 2012;110:1489–1501. doi: 10.1093/aob/mcs132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinzano P, Falchi F, Elvidge CD. The first World Atlas of the artificial night sky brightness. Monthly Notices of the Royal Astronomical Society. 2001;328:689–707. [Google Scholar]
- Conrad KF, Woiwod IP, Parsons M, Fox R, Warren MS. Long-term population trends in widespread British moths. Journal of Insect Conservation. 2004;8:119–136. [Google Scholar]
- Conrad KF, Warren MS, Fox R, Parsons MS, Woiwod IP. Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biological Conservation. 2006;132:279–291. [Google Scholar]
- Costanza R, d'Arge R, de Groot R, Farber S, Grasso M, Hannon B, et al. The value of the world's ecosystem services and natural capital. Nature. 1997;387:253–260. [Google Scholar]
- Cowan T, Gries G. Ultraviolet and violet light: attractive orientation cues for the Indian meal moth, Plodia interpunctella. Entomologia Experimentalis et Applicata. 2009;131:148–158. [Google Scholar]
- Cruden RW. Reproductive biology of weedy and cultivated Mirabilis (Nyctaginaceae) American Journal of Botany. 1973;60:802–809. [Google Scholar]
- Davies TW, Bennie J, Gaston KJ. Street lighting changes the composition of invertebrate communities. Biology Letters. 2012;8:764–767. doi: 10.1098/rsbl.2012.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TW, Bennie J, Inger R, de Ibarra NH, Gaston KJ. Artificial light pollution: are shifting spectral signatures changing the balance of species interactions? Global Change Biology. 2013;19:1417–1423. doi: 10.1111/gcb.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle J, West RJ, Bowers WW. The relative performance of pheromone and light traps in monitoring the seasonal activity of both sexes of the eastern hemlock looper, Lambdina fiscellaria fiscellaria. Entomologia Experimentalis et Applicata. 1998;89:87–98. [Google Scholar]
- Devoto M, Bailey S, Memmott J. The ‘night shift’: nocturnal pollen-transport networks in a boreal pine forest. Ecological Entomology. 2011;36:25–35. [Google Scholar]
- Dicks LV, Corbet SA, Pywell RF. Compartmentalization in plant–insect flower visitor webs. Journal of Animal Ecology. 2002;71:32–43. [Google Scholar]
- Eaton JL, Tignor KR, Holtzman GI. Role of moth ocelli in timing flight initiation at dusk. Physiological Entomology. 1983;8:371–375. [Google Scholar]
- Eguchi E, Watanabe K, Hariyama T, Yamamoto K. A comparison of electrophysiologically determined spectral responses in 35 species of Lepidoptera. Journal of Insect Physiology. 1982;28:675–682. [Google Scholar]
- Eisenbeis G. Artificial night lighting and insects: attraction of insects to streetlamps in a rural setting in Germany. In: Rich C, Longcore T, editors. Ecological Consequences of Artificial Night Lighting. Washington, District of Columbia: Island Press; 2006. pp. 281–304. [Google Scholar]
- Evans DM, Pocock MJO, Memmott J. The robustness of a network of ecological networks to habitat loss. Ecology Letters. 2013;16:844–852. doi: 10.1111/ele.12117. [DOI] [PubMed] [Google Scholar]
- Fatzinger CW. Circadian rhythmicity of sex pheromone release by Dioryctria abietella (Lepidoptera: Pyralidae (Phycitinae)) and the effect of a diel light cycle on its precopulatory behaviour. Annals of the Entomological Society of America. 1973;66:1147–1153. [Google Scholar]
- Fontaine C, Dajoz I, Meriguet J, Loreau M. Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biology. 2006;4:e1. doi: 10.1371/journal.pbio.0040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forup ML, Henson KSE, Craze PG, Memmott J. The restoration of ecological interactions: plant–pollinator networks on ancient and restored heathlands. Journal of Applied Ecology. 2008;45:742–752. [Google Scholar]
- Fox R. The decline of moths in Great Britain: a review of possible causes. Insect Conservation and Diversity. 2013;6:5–19. [Google Scholar]
- Fox R, Randle Z, Hill L, Anders S, Wiffen L, Parsons MS. Moths count: recording moths for conservation in the UK. Journal of Insect Conservation. 2011;15:55–68. [Google Scholar]
- Fox R, Parsons MS, Chapman JW, Woiwod IP, Warren MS, Brooks DR. The State of Britain's Larger Moths 2013. U.K: Butterfly Conservation and Rothamsted Research; 2013. [Google Scholar]
- Fox R, Oliver TH, Harrower C, Parsons MS, Thomas CD, Roy DB. Long-term changes to the frequency of occurrence of British moths are consistent with opposing and synergistic effects of climate and land-use changes. Journal of Applied Ecology. 2014;51:949–957. doi: 10.1111/1365-2664.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KD. Effects of artificial night lighting on moths. In: Rich C, Longcore T, editors. Ecological Consequences of Artificial Night Lighting. Washington, District of Columbia: Island Press; 2006. pp. 305–344. [Google Scholar]
- Frick TB, Tallamy DW. Density and diversity of nontarget insects killed by suburban electric insect traps. Entomological News. 1996;107:77–82. [Google Scholar]
- Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 2013;339:1608–1611. doi: 10.1126/science.1230200. [DOI] [PubMed] [Google Scholar]
- Garris HW, Snyder JA. Sex-specific attraction of moth species to ultraviolet light traps. Southeastern Naturalist. 2010;9:427–434. [Google Scholar]
- Gaston KJ, Davies TW, Bennie J, Hopkins J. Reducing the ecological consequences of night-time light pollution: options and developments. Journal of Applied Ecology. 2012;49:1256–1266. doi: 10.1111/j.1365-2664.2012.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston KJ, Bennie J, Davies TW, Hopkins J. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biological Reviews. 2013;88:912–927. doi: 10.1111/brv.12036. [DOI] [PubMed] [Google Scholar]
- van Geffen KG, van Grunsven RHA, van Ruijven J, Berendse F, Veenendaal EM. Artificial light at night causes diapause inhibition and sex-specific life history changes in a moth. Ecology and Evolution. 2014;4:2082–2089. doi: 10.1002/ece3.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett MPT, Gardner AS. An unusual observation – attraction of caterpillars to mercury vapour light in the Abu Dhabi desert (Lepidoptera: Pyralidae) Tribulus. 2009;18:56–59. [Google Scholar]
- Groenendijk D, Ellis WN. The state of the Dutch larger moth fauna. Journal of Insect Conservation. 2011;15:95–101. [Google Scholar]
- van Grunsven RHA, Lham D, van Geffen KG, Veenendaal EM. Range of attraction of a 6-W moth light trap. Entomologia Experimentalis et Applicata. 2014;152:87–90. [Google Scholar]
- Hamdorf K, Höglund G. Light induced retinal screening pigment migration independent of visual cell activity. Journal of Comparative Physiology. 1981;143:305–309. [Google Scholar]
- Hartstack AW, Jr, Hollingsworth JP, Lindquist DA. A technique for measuring trapping efficiency of electric insect traps. Journal of Economic Entomology. 1968;61:546–552. [Google Scholar]
- Heiling AM. Why do nocturnal orb-web spiders (Araneidae) search for light? Behavioral Ecology and Sociobiology. 1999;46:43–49. [Google Scholar]
- Henderson RW, Powell R. Responses by the West Indian herpetofauna to human-influenced resources. Caribbean Journal of Science. 2001;37:41–54. [Google Scholar]
- Hölker F, Moss T, Griefahn B, Kloas W, Voigt CC, Henckel D, et al. The dark side of light: a transdisciplinary research agenda for light pollution policy. Ecology and Society. 2010a;15:13. [Google Scholar]
- Hölker F, Wolter C, Perkin EK, Tockner K. Light pollution as a biodiversity threat. Trends in Ecology & Evolution. 2010b;25:681–682. doi: 10.1016/j.tree.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Howe WH. A swarm of noctuid moths in southeastern Kansas. Journal of the Lepidopterists' Society. 1959;13:26. [Google Scholar]
- Hsiao HS. Flight paths of night-flying moths to light. Journal of Insect Physiology. 1973;19:1971–1976. doi: 10.1016/0022-1910(73)90191-1. [DOI] [PubMed] [Google Scholar]
- Ingalls V. Startle and habituation responses of Blue Jays (Cyanocitta cristata) in a laboratory simulation of anti-predator defenses of Catocala moths (Lepidoptera: Noctuidae) Behaviour. 1993;126:77–96. [Google Scholar]
- Ings TC, Montoya JM, Bascompte J, Blüthgen N, Brown L, Dormann CF, et al. Ecological networks – beyond food webs. Journal of Animal Ecology. 2009;78:253–269. doi: 10.1111/j.1365-2656.2008.01460.x. [DOI] [PubMed] [Google Scholar]
- Jennersten O, Morse DH. The quality of pollination by diurnal and nocturnal insects visiting Common Milkweed, Asclepias syriaca. American Midland Naturalist. 1991;125:18–28. [Google Scholar]
- Johnson SD, Neal PR, Peter CI, Edwards TJ. Fruiting failure and limited recruitment in remnant populations of the hawkmoth-pollinated tree Oxyanthus pyriformis subsp. pyriformis (Rubiaceae) Biological Conservation. 2004;120:31–39. [Google Scholar]
- Jürgens A, Witt T, Gottsberger G. Pollen grain numbers, ovule numbers and pollen–ovule ratios in Caryophylloideae: correlation with breeding system, pollination, life form, style number, and sexual system. Sexual Plant Reproduction. 2002;14:279–289. [Google Scholar]
- Kato M, Kawakita A. Plant–pollinator interactions in New Caledonia influenced by introduced honey bees. American Journal of Botany. 2004;91:1814–1827. doi: 10.3732/ajb.91.11.1814. [DOI] [PubMed] [Google Scholar]
- Kato M, Inoue T, Nagamitsu T. Pollination biology of Gnetum (Gnetaceae) in a lowland mixed dipterocarp forest in Sarawak. American Journal of Botany. 1995;82:862–868. [Google Scholar]
- King C, Ballantyne G, Willmer PG. Why flower visitation is a poor proxy for pollination: measuring single-visit pollen deposition, with implications for pollination networks and conservation. Methods in Ecology and Evolution. 2013;4:811–818. [Google Scholar]
- van Langevelde F, Ettema JA, Donners M, Wallis De Vries MF, Groenendijk D. Effect of spectral composition of artificial light on the attraction of moths. Biological Conservation. 2011;144:2274–2281. [Google Scholar]
- LeCroy KA, Shew HW, van Zandt PA. Pollen presence on nocturnal moths in the Ketona Dolomite glades of Bibb County, Alabama. Southern Lepidopterists' News. 2013;35:136–142. [Google Scholar]
- Lewanzik D, Voigt CC. Artificial light puts ecosystem services of frugivorous bats at risk. Journal of Applied Ecology. 2014;51:388–394. [Google Scholar]
- Longcore T, Rich C. Ecological light pollution. Frontiers in Ecology and the Environment. 2004;2:191–198. [Google Scholar]
- Lyytimäki J. Nature's nocturnal services: light pollution as a non-recognised challenge for ecosystem services research and management. Ecosystem Services. 2013;3:e44–e48. [Google Scholar]
- Martinell MC, Dötterl S, Blanché C, Rovira A, Massó S, Bosch M. Nocturnal pollination of the endemic Silene sennenii (Caryophyllaceae): an endangered mutualism? Plant Ecology. 2010;211:203–218. [Google Scholar]
- Mattila N, Kaitala V, Komonen A, Kotiaho JS, Päivinen J. Ecological determinants of distribution decline and risk of extinction in moths. Conservation Biology. 2006;20:1161–1168. doi: 10.1111/j.1523-1739.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- Mattila N, Kotiaho JS, Kaitala V, Komonen A. The use of ecological traits in extinction risk assessments: a case study on geometrid moths. Biological Conservation. 2008;141:2322–2328. [Google Scholar]
- Mazokhin-Porshnyakov GA. Why insects fly to light by night. Anzeiger für Schädlingskunde. 1961;34:47. [Google Scholar]
- Memmott J, Waser NM, Price MV. Tolerance of pollination networks to species extinctions. Proceedings of the Royal Society B. 2004;271:2605–2611. doi: 10.1098/rspb.2004.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmott J, Craze PG, Waser NM, Price MV. Global warming and the disruption of plant–pollinator interactions. Ecology Letters. 2007;10:710–717. doi: 10.1111/j.1461-0248.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Merckx T, Slade EM. Macro-moth families differ in their attraction to light: implications for light-trap monitoring programmes. Insect Conservation and Diversity. 2014;7:453–461. DOI: 10.1111/icad.12068. [Google Scholar]
- Miyake T, Yahara T. Why does the flower of Lonicera japonica open at dusk? Canadian Journal of Botany. 1998;76:1806–1811. [Google Scholar]
- Miyake T, Yahara T. Theoretical evaluation of pollen transfer by nocturnal and diurnal pollinators: when should a flower open? Oikos. 1999;86:233–240. [Google Scholar]
- Mizunami M. Functional diversity of neural organization in insect ocellar systems. Vision Research. 1995;35:443–452. doi: 10.1016/0042-6989(94)00192-o. [DOI] [PubMed] [Google Scholar]
- Montoya JM, Pimm SL, Solé RV. Ecological networks and their fragility. Nature. 2006;442:259–264. doi: 10.1038/nature04927. [DOI] [PubMed] [Google Scholar]
- Morse DH, Fritz RS. Contributions of diurnal and nocturnal insects to the pollination of common milkweed (Asclepias syriaca L.) in a pollen-limited system. Oecologia. 1983;60:190–197. doi: 10.1007/BF00379521. [DOI] [PubMed] [Google Scholar]
- Navara KJ, Nelson RJ. The dark side of light at night: physiological, epidemiological and ecological consequences. Journal of Pineal Research. 2007;43:215–224. doi: 10.1111/j.1600-079X.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- Nemec SJ. Use of artificial lighting to reduce Heliothis spp. populations in cotton fields. Journal of Economic Entomology. 1969;62:1138–1140. [Google Scholar]
- Olesen JM, Bascompte J, Dupont YL, Jordano P. The modularity of pollination networks. Proceedings of the National Academy of Sciences. 2007;104:19891–19896. doi: 10.1073/pnas.0706375104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals? Oikos. 2011;120:321–326. [Google Scholar]
- Pauw A. Collapse of a pollination web in small conservation areas. Ecology. 2007;88:1759–1769. doi: 10.1890/06-1383.1. [DOI] [PubMed] [Google Scholar]
- Pfrimmer TR, Lukefahr MJ, Hollingsworth JP. Experiments with Light Traps for Control of the Pink Bollworm. Washington, District of Columbia: U.S. Department of Agriculture, Agricultural Research Service; 1955. ARS-33-6. [Google Scholar]
- Philipp M, Böcher J, Siegismund HR, Nielsen LR. Structure of a plant–pollinator network on a pahoehoe lava desert of the Galápagos Islands. Ecography. 2006;29:531–540. [Google Scholar]
- Pocock MJO, Evans DM, Memmott J. The robustness and restoration of a network of ecological networks. Science. 2012;335:973–977. doi: 10.1126/science.1214915. [DOI] [PubMed] [Google Scholar]
- Popic TJ, Wardle GM, Davila YC. Flower-visitor networks only partially predict the function of pollen transport by bees. Austral Ecology. 2013;38:76–86. [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: trends, impacts and drivers. Trends in Ecology & Evolution. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Raguso RA, Willis MA. Synergy between visual and olfactory cues in nectar feeding by wild hawkmoths, Manduca sexta. Animal Behaviour. 2005;69:407–418. [Google Scholar]
- Ramirez N. Pollination specialization and time of pollination on a tropical Venezuelan plain: variation in time and space. Botanical Journal of the Linnean Society. 2004;145:1–16. [Google Scholar]
- Rathke BJ, Jules ES. Habitat fragmentation and plant–pollinator interactions. Current Science. 1993;65:273–277. [Google Scholar]
- Riordan DF. High-intensity flash discharge as a source of radiant energy for sterilizing insects. Nature. 1964;204:1332. [Google Scholar]
- Robinson HS, Robinson PJM. Some notes on the observed behavior of Lepidoptera in flight in the vicinity of light sources. Entomologist's Gazette. 1950;1:3–20. [Google Scholar]
- Rydell J. Exploitation of insects around streetlamps by bats in Sweden. Functional Ecology. 1992;6:744–750. [Google Scholar]
- Schlenoff DH. The startle responses of blue jays to Catocala (Lepidoptera: Noctuidae) prey models. Animal Behaviour. 1985;33:1057–1067. [Google Scholar]
- Solé RC, Montoya M. Complexity and fragility in ecological networks. Proceedings of the Royal Society B. 2001;268:2039–2045. doi: 10.1098/rspb.2001.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers-Yeates R, Hodgson D, McGregor PK, Spalding A, ffrench-Constant RH. Shedding light on moths: shorter wavelengths attract noctuids more than geometrids. Biology Letters. 2013;9:20130376. doi: 10.1098/rsbl.2013.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotthibandhu S, Baker RR. Celestial orientation by the large yellow underwing moth, Noctua pronuba L. Animal Behaviour. 1979;27:786–800. [Google Scholar]
- Sower LL, Shorey HH, Gaston LK. Sex pheromones of noctuid moths. XXI. Light : dark cycle regulation and light inhibition of sex pheromone release by females of Trichoplusia ni. Annals of the Entomological Society of America. 1970;63:1090–1092. doi: 10.1093/aesa/63.4.1090. [DOI] [PubMed] [Google Scholar]
- Sutherland WJ, Armstrong-Brown S, Armsworth PR, Brereton T, Brickland J, Campbell CD, et al. The identification of 100 ecological questions of high policy relevance in the UK. Journal of Applied Ecology. 2006;43:617–627. [Google Scholar]
- Svensson AM, Rydell J. Mercury vapour lamps interfere with the bat defence of tympanate moths (Operophtera spp.; Geometridae) Animal Behaviour. 1998;55:223–226. doi: 10.1006/anbe.1997.0590. [DOI] [PubMed] [Google Scholar]
- Syed RA. Studies on oil palm pollination by insects. Bulletin of Entomological Research. 1979;69:213–224. [Google Scholar]
- Truxa C, Fiedler K. Attraction to light – from how far do moths (Lepidoptera) return to weak artificial sources of light? European Journal of Entomology. 2012;109:77–84. [Google Scholar]
- Tylianakis JM, Didham RK, Bascompte J, Wardle DA. Global change and species interactions in terrestrial ecosystems. Ecology Letters. 2008;11:1351–1363. doi: 10.1111/j.1461-0248.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- Tylianakis JM, Laliberté E, Nielsen A, Bascompte J. Conservation of species interaction networks. Biological Conservation. 2010;143:2270–2279. [Google Scholar]
- Wallis De Vries MF, Van Swaay CAM, Plate CL. Changes in nectar supply: a possible cause of widespread butterfly decline. Current Zoology. 2012;58:384–391. [Google Scholar]
- Warren AD. Predation of five species of Noctuidae at ultraviolet light by the western yellowjacket (Hymenoptera: Vespidae) Journal of the Lepidopterists' Society. 1990;44:32. [Google Scholar]
- Wasserthal LT. The pollinators of the Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Botanica Acta. 1997;110:343–359. [Google Scholar]
- Williams PH. The distribution and decline of British bumble bees (Bombus Latr.) Journal of Apicultural Research. 1982;21:236–245. [Google Scholar]
- Willmer P. Pollination and Floral Ecology. Princeton, New Jersey: Princeton University Press; 2011. [Google Scholar]
- Winfree R, Bartomeus I, Cariveau DP. Native pollinators in anthropogenic habitats. Annual Review of Ecology, Evolution, and Systematics. 2011;42:1–22. [Google Scholar]
- Young HJ. Diurnal and nocturnal pollination of Silene alba (Caryophyllaceae) American Journal of Botany. 2002;89:433–440. doi: 10.3732/ajb.89.3.433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. References for Fig. 1.
Appendix S2. Further tables summarising results of the moth-pollination review.


