Abstract
Aim
The present study aimed to investigate whether cognitive reserve (CR), referring here to education and premorbid intelligence (IQ), is associated with the risk for progression from mild cognitive impairment (MCI) to Alzheimer's disease (AD).
Methods
A total of 51 patients with MCI and 59 patients with AD were prospectively enrolled for assessment with the Mini-Mental State Examination, the Japanese version of the cognitive subscale of the Alzheimer's Disease Assessment Scale, the Japanese version of the Nelson Adult Reading Test (JART), magnetic resonance imaging (MRI) and single-photon emission computed tomography (SPECT), adjusting for sex, age at diagnosis, age at onset and duration of illness.
Results
SPECT findings showed hypoperfusion in the posterior cingulate gyri and precunei, suggesting that the participants were in the early or mild stage of AD or MCI. Voxel-based morphometry MRI showed no statistical differences between the two groups in gray matter loss in the entorhinal and hippocampal areas; however, multiple logistic regression analysis showed a significant difference in premorbid IQ measured with JART.
Conclusion
Despite the limitations of the cross-sectional design, the findings suggest that premorbid intellectual function might explain the discrepancy in clinical status between MCI and AD patients with a similar magnitude of brain pathology and comorbid medical disorders. Geriatr Gerontol Int 2015; 15: 428–434.
Keywords: Alzheimer's disease, cognitive reserve, education, mild cognitive impairment, premorbid IQ
Introduction
Alzheimer's disease (AD) is the most common cause of dementia in elderly people. However, the relevance and independent contribution of risk factors, and of possible early signs, such as mild cognitive impairment (MCI) on the progression of AD, have not been investigated. Many studies have shown a strong association between a lower level of education, and higher prevalence and incidence of AD.1,2 Higher education has also been reported to slow down cognitive and functional decline.3 The concept of cognitive reserve (CR) suggests that brain pathology and age-related changes can be protected as a result of the way in which tasks are processed.4 Although there is a wide range of putative indices of CR, the main indicator is thought to be premorbid intelligence (IQ).5 However, its effect on the subsequent course of MCI to AD is less clear. Neuroimaging studies have begun to identify the neural substrate of CR, and numerous structural magnetic resonance imaging (MRI) studies have shown that atrophy of the medial temporal lobe, including the hippocampus and entorhinal cortex, is a sensitive marker of early AD.6,7 However, the interaction between neuropsychological performance, brain pathology and CR has yet to be established.
The aim of the present study, therefore, was to investigate the relationship between neuropsychological performance, brain pathology and CR, including associated medical diseases.
Methods
The present study was approved by the ethics committee of Dokkyo Medical University School of Medicine. Patients were referred to our Center for Dementia-Related Diseases by their physician (40%) or were self-referred (60%). Study participants were 110 patients (41 men and 69 women; mean age [SD] 78.5 years [5.6 years] across all patients, 77.8 years [5.1 years) for men and 79.0 years [5.9 years] for women). All MCI and AD patients were Japanese, were enrolled consecutively, were informed about the purpose and procedures of the study, and signed an informed consent form before their participation in the study. Patients received a standardized clinical evaluation consisting of a physical examination, laboratory tests, and cognitive and functional assessments at the initial visit. Patients who took benzodiazepines, antidepressants or choline esterase inhibitors were excluded. Diagnoses were made at a clinical consensus meeting using relevant diagnostic guidelines, such as the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria,8 for the diagnosis of AD and criteria outlined in Petersen et al.9 or a 0.5 Clinical Dementia Rating (CDR)10 for MCI. All the MCI patients were diagnosed with amnestic single-domain MCI.11 Subjective and objective (corroborated by an informant) anamnestic evidence of progressive cognitive impairment were also required. In addition, hypoperfusion in the posterior cingulate cortex and precuneus were examined by single-photon emission computed tomography (SPECT) in MCI and AD patients, because the presence of hypoperfusion in parietal association areas and at the MCI stage have been reported to predict a rapid conversion to AD.12 The patients were also asked to complete the Geriatric Depression Scale (short version), translated and localized for Japanese speakers.13 Patients with major depressive or other severe psychiatric disorders were excluded.
The National Adult Reading Test (NART),14,15 is widely used to measure premorbid IQ of English-speaking patients with dementia. Previous studies have also been carried out to examine the relationship between premorbid IQ and progression of AD2 or functional outcome in AD16 measured using the American version of the Nelson Adult Reading Test or NART. We used the Japanese version of the NART (JART), which has been proven to show good validity for measuring premorbid IQ and to be independent of present cognitive function in AD patients in Japan,17 and the Mini-Mental State Examination (MMSE) scale,18 a widely used index of cognitive status that has been validated for use with Japanese subjects.19
The cognitive subscale of the Alzheimer's Disease Assessment Scale is the most widely used tool in clinical trials of AD.20 For the Japanese version of the Alzheimer's Disease Assessment Scale (ADAS-J cog), mean scores (SD) were 12.8 (3.7) for MCI (CDR = 0.5), 21.2 (7.4) for mild AD (CDR = 1), 29.0 (11.7) for moderate AD (CDR = 2) and 42.1 (10.8) for severe AD (CDR = 3), and the test showed high sensitivity (98.1%) and specificity (95.1%).21 In the present study, the ADAS-J cog was administered by two skilled clinical psychologists (R A and R H).
MRI procedures
MRI was carried out using a 1.5-T system (Magnetom Symphony Quantum or Sonata; Siemens, Erlangen, Germany). For diagnosis, magnetization-prepared rapid gradient echo of sagittal 3-D sequence images (repetition time [TR] 1700 ms; echo time [TE] 3.93 ms; 4-mm thickness; 15° flip angle; 230-mm field of view; inversion time [TI], 1800 ms; 256 × 256 matrix; 176 × 1.25-mm contiguous sections; gapless) and axial T2-weighted turbo spin echo (TSE) images (TR 3800 ms; TE 99.0 ms; 6-mm thickness; 1.5-mm slice-gap) were obtained for diagnosis. Coronal TSE imaging (TR 9000 ms; TE 107 ms; 4-mm thickness; 1-mm slice-gap) was also carried out for voxel-based morphometry (VBM) analysis. The acquired MR images were reformatted to gapless 4-mm thickness trans-axial images for analysis with Statistical Parametric Mapping 2002 (SPM2; Wellcome Department of Imaging Neuroscience, London, UK) running on MATLAB (The MathWorks, Sherborn, MA, USA). We used a software program running on Windows XP and Vista called the voxel-based specific region analysis system for Alzheimer's disease (VSRAD plus; Eisai, Tokyo, Japan),22 which automatically analyzes 3-D T1-weighted MRI data as a series of segmentation, anatomical standardization and smoothing using SPM2, and that has high discrimination accuracy of 87.8%.23 The Z-score of VSRAD, defined as (normal mean – patient value) / (normal SD), representing the magnitude of gray matter density discrepancy, n × SD, was used as an indicator of the degree of atrophy of the entorhinal cortex and hippocampus. The Z-score maps were displayed by overlay on tomographic sections.
Statistical analysis
Group differences in demographic variables were evaluated using independent sample t-tests for continuous data, and with the odds ratio (OR) and 95% confidence interval (CI) for categorical data. Pearson's correlation coefficient was calculated to examine the relationship between years of education and premorbid IQ, and multiple regression analysis was carried out to explore the relationship between MCI and AD scores. Analyses were carried out using the spss version 20 statistical software package for Mac (IBM, Armonk, NY, USA). An alpha level of 0.05 was considered statistically significant.
Results
Table 1 summarizes patient demographic data and Table 2 shows sex differences in the 110 patients in the present study. There were no significant differences in sex, mean age at onset, mean onset of illness or mean duration of illness between the MCI and AD groups, or between men and women in general. However, statistical differences were found in MMSE score (t = 8.95, df = 108, P = 0.00) and ADAS-J cog score (t = 5.65, df = 108, P = 0.00) between the MCI and AD groups, showing that MCI patients had better cognitive performance than AD patients. In terms of education, MCI patients had more years of education (t = 2.26, df = 108, P = 0.03). All the patients in the AD group showed late-onset mild AD, where NART is a valid estimator of premorbid ability.24 JART-predicted IQ was higher in MCI patients than in AD patients (t = 3.85, df = 108, P = 0.00).
Table 1.
Demographic characteristics and other variables of patients with mild cognitive impairment or Alzheimer disease
| MCI (n = 51) | AD (n = 59) | P | |
|---|---|---|---|
| Sex (male/female) | 19/32 | 22/37 | 1.00 (0.46–2.17) |
| Age at diagnosis (years) | 77.7 (5.0) | 79.3 (6.0) | 0.14 |
| Age at onset (years) | 76.0 (5.0) | 77.1 (7.4) | 0.69 |
| Duration of illness (months) | 18.6 (6.0) | 20.0 (19.3) | 0.69 |
| Education (years) | 11.4 (2.6) | 10.2 (2.8) | 0.03 |
| <12 | 25 (49.0%) | 21 (35.6%) | 0.57 (0.27–1.23) |
| 12–16 | 18 (35.3%) | 33 (55.9%) | 2.32 (1.08–5.03) |
| ≥16 | 8 (15.7%) | 5 (8.5%) | 0.50 (0.15–1.63) |
| MMSE score | 24.3 (2.9) | 18.7 (3.2) | 0.00 |
| ADAS-J cog score | 10.1 (4.6) | 18.3 (6.9) | 0.00 |
| JART score | 100.8 (12.8) | 91.2 (13.1) | 0.00 |
| Hypertension | 31 (60.8%) | 27 (45.8%) | 0.54 (0.25–1.16) |
| Diabetes mellitus | 8 (15.7%) | 9 (15.3%) | 1.00 (0.34–2.73) |
| Hyperlipidemia | 16 (31.4%) | 10 (16.9%) | 0.45 (0.18–1.10) |
| VSRAD Z-score | 2.49 (1.31) | 2.96 (1.20) | 0.06 |
| 0–1 | 5 (9.8%) | 4 (6.8%) | 0.67 (0.17–2.64) |
| 1–2 | 17 (33.3%) | 9 (15.3%) | 0.36 (0.14–0.90) |
| 2–3 | 17 (33.3%) | 22 (37.3%) | 1.19 (0.54–2.61) |
| >3 | 12 (23.5%) | 24 (40.7%) | 2.22 (0.97–5.11) |
Data presented as mean (SD) or n (%). P-values were calculated using the t-test or were odds ratios (95% confidence interval). ADAS-J cog, Cognitive subscale of the Japanese version of the Alzheimer's Disease Assessment Scale; JART, Japanese Adult Reading Test; MMSE, Mini-Mental State Examination; VSRAD, voxel-based specific region analysis system for Alzheimer's disease software program, where Z-scores show the degree of gray matter loss in the entorhinal and hippocampal areas (clinical criteria are: 0–1, normal; 1–2, slight; 2–3, moderate; >3, severe)
Table 2.
Sex differences for the total 110 patients with either mild cognitive impairment or Alzheimer disease
| Men (n = 41) | Women (n = 69) | P | |
|---|---|---|---|
| MCI/AD | 019/22 | 32/37 | 0.99 (0.46–2.17) |
| Age at diagnosis (years) | 78.5 (5.6) | 79.0 (5.9) | 0.19 |
| Age at onset (years) | 76.6 (6.4) | 77.1 (6.9) | 0.27 |
| Duration of illness (months) | 22.0 (21.4) | 17.4 (14.8) | 0.23 |
| Education (years) | 11.8 (3.1) | 10.1 (2.3) | 0.00 |
| <12 | 22 (53.7%) | 24 (34.8%) | 2.17 (0.99–4.78) |
| 12–16 | 9 (35.3%) | 42 (60.9%) | 5.53 (2.29–13.38) |
| >16 | 10 (24.4%) | 3 (4.3%) | 7.10 (1.82–27.62) |
| MMSE score | 21.2 (3.7) | 21.3 (4.3) | 0.89 |
| ADAS-J cog score | 13.3 (6.2) | 12.9 (7.2) | 0.80 |
| JART score | 97.7 (13.6) | 94.5 (13.9) | 0.25 |
| Hypertension | 22 (53.7%) | 36 (52.2%) | 1.06 (0.49–2.30) |
| Diabetes mellitus | 8 (19.5%) | 9 (13.0%) | 1.62 (0.57–4.59) |
| Hyperlipidemia | 8 (19.5%) | 18 (26.1%) | 0.69 (0.27–1.76) |
| VSRAD Z-score | 2.68 (1.24) | 2.77 (1.31) | 0.74 |
| 0–1 | 3 (7.3%) | 6 (8.7%) | 0.83 (0.20–3.51) |
| 1–2 | 10 (24.4%) | 16 (23.3%) | 1.07 (0.43–2.64) |
| 2–3 | 16 (39.0%) | 23 (33.3%) | 1.28 (0.57–2.86) |
| >3 | 12 (29.3%) | 24 (34.8%) | 0.78 (0.34–1.79) |
Data presented as mean (SD) or n (%). P-values were calculated using the t-test or were odds ratios (95% confidence interval). AD, Alzheimer's disease; ADAS-J cog, Cognitive subscale of the Japanese version of the Alzheimer's Disease Assessment Scale; JART, Japanese Adult Reading Test; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; VSRAD, voxel-based specific region analysis system for Alzheimer's disease software program, where Z-scores show the degree of gray matter loss in the entorhinal and hippocampal areas (clinical criteria are: 0–1, normal; 1–2, slight; 2–3, moderate; >3, severe)
There were no statistical differences between the MCI and AD groups in terms of lifestyle-related diseases, such as hypertension, diabetes mellitus and hyperlipidemia. VSRAD Z-score for MCI (mean 2.49) was lower than that for AD (mean 2.96), suggesting a tendency for less atrophy compared with AD patients. However, Z-score and degree of atrophy did not differ significantly between the MCI (mean 2.49, SD 1.31) and AD (mean 2.96, SD 1.20) groups, suggesting a similar magnitude of severity, namely, moderate atrophy. The only differences between the MCI and AD patients other than the diagnostic variables were years of education (P = 0.03) and JART-predicted IQ score (P = 0.00).
Figure 1 shows the relationship between VSRAD Z-score and ADAS-J cog score divided by a premorbid IQ of 100. Pearson's correlation coefficient was 0.42 for the patients with low premorbid IQ (<100), suggesting a weak correlation between VSRAD Z-score and ADAS-J cog score. However, Pearson's correlation coefficient was 0.01 for the patients with high premorbid IQ (≥100).
Figure 1.
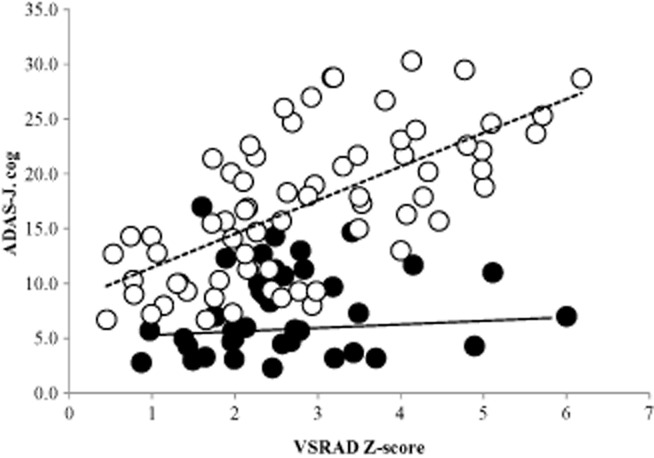
Correlation between voxel-based specific region analysis system for Alzheimer's disease (VSRAD) Z-score and the Japanese version of the Alzheimer's Disease Assessment Scale (ADAS-J cog) score. The patients were divided into two groups according to premorbid intelligence (IQ) measured with the Japanese version of the Nelson Adult Reading Test (<100 or ≥100). Pearson's r was 0.42 (dashed line) for patients with low premorbid IQ (<100), suggesting a weak positive correlation. The correlation coefficient for patients with high premorbid IQ (≥100) was much lower (Pearson's r = 0.01, solid line) than that for patients with low premorbid IQ, suggesting a protective effect by cognitive reserve. VSRAD, a voxel-based specific region analysis system for Alzheimer's disease software program, where Z-scores show the degree of gray matter loss in the entorhinal and hippocampal areas (clinical criteria are: 0–1, normal; 1–2, slight; 2–3, moderate; >3, severe).
Figure 2 shows the relationship between years of education and premorbid IQ measured with JART. Pearson's correlation coefficient was 0.41 for the total 110 patients, suggesting a weak correlation between years of education and premorbid IQ. However, a sex difference was observed; Pearson's correlation coefficient for men (0.44) was higher than that for women (0.38).
Figure 2.
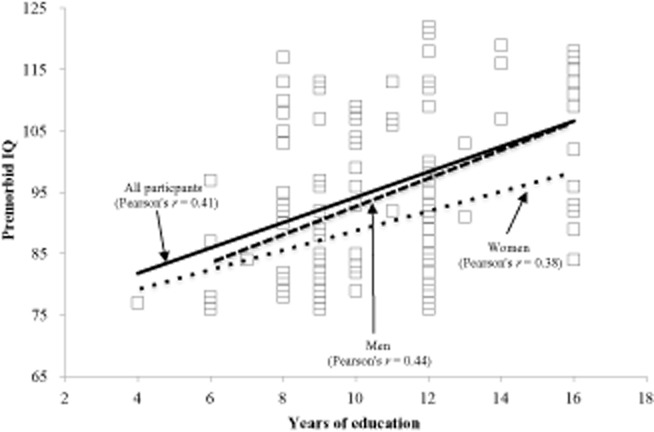
Correlation between years of education and premorbid intelligence (IQ) measured using the Japanese version of the Nelson Adult Reading Test. Note that Pearson's r was 0.41 for the total 110 patients, suggesting a weak correlation, whereas the correlation for men (r = 0.44) was higher than that for women (r = 0.38), suggesting a sex difference.
Discussion
Many studies have examined the risk and protective factors that underlie AD pathology by neuroimaging or using biomarkers. In particular, considerable efforts have been made to identify patients with early-stage AD with the aim of initiating effective treatment at an early stage.6 Studies have also reported a relationship between premorbid IQ and cerebral glucose metabolism in patients with AD.1 However, no studies have measured CR, namely, the ability to cope with this pathology.4
Although we used JART to assess premorbid intellectual function, the test has some limitations. For example, as pointed out by Matsuoka et al., JART-predicted IQ scores have a smaller range (75.9−124.1) than the original NART (68.6−130.6).17 As a result, the JART-predicted IQ scores for participants with very high intelligence will be underestimated, whereas those of participants with very low intelligence will be overestimated. In addition, there are many definitions of IQ, and JART might reflect only one aspect of IQ, especially literacy.
As shown in Figure 1, for patients with low premorbid IQ (<100), the more severe the degree of entorhinal and hippocampal atrophy, the worse the cognitive performance, suggesting a lower protective effect by CR. In patients with high premorbid IQ (≥100), however, CR might compensate for increasing entorhinal and hippocampal atrophy.
As shown in Figure 2, unlike premorbid IQ, years of education typically had limited variance, especially among women,16 and women in Japan had less opportunity to receive education than men before the end of World War II. Such unfavorable socioeconomic circumstances might, therefore, have limited an individual's opportunities to receive appropriate education. There was no statistical significance (t = 1.23, df = 108, P = 0.29) in age at diagnosis between the 41 men (mean 77.5 years, SD 5.1 years) and 69 women (mean 79.0 years, SD 5.9 years) participating in the present study. Although men received more years of education (mean 11.8 years, SD 3.1 years) than women (mean 10.1 years, SD 2.3 years; t = 3.00, df = 108, P = 0.004), there was no statistical significance (t = 1.17, df = 108, P = 0.25) in premorbid IQ between men (mean 97.7, SD 13.6) and women (mean 94.5, SD 13.9). These results suggest that years of education is not a determinant factor of premorbid IQ. The effect of the education level on the risk for AD is controversial, with a recent review reporting that more years of education did not uniformly attenuate the risk for dementia, consistent with the present findings.25
Analyses of comorbid medical conditions in AD patients have suggested a strong association between medical comorbidity and cognitive status in AD patients.26 Furthermore, a prospective cohort study showed that coexisting medical conditions could have some effect on the progression of AD.27 Furthermore, lifestyle-related diseases, such as hypertension, diabetes mellitus and hyperlipidemia, are associated with a risk of AD.28–30 Although the comorbid rate seemed to be higher in MCI patients in the present study, there was no statistical difference between the MCI and AD groups, suggesting that there is no association with the progression of MCI to AD. However, further longitudinal studies are still required.
In the 1990s, anatomical studies suggested that atrophic progression from the transentorhinal region to entorhinal and transentorhinal regions was correlated with a gradual worsening of clinical symptoms.31 However, it is difficult to evaluate atrophy of the entorhinal cortex by visual inspection alone. Accordingly, numerous MRI studies have since shown that atrophy of the medial temporal lobe, including the hippocampus as well as the entorhinal cortex, is indeed associated with the onset of symptoms of early AD.6,7,32 This was shown to be especially true with the earliest pathological change, which occurs in the entorhinal cortex. Z-score images obtained with VBM are a powerful diagnostic tool for detecting mild AD.33 That is, the purpose of neuroimaging in AD is not for diagnosis of advanced AD, but rather for very early AD in the form of conversion from MCI to AD. This finding might be related to anatomical findings showing that the regions are linked through the circuit of Papez to not only emotions, but also memory function.22 However, brain reserve,34 such as that indicated by anatomical or neuroimaging results, does not directly reflect individual memory function, as shown in the present study. In contrast, CR suggests that the brain actively attempts to cope with brain damage by using pre-existing cognitive processes or by enlisting compensatory processes.35
With sex, age at onset, duration of illness, years of education, magnitude of atrophy of entorhinal and hippocampal areas, and three coexisting lifestyle-related diseases as independent variables, and MCI and AD as dependent variables, multiple logistic regression analysis showed that the only difference between the MCI and AD groups was in premorbid IQ (P = 0.00, OR 1.06, 95% CI 1.02−1.09, partial regression coefficient 0.05), and that years of education had no statistical significance. This suggests that premorbid IQ represents a more robust CR than education, with a seemingly protective effect on the progression of MCI to AD through increased CR.
The concept of CR arose from observations that there appears to be no direct relationship between the magnitude of brain pathology and clinical manifestation.35 It has been hypothesized that CR modifies the relationship between pathology and clinical symptoms, and the present results suggest that the main factor affecting CR is premorbid IQ. Consideration of CR could, therefore, improve our understanding of individual differences in the progression of AD. Further longitudinal studies of, for example, occupational exposure and leisure activities are now required to clarify the main factors involved in the progression of cognitive decline.
Acknowledgments
We thank all patients and staff who took part in this study.
Disclosure statement
The authors declare no conflicts of interest.
References
- 1.Alexander GE, Furey ML, Grady CL, et al. Association of premorbid intellectual function with cerebral metabolism in Alzheimer's disease: implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154:165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- 2.Pavlik VN, Doody RS, Massman PJ, Chan W. Influence of premorbid IQ and education on progression of Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;22:367–377. doi: 10.1159/000095640. [DOI] [PubMed] [Google Scholar]
- 3.Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 4.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 5.Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. 2005;58:617–622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- 6.Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 10.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda H. Role of neuroimaging in Alzheimer's disease, with emphasis on brain perfusion SPECT. J Nucl Med. 2007;48:1289–1300. doi: 10.2967/jnumed.106.037218. [DOI] [PubMed] [Google Scholar]
- 13.Sugishita M, Asada T. Geriatric Depression Scale-Short Version-Japanese,GDS-S-J. Jpn J Cogn Neurosci. 2009;11:87–90. (in Japanese) [Google Scholar]
- 14.Nelson HE. National Adult Reading Test (NART) Windsor, UK: NFER-Nelson; 1982. [Google Scholar]
- 15.Nelson H, Willison J. National Adult Reading Test (NART) 2nd edn. Windsor, UK: NFER-Nelson; 1991. [Google Scholar]
- 16.Starr JM, Lonie J. Estimated pre-morbid IQ effects on cognitive and functional outcomes in Alzheimer disease: a longitudinal study in a treated cohort. BMC Psychiatry. 2008;8:27. doi: 10.1186/1471-244X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuoka K, Uno M, Kasai K, Koyama K, Kim Y. Estimation of premorbid IQ in individuals with Alzheimer's disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin Neurosci. 2006;60:332–339. doi: 10.1111/j.1440-1819.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Mori E, Mitani Y, Yamadori A. The validity of Japanese version of Mini-Mental State Examination in patients with nervous disorder. Shinkei Shinri. 1985;1:2–10. (in Japanese) [Google Scholar]
- 20.Cano SJ, Posner HB, Moline ML, et al. The ADAS-cog in Alzheimer's disease clinical trials: psychometric evaluation of the sum and its parts. J Neurol Neurosurg Psychiatry. 2010;81:1363–1368. doi: 10.1136/jnnp.2009.204008. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita M, Hirono N, Ikejiri Y, et al. Examining the diagnostic utility of the Japanese version of the Alzheimer's Disease Assessment Scale (ADAS-J cog.) Jpn J Geriatr. 1998;9:187–194. (in Japanese) [Google Scholar]
- 22.Matsuda H. The role of neuroimaging in mild cognitive impairment. Neuropathology. 2007;27:570–577. doi: 10.1111/j.1440-1789.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 23.Hirata Y, Matsuda H, Nemoto K, et al. Voxel-based morphometry to discriminate early Alzheimer's disease from controls. Neurosci Lett. 2005;382:269–274. doi: 10.1016/j.neulet.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 24.McGurn B, Starr JM, Topfer JA, et al. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology. 2004;62:1184–1186. doi: 10.1212/01.wnl.0000103169.80910.8b. [DOI] [PubMed] [Google Scholar]
- 25.Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25:289–304. doi: 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doraiswamy PM, Leon J, Cummings JL, Marin D, Neumann PJ. Prevalence and impact of medical comorbidity in Alzheimer's disease. J Gerontol Ser A Biol Sci Med Sci. 2002;57:M173–M177. doi: 10.1093/gerona/57.3.m173. [DOI] [PubMed] [Google Scholar]
- 27.Boksay I, Boksay E, Reisberg B, Torossian C, Krishnamurthy M. Alzheimer's disease and medical disease conditions: a prospective cohort study. J Am Geriatr Soc. 2005;53:2235–2236. doi: 10.1111/j.1532-5415.2005.00512_4.x. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai H, Hanyu H, Kanetaka H, et al. Prevalence of coexisting diseases in patients with Alzheimer's disease. Geriatr Gerontol Int. 2010;10:216–217. doi: 10.1111/j.1447-0594.2010.00609.x. [DOI] [PubMed] [Google Scholar]
- 29.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 30.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 31.Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand Suppl. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 32.Chetelat G, Baron JC. Early diagnosis of Alzheimer's disease: contribution of structural neuroimaging. Neuroimage. 2003;18:525–541. doi: 10.1016/s1053-8119(02)00026-5. [DOI] [PubMed] [Google Scholar]
- 33.Ishii K, Kawachi T, Sasaki H, et al. Voxel-based morphometric comparison between early- and late-onset mild Alzheimer's disease and assessment of diagnostic performance of z score images. AJNR Am J Neuroradiol. 2005;26:333–340. [PMC free article] [PubMed] [Google Scholar]
- 34.Katzman R. Education and the prevalence of dementia and Alzheimer's disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- 35.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc JINS. 2002;8:448–460. [PubMed] [Google Scholar]


