Abstract
We know surprisingly little about whole-tree nonstructural carbon (NSC; primarily sugars and starch) budgets. Even less well understood is the mixing between recent photosynthetic assimilates (new NSC) and previously stored reserves. And, NSC turnover times are poorly constrained.
We characterized the distribution of NSC in the stemwood, branches, and roots of two temperate trees, and we used the continuous label offered by the radiocarbon (carbon-14, 14C) bomb spike to estimate the mean age of NSC in different tissues.
NSC in branches and the outermost stemwood growth rings had the 14C signature of the current growing season. However, NSC in older aboveground and belowground tissues was enriched in 14C, indicating that it was produced from older assimilates. Radial patterns of 14C in stemwood NSC showed strong mixing of NSC across the youngest growth rings, with limited ‘mixing in’ of younger NSC to older rings.
Sugars in the outermost five growth rings, accounting for two-thirds of the stemwood pool, had a mean age < 1 yr, whereas sugars in older growth rings had a mean age > 5 yr. Our results are thus consistent with a previously-hypothesized two-pool (‘fast’ and ‘slow’ cycling NSC) model structure. These pools appear to be physically distinct.
Keywords: carbohydrates, carbon allocation, Harvard Forest, radiocarbon (14C), storage, tree rings, wood
Introduction
Although plant photosynthesis itself is well understood, much less is known about processes regulating the allocation of photosynthetic assimilates to growth, storage, and other metabolic functions (Wiley & Helliker, 2012; Dietze et al., 2014). Storage of nonstructural carbon (NSC, principally sugars and starch; Hoch et al., 2003) is critically important for woody plants, because these reserves enable sessile, long-lived organisms to tolerate biotic and abiotic stress, including pests, disturbance, and drought (Körner, 2003; Dietze et al., 2014). Thus, NSC reserves are highly relevant in the context of tree resilience to many global change factors. But, critical questions about the size and turnover of these reserves remain unanswered (Carbone et al., 2013; Muhr et al., 2013; Richardson et al., 2013; Dietze et al., 2014).
Isotope labeling experiments have provided key insights into the use of NSC to support tree growth and metabolism (Epron et al., 2012). This work has conclusively shown that new tissue growth typically relies on some mixture of both ‘old’ (previously stored) and ‘new’ (recent photosynthetic assimilates) NSC, although the reliance on stored reserves varies depending on the tissue in question, the time of year, and also among species (Kagawa et al., 2006; Keel et al., 2006, 2007; Carbone et al., 2007; Kuptz et al., 2011; Krepkowski et al., 2013). For logistical reasons, few labeling studies have been conducted on large, mature trees (Keel et al., 2006; Carbone et al., 2007). And, while previous studies have provided important insight into the short-term (hours–months) patterns of carbon (C) allocation and use, the long-term (years–decades) fate of the labeled C in structural and nonstructural pools has not been characterized (Kagawa et al., 2006; Keel et al., 2006; Högberg et al., 2008).
The radiocarbon (carbon-14, 14C) bomb spike (Levin & Kromer, 2004) provides an alternative to conventional isotope labeling for tracking the long-term fate of assimilated CO2 (Gaudinski et al., 2001). Using this method, we previously identified NSC more than a decade old in the stemwood of two temperate tree species (Richardson et al., 2013). Other work has shown that stored, decade-old C can be used to regrow root (Vargas et al., 2009) and shoot (Carbone et al., 2013) tissues following major disturbance. These are surprising results, given that short-term pulse-chase labeling studies have generally shown rapid use of new (labeled) NSC and inferred fast mixing between old and new NSC, both of which suggest quick turnover of storage reserves (Keel et al., 2007; Von Felten et al., 2007; Krepkowski et al., 2013).
Because whole-tree budgets of NSC are also scarce, we have few data with which to constrain estimates of NSC pool sizes and turnover times (Körner, 2003; Trumbore, 2006; Gaudinski et al., 2009; Muhr et al., 2013). Thus, to better understand how NSC reserves are distributed, the degree to which these reserves represent a mix of older vs newer NSC, and how this mixing varies throughout the tree, we destructively sampled a 23-yr-old white pine (Pinus strobus) and a 30-yr-old red oak (Quercus rubra) from Harvard Forest, an oak-dominated temperate forest in the north-eastern Unites States. This study thus builds on our previous work (Carbone et al., 2013; Richardson et al., 2013) by constructing whole-tree NSC budgets and by examining the radial patterns in NSC concentration and age, which allow us to directly examine the evidence for different mixing scenarios.
We analyzed stemwood samples (divided into individual rings, bark, and phloem), current- and multi-year branches, and three different root diameter size classes, for concentrations of total sugars and starch, and integrated these to whole-tree budgets using allometric scaling (Jenkins et al., 2004). Then, by comparing the age of stored NSC (estimated via 14C analysis) to the age of the tissue from which it was extracted, we evaluated four candidate conceptual models for the mixing of old and new NSC (Fig.1). The ‘complete mixing’ model is what is implicitly assumed in most contemporary ecosystem and tree growth models that include a storage pool (Riley et al., 2009; Richardson et al., 2013; Dietze et al., 2014), whereas the limited evidence to date is more consistent with the ‘no mixing’ model (Taylor et al., 2007). Our previous work has been unable to distinguish between these models (Carbone et al., 2013; Richardson et al., 2013).
Figure 1.
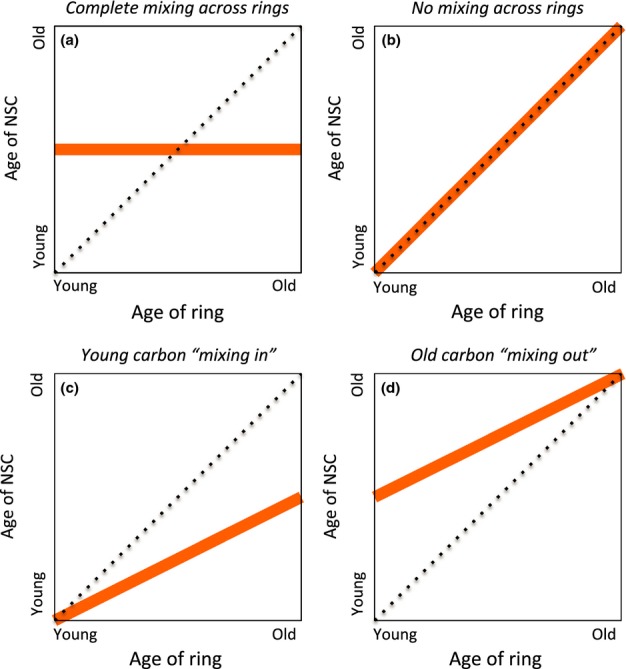
Conceptual models indicating possible scenarios for mixing of nonstructural carbon (NSC) across tree ring boundaries. In the case where there is complete mixing of ‘old’ (stored) NSC and ‘new’ (recent photosynthetic assimilates) NSC across the radial profile, the measured age of extracted NSC should be constant with depth, that is, across rings of different ages (a). When there is no mixing of old and new, and NSC is effectively ‘locked’ into the ring in which it is first deposited, then the measured age of extracted NSC should increase in parallel with ring age (b). However, if younger NSC ‘mixes in’ to older rings, the age of NSC should always be younger than the ring age (c), whereas if older NSCs ‘mixes out’ to younger rings, the age of NSC should always be older than the ring age (d). Intermediate cases are possible, for example, partial mixing would be characterized by young rings where the NSC is older than the ring age, and old rings where the NSC is younger than the ring age. The dashed line indicates the 1:1 line, and the orange line indicates the mixing scenario.
Materials and Methods
Study site
We conducted field sampling in November 2012 within the Tom Swamp (42°30.8′N, 72°13.1′W) tract of the Harvard Forest, c. 110 km west of the city of Boston, MA, USA. The mean annual temperature is 6.5°C, and mean annual precipitation is 1000 mm, distributed evenly throughout the year. The soil is a well-drained sandy loam derived from glacial till. The stand was previously a red pine (Pinus resinosa Ait.) plantation that was thinned in 1983 and harvested in 1990. The trees we sampled regenerated naturally and are young enough to contain no pre-bomb (before ad 1950) C. The forest in the vicinity of our sampling site is dominated by the two study species, white pine (Pinus strobus L.) and red oak (Quercus rubra L.). These species were selected primarily because the annual growth rings in stemwood disks were conspicuous and could be clearly identified by the naked eye, with no sanding or other preparation required. White pine is an evergreen conifer, while red oak is a ring porous, deciduous hardwood. Both species are of intermediate shade tolerance, have a wide distribution across the eastern half of North America, and are reproductively mature by c. 25 yr of age.
Field collection
We selected one tree of each species for destructive harvesting. The cost of conducting the 14C analyses precluded the sampling of additional trees. The main criteria for selection were (1) evidence of vigorous growth and (2) a well-developed crown that was not overtopped by surrounding trees. The pine was 12 m high, 23 yr of age, 18.5 cm diameter at breast height (DBH), and established following the harvest in 1990. The oak was 20 m high, 30 yr of age, and 17.0 cm DBH, and established following the thinning in 1983. Trees were felled and stemwood bolts (c. 50 cm in length) collected at breast height, mid-way up the stem, and near the top of the stem. We also collected current-year twigs, as well as multi-year branches, from throughout the crown. We then partially excavated the stump and root system of each tree, and collected samples of large coarse roots (ϕ > 5 cm), as well as medium coarse roots (ϕ ≈ 1 cm) and fine roots (ϕ ≤ 1 mm) that were directly attached to the large coarse roots (and thus undoubtedly from the same tree).
Laboratory preparations
Samples were processed in the laboratory on the same day that they were collected in the field. Cookies, or stemwood sections c. 2 cm in thickness, were cut from the stemwood bolts. Using a hammer and chisel, we separated the outer bark and phloem, and then separated individual annual growth rings from each cookie. We identified the heartwood–sapwood transition based on wood color.
In the large and intermediate coarse roots we could not easily count rings. We therefore separated the large coarse root wood by thirds into three depths, with D1 denoting the outer (most recent) wood and D3 the center (oldest) wood. For all root samples, in lieu of ring counts we determined the mean age of the cellulose (i.e. structural C) via 14C analysis as described later.
Samples were frozen at −80°C and then freeze-dried (FreeZone 2.5; Labconco, Kansas City, MO, USA, and Hybrid Vacuum Pump, Vacuubrand, Wertheim, Germany) before being homogenized and milled to 20 mesh (Wiley Mini Mill; Thomas Scientific, Swedesboro, NJ, USA). Samples were then kept at −80°C until the NSC extraction was conducted. Additional sample material was kept frozen at −80°C.
NSC analysis
For total soluble sugar concentration (following Chow & Landhäusser, 2004), 50 mg of tissue was freeze-dried a second time (24 h; for determination of precise weight) and subjected to hot ethanol extraction followed by colorimetric analysis with phenol-sulfuric acid. The resulting extract was read at 490 nm with a microplate reader (Epoch Microplate Spectrophotometer; Bio-Tek Instruments, Winooski, VT, USA) with sugar concentration (expressed as milligrams of sugar per gram of dry wood) calculated from a standard curve of 1:1:1 glucose–fructose–galactose (Sigma Chemicals, St Louis, MO, USA).
For starch analysis (following Wargo et al., 2002), the remaining tissue residue was boiled in potassium hydroxide (KOH) followed by neutralization with acetic acid and enzymatic digestion with amyloglucosidase. Glucose hydrolyzate was determined using a glucose hexokinase kit (Pointe Scientific, Canton, MI, USA) and read at 340 nm with the microplate reader. Starch concentration (expressed as milligrams of starch per gram of dry wood) was calculated based on glucose standard (Pointe Scientific, Canton, MI, USA) curves.
The NSC concentration data for each sample are reported in Supporting Information Table S1.
14C analysis
Following Richardson et al. (2013) and Carbone et al. (2013) we used the 14C bomb spike to estimate the age of extracted NSC, and in the case of root wood, which could not be easily aged by ring counting, to estimate the age of the cellulose (i.e. structural C). The bomb spike method is based on the fact that aboveground testing of thermonuclear weapons between 1955 and 1963 approximately doubled the amount of 14CO2 in the atmosphere (Levin & Kromer, 2004). Since 1963, when the Limited Nuclear Test Ban Treaty was signed, the 14C content of atmospheric CO2 has decreased through mixing with oceanic and terrestrial C reservoirs, and by addition of 14C-free CO2 from fossil fuel burning (Levin et al., 2010). The C in photosynthetic products reflects the 14C content of atmospheric CO2 in the year assimilation occurred, and hence these products are labeled with a unique isotopic signature. Thus, the mean 14C age of a sample can be determined by measuring its 14C content, and comparing this to the atmospheric 14CO2 record.
We conducted 14C analysis on sugars and starch extracted following Czimczik et al. (2014). Soluble NSC (mostly sugars) was isolated by hot water extraction, and insoluble NSC (mostly starch) was isolated by acid digestion following lipid removal by boiling in ethanol. The extractions were conducted sequentially from each tissue sample. We conducted the 14C analysis on cellulose extracted using a Soxhlet apparatus (Leavitt & Danzer, 1993).
For the 14C analysis of both NSC and cellulose, extracts were combusted to CO2, purified on a vacuum line, and converted to graphite (Xu et al., 2007). Graphite was analyzed for its 14C content at the W.M. Keck Carbon Cycle Accelerator Mass Spectrometry facility at University of California, Irvine (Southon et al., 2007).
We used the northern hemisphere atmospheric record (Levin et al., 2008) for dating, following Gaudinski et al. (2001). Previous 14CO2 samples (S. Trumbore, unpublished; Carbone et al., 2013; Richardson et al., 2013) have shown that the background air at Harvard Forest is consistent with the atmospheric record. For further verification, we have since 2010 collected annual plant samples (Jewelweed, Impatiens capensis Meerb. and ragweed, Ambrosia artemisiifolia L.) each year. Annual plants are natural ‘isometers’ because the 14C content in their structural tissues reflects an average daytime 14CO2 value of the atmosphere, integrated over weeks-to-months, for the current growing season. Because they live for only 1 yr, they have no stored NSC that could be carried over from previous years, and any previous-year seed signal is overwhelmed by current-year assimilation.
The 14C data for each sample are reported in Supporting Information Table S2. An analysis of the uncertainty in the 14C measurements is contained in Supporting Information Methods S1.
Allometric scaling from concentrations to whole-tree budgets
We sanded stemwood disks, from breast height, using progressively finer sandpaper until all ring boundaries could be precisely identified under a dissecting microscope (Stereozoom; Leica Microsystems, Wetzlar, Germany). We measured ring widths (mean of three radii for pine, four radii for oak) under the microscope, using a sliding stage and linear encoder (TA Tree Ring System; Velmex Inc., Bloomfield, NY, USA) with a resolution of 0.001 mm and accuracy of 0.010 mm m−1. With these data, we could then estimate the stem biomass attributable to each year of growth (from the current growing season, 2012, to the first year at which each tree reached breast height – 1990 for the pine, and 1983 for the oak) using standard allometric scaling theory (Whittaker et al., 1974). For this we used equations and coefficients presented by Jenkins et al. (2004) to estimate stemwood, branch, and root biomass (in kilograms of dry wood).
We paired our tissue-specific (or in the case of stemwood rings, ring-specific) concentration measurements with our estimate of the woody biomass in each tissue type (or ring). We then determined the total amount (= biomass × concentration) of sugars and starch in each biomass component (stemwood, roots, and branches), and added these together to estimate the aggregate, whole-tree NSC pool stored in woody tissues. For additional details, see Supporting Information Methods S2.
Results and discussion
In the stemwood, radial patterns of NSC concentrations differed between the two trees, although the highest concentrations of both sugars and starch were always observed in the phloem and/or outermost rings (Fig.2) (Hoch et al., 2003; Niamké et al., 2011). And, the degree to which the sapwood/heartwood transition was related to the radial patterns differed between trees (Barbaroux & Bréda, 2002). At 1 m height, concentrations of sugars in the pine dropped off rapidly within the first few rings (Fig.2a), whereas starch concentrations were low in all rings (Fig.2c). In the oak, concentrations of sugars were high until the sapwood/heartwood boundary (Fig.2b), and low thereafter, whereas starch concentrations declined steeply until the sapwood/heartwood boundary (Fig.2d). Similar patterns were observed for samples from the middle and top of the stem (Fig.2a–d).
Figure 2.
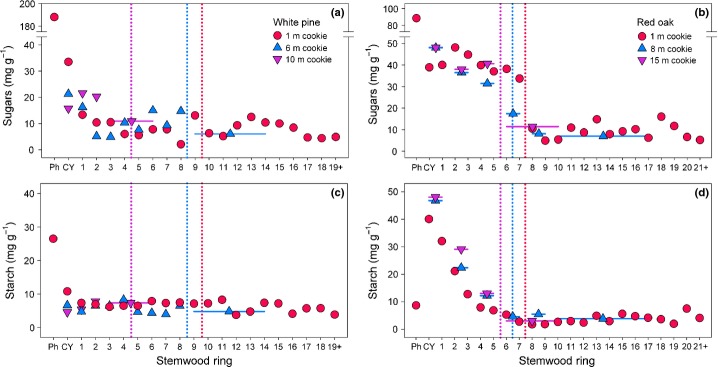
Radial patterns in stemwood concentrations of sugars (a, b) and starch (c, d) for white pine (Pinus strobus, a, c) and red oak (Quercus rubra, b, d). Samples were collected at three different heights in the tree. The vertical dotted lines indicate the sapwood–heartwood boundary at these heights, with the color corresponding to the sampling height. Thin horizontal lines indicate the range of rings included, for cases where multiple rings were pooled to produce a single sample for analysis. Ph is phloem tissue, and CY is the current-year (2012) ring.
In large coarse roots, NSC concentrations did not vary with radial depth in either species (Table S3). However, large and intermediate coarse root concentrations of both sugars and starch were generally much lower in the pine than the oak. By comparison, fine root concentrations of both sugars and starch were about equal in pine and oak.
In branches, concentrations of sugars and starch were lower in pine than oak (Table S3). In both trees, branch concentrations of sugars were higher than those of starch, particularly in current-year branches.
Scaled up to whole-tree budgets for NSC stored in woody tissues, we observed substantial differences between trees in total NSC, and how this total was partitioned between aboveground and belowground tissues (Table1; see also Tables S4–S6). For example, although the estimated whole-tree woody biomass of the pine (103 kg) was only 30% smaller than that of the oak (144 kg), the oak stored nearly four times as much NSC in its woody tissues. Thus, in the pine, NSC reserves were estimated at 1.5 kg of sugars and 1.1 kg of starch, compared with 4.8 kg of sugars and 5.3 kg of starch in the oak. In both trees, stemwood was the largest reserve of sugars, and roots the largest reserve of starch, but there was only one-eighth as much starch in the pine's roots (0.5 kg, 45% of the total starch pool) compared to the oak's roots (3.9 kg, 73% of the total starch pool). Furthermore, in both trees, the amount of NSC stored in the heartwood of the stem was relatively small: it accounted for < 15% of the stemwood total in the pine, and < 7% of the stemwood total in the oak. However, since parenchyma tissue in the heartwood is by definition dead, these reserves are therefore likely unavailable to the tree (Spicer, 2005). Finally, this scaling showed that branches are an important store of sugars, accounting for about one-quarter of the total stored in woody tissues, in both trees. Thus this analysis highlights the importance of employing a relatively detailed accounting scheme – including roots and branches – for scaling up to whole-tree NSC budgets (Gholz & Cropper, 1991; Barbaroux et al., 2003; Würth et al., 2005).
Table 1.
Whole-tree budgets for nonstructural carbon (NSC) reserves stored in the woody tissues of a 23-yr-old white pine (Pinus strobus, top) and 30-yr-old red oak (Quercus rubra, bottom)
| Component | Woody biomass (kg) | NSC content (g) | Per cent of tree total | Effective mean concentration (mg g−1) | |||
|---|---|---|---|---|---|---|---|
| Sugars | Starch | Sugars | Starch | Sugars | Starch | ||
| White pine | |||||||
| Stem | 60 | 644 | 428 | 42 | 38 | 11 | 7 |
| Root | 24 | 460 | 501 | 30 | 45 | 19 | 21 |
| Branches | 19 | 427 | 185 | 28 | 17 | 23 | 10 |
| Total | 103 | 1531 | 1114 | 100 | 100 | 15 | 11 |
| Red oak | |||||||
| Stem | 71 | 2115 | 997 | 44 | 19 | 30 | 14 |
| Root | 30 | 1638 | 3897 | 34 | 73 | 56 | 132 |
| Branches | 44 | 1075 | 426 | 22 | 8 | 25 | 10 |
| Total | 144 | 4828 | 5320 | 100 | 100 | 33 | 37 |
In both pine and oak, and for both sugars and starch, NSC was always the same age or younger than the structural tissue from which it was extracted (Fig.3; Table S3). Notably, in the stemwood of both species, sugars in the outermost few rings were comprised primarily of current-year photosynthate: from the current-year ring to the 4-yr-old ring in pine, and to the 2-yr-old ring in oak, the mean age of extracted sugars in each ring was ≤ 1 yr. However, beyond these outermost rings, there was a pattern of sugars increasing steadily in age with increasing ring age. For pine, the age of sugars increased linearly (r = 0.98, slope = 0.75 ± 0.05 yr per ring, n = 5) from 2.3 yr in the 6-yr-old ring, to 11.0 yr in the 18-yr-old ring (Fig.3a). By comparison, in oak, the age of sugars also increased linearly (r = 0.99, slope = 0.98 ± 0.03 yr per ring, n = 6) but at a faster rate, from 2.1 yr in the 4-yr-old ring, to 17.8 yr in the 20-yr-old ring (Fig.3b). In both species, these data suggest strong mixing of relatively new sugars across the outermost rings (Fig.1a). However, it appears that mixing is more limited beyond about the 5-yr-old ring. There is no evidence in the stemwood data for old NSC ‘mixing out’ (Fig.1d), but in the case of pine, the data are consistent with some younger NSC ‘mixing in’ to older rings (Fig.1c), whereas in oak the data suggest much more limited mixing of younger NSC into older rings (Fig.1b). These results are surprising because oak wood has large and prominent ray cells (accounting for > 25% of wood volume; Brown et al., 1949), which might be expected to facilitate radial mixing, compared to the extremely small ray cells in pine (accounting for only 5% of wood volume; Brown et al., 1949). The higher total NSC amount and the apparently more limited mixing of younger NSC into older stemwood rings in the hardwood (deciduous oak) than the softwood (evergreen pine) reinforces the idea that wood anatomy and leaf habit are key drivers governing NSC allocation and availability (Hoch et al., 2003). Disturbance ecology could also play a role, in that red oak can resprout vigorously from the stump or root collar, whereas white pine does not resprout and so maintaining large reserves of NSC in the root system would be of limited value.
Figure 3.
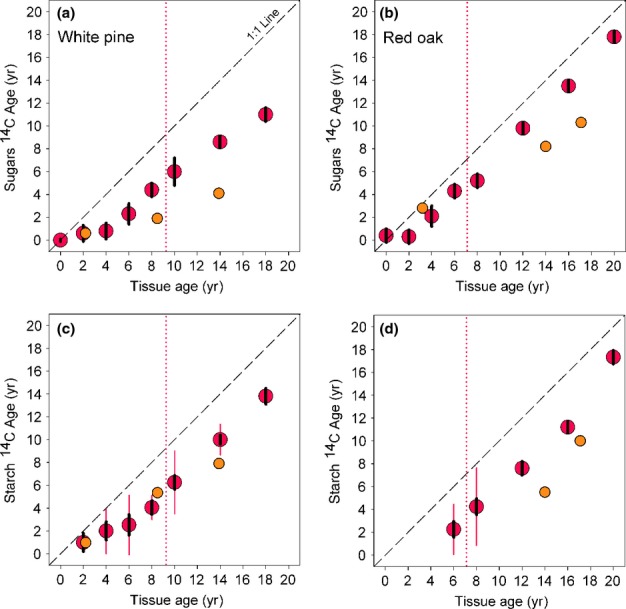
Radial trends in radiocarbon (14C) age (see the Materials and Methods section) of extracted sugars (a, b) and starch (c, d) in stemwood and large coarse root samples of white pine (Pinus strobus, a, c) and red oak (Quercus rubra, b, d). For stemwood, tissue age was determined by direct ring counts. For roots, tissue age was determined by 14C analysis of extracted cellulose (see the Materials and Methods section). The vertical dotted line indicates the sapwood–heartwood boundary at breast height. The thin red vertical error bars indicate the standard deviation of 14C age across repeat samples (where available; see Supporting Information Methods S1). The heavier black vertical bars indicate the analytical uncertainty (one standard deviation, 1 SD) of the accelerator mass spectrometry (AMS) measurements (see Methods S1).
Overall, because the stem biomass is dominated by more recent growth rings (Table S4), which contain high concentrations of very young NSC, the weighted mean age (across all rings) of stemwood sugars was 2.2 yr in the pine and 2.4 yr in the oak. This mean age of stemwood sugars is younger than we previously reported for NSC extracted from 2 cm stemwood cores of red maple and eastern hemlock (Richardson et al., 2013). However, for the red maple in that analysis, there was a significant positive correlation (r = 0.66, P < 0.001, n = 26) between the age of extracted sugars (range: 2–24 yr) and tree age (range 70–194 yr). Consequently, the most likely reason we obtained younger ages here was because we sampled younger trees.
Additionally, we previously reported that stemwood NSC is highly dynamic on seasonal timescales, but also (on average) substantially older (mean ± 1 SD: 11 ± 7 yr for sugars + starch) than expected or predicted by most simulation models (Richardson et al., 2013). We resolved this apparent paradox of a dynamic, but old, pool by hypothesizing that there is not a single NSC pool, but rather two pools with functionally different roles: a ‘fast’ pool that turns over quickly and is preferentially used to support growth and metabolism, and a ‘slow’ pool, comprised of older NSC, that can be drawn on if reserves in the fast pool drop too low. The data presented here for stemwood sugars are consistent with this hypothesis: in both trees, the outermost five (current year to 4-yr-old) rings contain about two-thirds of the stemwood sugars, and together have a weighted mean age of < 1 yr, while the remaining one-third of stemwood sugars are found deeper in the stem and have a mean age of > 5 yr. Overall, the weighted mean age is thus 2–3 yr, but with this interpretation the ‘fast’ and ‘slow’ cycling components are stored in different rings. NSC in the outermost rings is likely considerably more accessible to the tree than NSC in deeper rings. But, previous studies have shown that old reserves are available to support growth following major disturbance (Vargas et al., 2009; Carbone et al., 2013).
The starch age data are not as complete because some samples were lost in processing, but they are consistent with the patterns for sugars, in that starch age increased steadily with radial depth and was always younger than the tissue from which it was extracted (Fig.3c,d). Pooling stemwood data for both species shows a strong linear relationship (r = 0.97; intercept not significantly different from zero, slope not significantly different from one, both P > 0.50 by t-test, n = 12) between the age of extracted sugars and the age of extracted starch. This is consistent with previous observations (Richardson et al., 2013) indicating seasonal cycles of substantial interconversion of sugar and starch in the stemwood of a variety of different temperate tree species. However, we interpret the radial patterns of NSC ages (Fig.3) to suggest that this mixing does not cross ring boundaries, except for possibly within the outermost few rings.
In roots, we found both young (< 1 yr) and old (> 5 yr) NSC, again suggesting poor mixing and possibly the presence of C reserves that turn over on different timescales (Table S3). But whereas in the stemwood old NSC was found only in older rings, where concentrations were low, old NSC was found at relatively high concentrations in the medium coarse roots and fine roots of pine, and in all roots of oak (Table S3). As was the case aboveground, NSC age in roots increased with increasing tissue age, and in large coarse roots with increasing radial depth. And, especially in older tissues, both sugars and starch tended to be substantially younger than the tissues from which they were extracted, which is consistent with the ‘mixing in’ of newer NSC (Fig.3). But, unlike what we found aboveground, the age of root sugars was poorly correlated with the age of root starch (r = 0.53, P = 0.17, n = 8), suggesting that less seasonal interconversion of sugars and starch occurs belowground, compared to aboveground.
Conclusions
By using radiocarbon (14C) methods, this study has provided unique insight into the long-term fate of NSC allocated to storage, and how new NSC (recent photosynthetic assimilates) mixes with old NSC (previously stored reserves) within the woody tissues of two temperate trees. In our previous analyses (Carbone et al., 2013; Richardson et al., 2013), in which we determined the mean age of NSC in a 2 cm stemwood core, we were unable to distinguish among candidate models for mixing of old and new reserves (Fig.1). Here, by extracting and aging NSC from individual growth rings in the stemwood, we have shown evidence for strong mixing of NSC across the youngest growth rings (where NSC concentrations are highest), but what we interpret to be much more limited ‘mixing in’ of younger NSC to older rings (where NSC concentrations are lower). Our results are consistent with the idea that while most of the NSC in trees is stored in a pool that is, well-mixed and turns over quickly, a substantial fraction of the reserves are much older and are not actively mixing with new assimilates.
Allocation and storage have long been recognized as an ‘Achilles’ Heel’ of simulation models (LeRoux & Lacointe, 2001), reflecting a general lack of understanding of these processes. Our results give support for a previously-hypothesized (Richardson et al., 2013) two-pool model representation of NSC reserves, with ‘fast’ and ‘slow’ cycling pools, which play functionally different roles in supporting growth and metabolism. Additionally, if the ‘fast’ and ‘slow’ cycling pools are physically distinct because they are stored in younger vs older rings, these results help to explain observations (Carbone et al., 2013) that NSC less than a year old is preferentially used for growth and metabolic demands, even when the mean age of NSC is much older. However, the slow pool is large enough that it cannot be ignored as a store of reserves. We conclude that it is overly simplistic to view (and for models to represent) NSC reserves as a single pool that is well mixed and turns over quickly.
Acknowledgments
The authors thank A. Barker-Plotkin for assistance in locating a suitable stand in which to conduct field collections. A.D.R. acknowledges support for research at Harvard Forest from the National Science Foundation through the Long-Term Ecological Research program (grant DEB-1237491). This work was partially supported by the US Forest Service Northern Research Station. Trevor Keenan is thanked for providing feedback on a draft of the manuscript.
Supporting Information
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Table S1 Sugar and starch concentration data for white pine and red oak
Table S2 Radiocarbon data for white pine and red oak
Table S3 Tissue age, concentrations of extracted nonstructural carbon (NSC), and the radiocarbon (14C) age of extracted NSC, for roots and branches of a white pine and a red oak tree
Table S4 Woody biomass and nonstructural carbon (NSC) content in the stemwood of a white pine and red oak tree
Table S5 Woody biomass and nonstructural carbon (NSC) content in the root system of a white pine and red oak tree.
Table S6 Woody biomass and nonstructural carbon (NSC) content in the branches of a white pine and red oak tree
Methods S1 Uncertainty of radiocarbon measurements.
Methods S2 Allometric scaling from nonstructural carbon (NSC) concentrations to whole-tree budgets, and uncertainty characterization.
References
- Barbaroux C, Bréda N. Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiology. 2002;22:1201–1210. doi: 10.1093/treephys/22.17.1201. [DOI] [PubMed] [Google Scholar]
- Barbaroux C, Breda N, Dufrene E. Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica. New Phytologist. 2003;157:605–615. doi: 10.1046/j.1469-8137.2003.00681.x. [DOI] [PubMed] [Google Scholar]
- Brown HP, Panshin AJ, Forsaith CC. Textbook of wood technology. NewYork, NY, USA: McGraw-Hill; 1949. [Google Scholar]
- Carbone MS, Czimczik CI, Keenan TF, Murakami PF, Pederson N, Schaberg PG, Xu X, Richardson AD. Age, allocation and availability of nonstructural carbon in mature red maple trees. New Phytologist. 2013;200:1145–1155. doi: 10.1111/nph.12448. [DOI] [PubMed] [Google Scholar]
- Carbone MS, Czimczik CI, McDuffee KE, Trumbore SE. Allocation and residence time of photosynthetic products in a boreal forest using a low-level 14C pulse-chase labeling technique. Global Change Biology. 2007;13:466–477. [Google Scholar]
- Chow PS, Landhäusser SM. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiology. 2004;24:1129–1136. doi: 10.1093/treephys/24.10.1129. [DOI] [PubMed] [Google Scholar]
- Czimczik CI, Trumbore SE, Xu XM, Carbone MS, Richardson AD. Extraction of nonstructural carbon and cellulose from wood for radiocarbon analysis. Bio-protocol. 2014;4:e1169. [Google Scholar]
- Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth Ja, Richardson AD, Vargas R. Nonstructural carbon in woody plants. Annual Review of Plant Biology. 2014;65:667–687. doi: 10.1146/annurev-arplant-050213-040054. [DOI] [PubMed] [Google Scholar]
- Epron D, Bahn M, Derrien D, Lattanzi FA, Pumpanen J, Gessler A, Högberg P, Maillard P, Dannoura M, Gérant D, et al. Pulse-labelling trees to study carbon allocation dynamics: a review of methods, current knowledge and future prospects. Tree Physiology. 2012;32:776–798. doi: 10.1093/treephys/tps057. [DOI] [PubMed] [Google Scholar]
- Gaudinski JB, Torn MS, Riley WJ, Swanston C, Trumbore SE, Joslin JD, Majdi H, Dawson TE, Hanson PJ. Use of stored carbon reserves in growth of temperate tree roots and leaf buds: analyses using radiocarbon measurements and modeling. Global Change Biology. 2009;15:992–1014. [Google Scholar]
- Gaudinski JB, Trumbore SE, Davidson EA, Cook AC, Markewitz D, Richter DD. The age of fine-root carbon in three forests of the eastern United States measured by radiocarbon. Oecologia. 2001;104:420–429. doi: 10.1007/s004420100746. [DOI] [PubMed] [Google Scholar]
- Gholz HL, Cropper WP. Carbohydrate dynamics in mature Pinus elliottii var. elliottii trees. Canadian Journal of Forest Research. 1991;21:1742–1747. [Google Scholar]
- Hoch G, Richter A, Körner C. Non-structural carbon compounds in temperate forest trees. Plant, Cell & Environment. 2003;26:1067–1081. [Google Scholar]
- Högberg P, Högberg MN, Göttlicher SG, Betson NR, Keel SG, Metcalfe DB, Campbell C, Schindlbacher A, Hurry V, Lundmark T, et al. High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytologist. 2008;177:220–228. doi: 10.1111/j.1469-8137.2007.02238.x. [DOI] [PubMed] [Google Scholar]
- Jenkins JC, Chojnacky DC, Heath LS, Birdsey RA. 2004. Comprehensive database of diameter-based biomass regressions for North American tree species. USDA Forest Service, Northeastern Research Station, Newtown Square, PA, USA.
- Kagawa A, Sugimoto A, Maximov TC. Seasonal course of translocation, storage and remobilization of 13C pulse-labeled photoassimilate in naturally growing Larix gmelinii saplings. New Phytologist. 2006;171:793–803. doi: 10.1111/j.1469-8137.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- Keel SG, Siegwolf RTW, Jäggi M, Körner C. Rapid mixing between old and new C pools in the canopy of mature forest trees. Plant, Cell & Environment. 2007;30:963–972. doi: 10.1111/j.1365-3040.2007.01688.x. [DOI] [PubMed] [Google Scholar]
- Keel SG, Siegwolf RTW, Körner C. Canopy CO2 enrichment permits tracing the fate of recently assimilated carbon in a mature deciduous forest. New Phytologist. 2006;172:319–329. doi: 10.1111/j.1469-8137.2006.01831.x. [DOI] [PubMed] [Google Scholar]
- Körner C. Carbon limitation in trees. Journal of Ecology. 2003;91:4–17. [Google Scholar]
- Krepkowski J, Gebrekirstos A, Shibistova O, Bräuning A. Stable carbon isotope labeling reveals different carry-over effects between functional types of tropical trees in an Ethiopian mountain forest. New Phytologist. 2013;199:441–451. doi: 10.1111/nph.12266. [DOI] [PubMed] [Google Scholar]
- Kuptz D, Fleischmann F, Matyssek R, Grams TEE. Seasonal patterns of carbon allocation to respiratory pools in 60-yr-old deciduous (Fagus sylvatica) and evergreen (Picea abies) trees assessed via whole-tree stable carbon isotope labeling. New Phytologist. 2011;191:160–172. doi: 10.1111/j.1469-8137.2011.03676.x. [DOI] [PubMed] [Google Scholar]
- Leavitt SW, Danzer SR. Method for batch processing small wood samples to holocellulose for stable-carbon isotope analysis. Analytical Chemistry. 1993;65:87–89. [Google Scholar]
- LeRoux X, Lacointe A. Carbon-based models of individual tree growth: a critical appraisal. Annals of Forest Science. 2001;58:469–506. [Google Scholar]
- Levin I, Hammer S, Kromer B, Meinhardt F. Radiocarbon observations in atmospheric CO2: determining fossil fuel CO2 over Europe using Jungfraujoch observations as background. Science of the Total Environment. 2008;391:211–216. doi: 10.1016/j.scitotenv.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Levin I, Kromer B. The tropospheric 14CO2 level in mid-latitudes of the Northern Hemisphere (1959–2003) Radiocarbon. 2004;46:1261–1272. [Google Scholar]
- Levin I, Naegler T, Kromer B, Diehl M, Francey RJ, Gomez-Pelaez AJ, Steele LP, Wagenbach D, Weller R, Worthy DE. Observations and modelling of the global distribution and long-term trend of atmospheric 14CO2. Tellus Series B. 2010;62:26–46. [Google Scholar]
- Muhr J, Angert A, Negrón-Juárez RI, Muñoz WA, Kraemer G, Chambers JQ, Trumbore SE. Carbon dioxide emitted from live stems of tropical trees is several years old. Tree Physiology. 2013;33:743–752. doi: 10.1093/treephys/tpt049. [DOI] [PubMed] [Google Scholar]
- Niamké FB, Amusant N, Charpentier J-P, Chaix G, Baissac Y, Boutahar N, Adima AA, Kati-Coulibaly S, Jay-Allemand C. Relationships between biochemical attributes (non-structural carbohydrates and phenolics) and natural durability against fungi in dry teak wood (Tectona grandis L. f.) Annals of Forest Science. 2011;68:201–211. [Google Scholar]
- Richardson AD, Carbone MS, Keenan TF, Czimczik CI, Hollinger DY, Murakami P, Schaberg PG, Xu X. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytologist. 2013;197:850–861. doi: 10.1111/nph.12042. [DOI] [PubMed] [Google Scholar]
- Riley WJ, Gaudinski JB, Torn MS, Joslin JD, Hanson PJ. Fine-root mortality rates in a temperate forest: estimates using radiocarbon data and numerical modeling. New Phytologist. 2009;184:387–398. doi: 10.1111/j.1469-8137.2009.02980.x. [DOI] [PubMed] [Google Scholar]
- Southon J, Santos G, Druffel-Rodriguez K, Druffel E, Trumbore S, Xu X, Mazo MM, Ali S, Mazon M. The Keck Carbon Cycle AMS Laboratory, University of California, Irvine; initial operation and a background surprise. Radiocarbon. 2007;46:41–49. [Google Scholar]
- Spicer R. Senescence in secondary xylem: heartwood fromation as an active developmental program. In: Holbrook NM, Zwieniecki MA, editors. Vascular transport in plants. Amsterdam, the Netherlands: Elsevier; 2005. pp. 457–475. [Google Scholar]
- Taylor AM, Brooks JR, Lachenbruch B, Morrell JJ. Radial patterns of carbon isotopes in the xylem extractives and cellulose of Douglas-fir. Tree Physiology. 2007;27:921–927. doi: 10.1093/treephys/27.6.921. [DOI] [PubMed] [Google Scholar]
- Trumbore S. Carbon respired by terrestrial ecosystems – recent progress and challenges. Global Change Biology. 2006;12:141–153. [Google Scholar]
- Vargas R, Trumbore SE, Allen MF. Evidence of old carbon used to grow new fine roots in a tropical forest. New Phytologist. 2009;182:710–718. doi: 10.1111/j.1469-8137.2009.02789.x. [DOI] [PubMed] [Google Scholar]
- Von Felten S, Hättenschwiler S, Saurer M, Siegwolf R. Carbon allocation in shoots of alpine treeline conifers in a CO2 enriched environment. Trees. 2007;21:283–294. [Google Scholar]
- Wargo PM, Minocha R, Wong BL, Long RP, Horsley SB, Hall TJ. Measuring changes in stress and vitality indicators in limed sugar maple on the Allegheny Plateau in north-central Pennsylvania. Canadian Journal of Forest Research. 2002;32:629–641. [Google Scholar]
- Whittaker RH, Bormann FH, Likens GE, Siccama TG. The Hubbard Brook Ecosystem Study: forest biomass and production. Ecological Monographs. 1974;44:641–650. [Google Scholar]
- Wiley E, Helliker B. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytologist. 2012;195:285–289. doi: 10.1111/j.1469-8137.2012.04180.x. [DOI] [PubMed] [Google Scholar]
- Würth MKR, Peláez-Riedl S, Wright SJ, Körner C. Non-structural carbohydrate pools in a tropical forest. Oecologia. 2005;143:11–24. doi: 10.1007/s00442-004-1773-2. [DOI] [PubMed] [Google Scholar]
- Xu X, Trumbore SE, Zheng S, Southon JR, McDuffee KE, Luttgen M, Liu JC. Modifying a sealed tube zinc reduction method for preparation of AMS graphite targets: reducing background and attaining high precision. Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions with Materials and Atoms. 2007;259:320–329. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Sugar and starch concentration data for white pine and red oak
Table S2 Radiocarbon data for white pine and red oak
Table S3 Tissue age, concentrations of extracted nonstructural carbon (NSC), and the radiocarbon (14C) age of extracted NSC, for roots and branches of a white pine and a red oak tree
Table S4 Woody biomass and nonstructural carbon (NSC) content in the stemwood of a white pine and red oak tree
Table S5 Woody biomass and nonstructural carbon (NSC) content in the root system of a white pine and red oak tree.
Table S6 Woody biomass and nonstructural carbon (NSC) content in the branches of a white pine and red oak tree
Methods S1 Uncertainty of radiocarbon measurements.
Methods S2 Allometric scaling from nonstructural carbon (NSC) concentrations to whole-tree budgets, and uncertainty characterization.


