Abstract
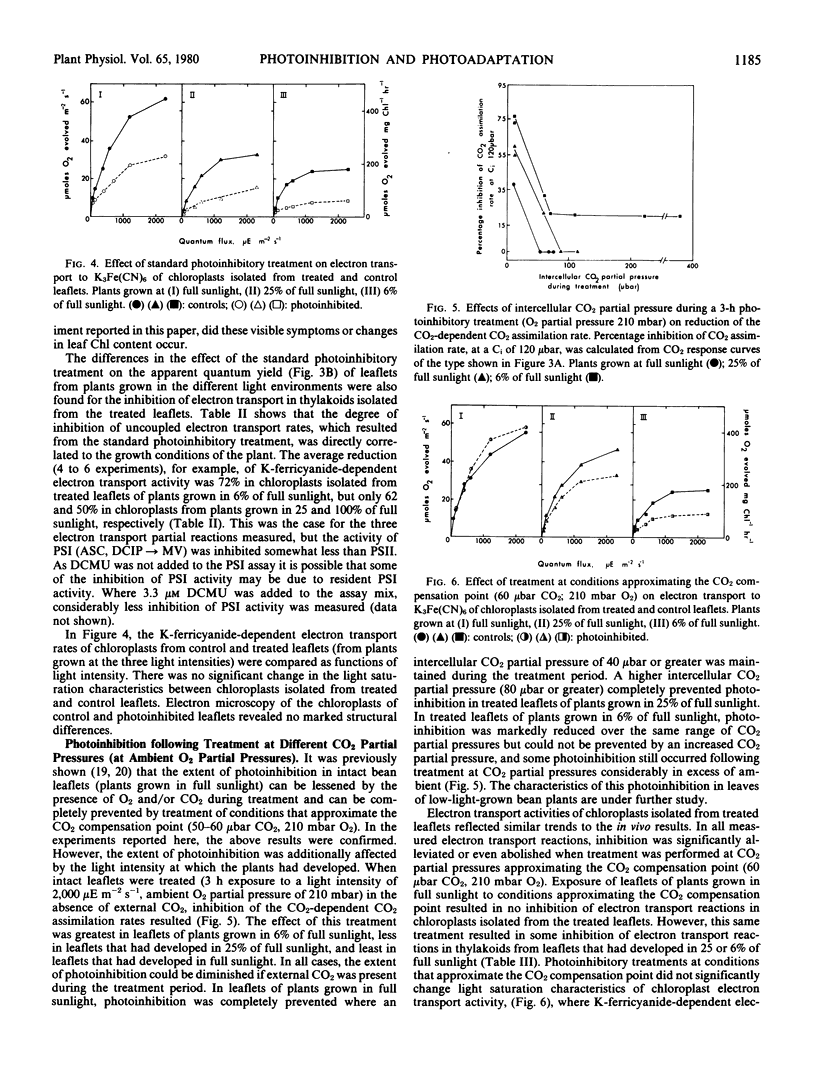
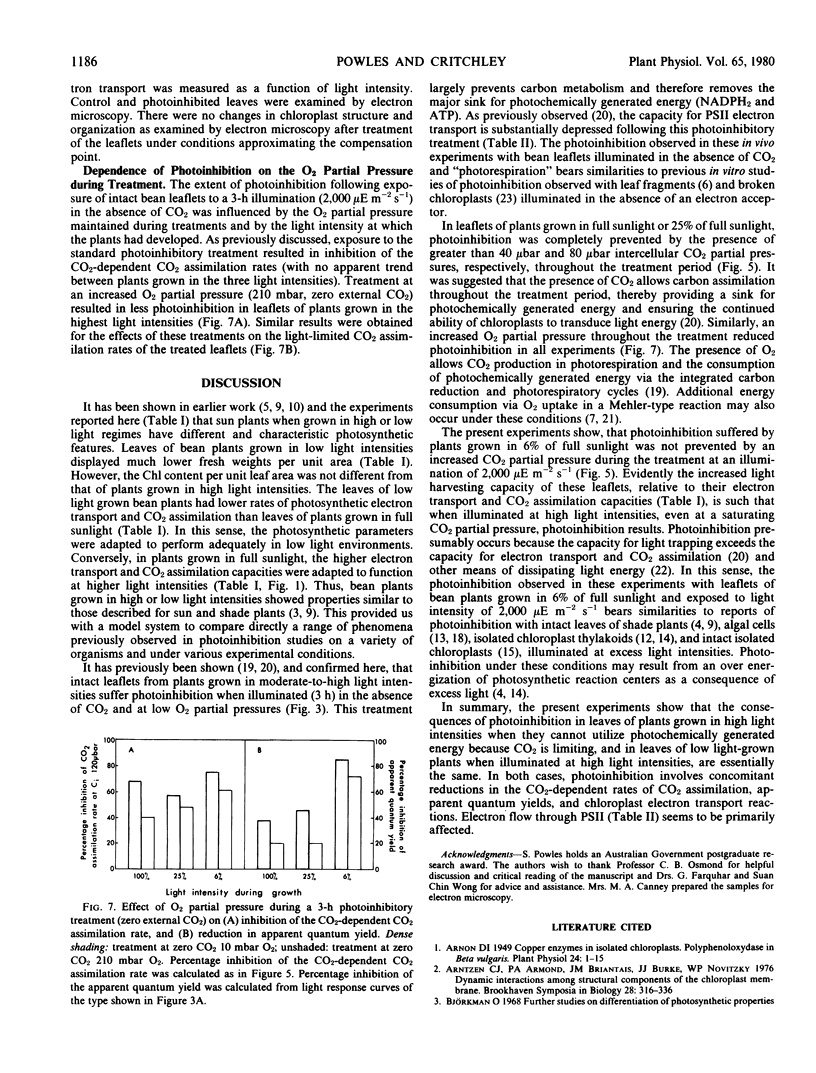
In the study reported here, two different photoinhibitory phenomena were compared within a single plant species. Bean plants were grown in three different light intensities to simulate sun and shade environments. The effects of photoinhibitory treatments on in vivo CO2 assimilation rates and in vitro chloroplast electron transport reactions were investigated and the extent to which carbon metabolism served to prevent photoinhibition was characterized. It was shown that the photoinhibition which follows exposure of intact leaflets of low light-grown bean plants to high light intensity in normal air is essentially similar to that which occurs when leaflets of plants grown in full sunlight are illuminated in the absence of CO2 at low O2 partial pressures.
Full text
PDF
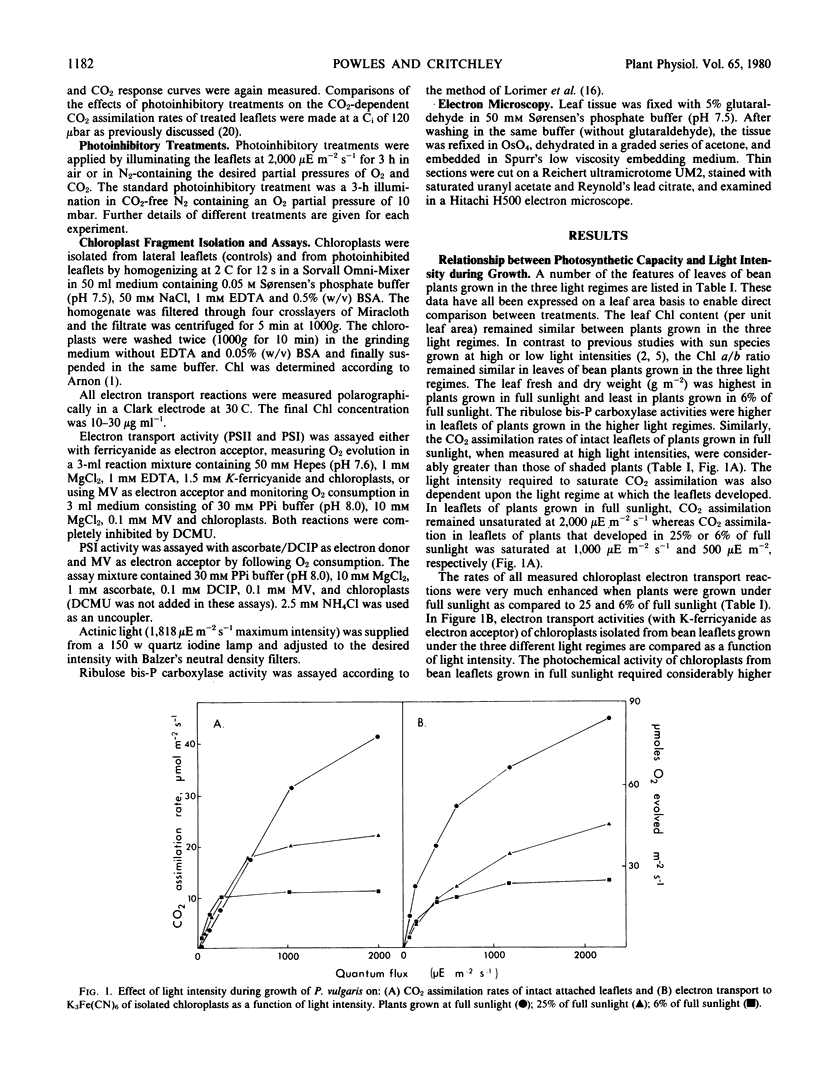
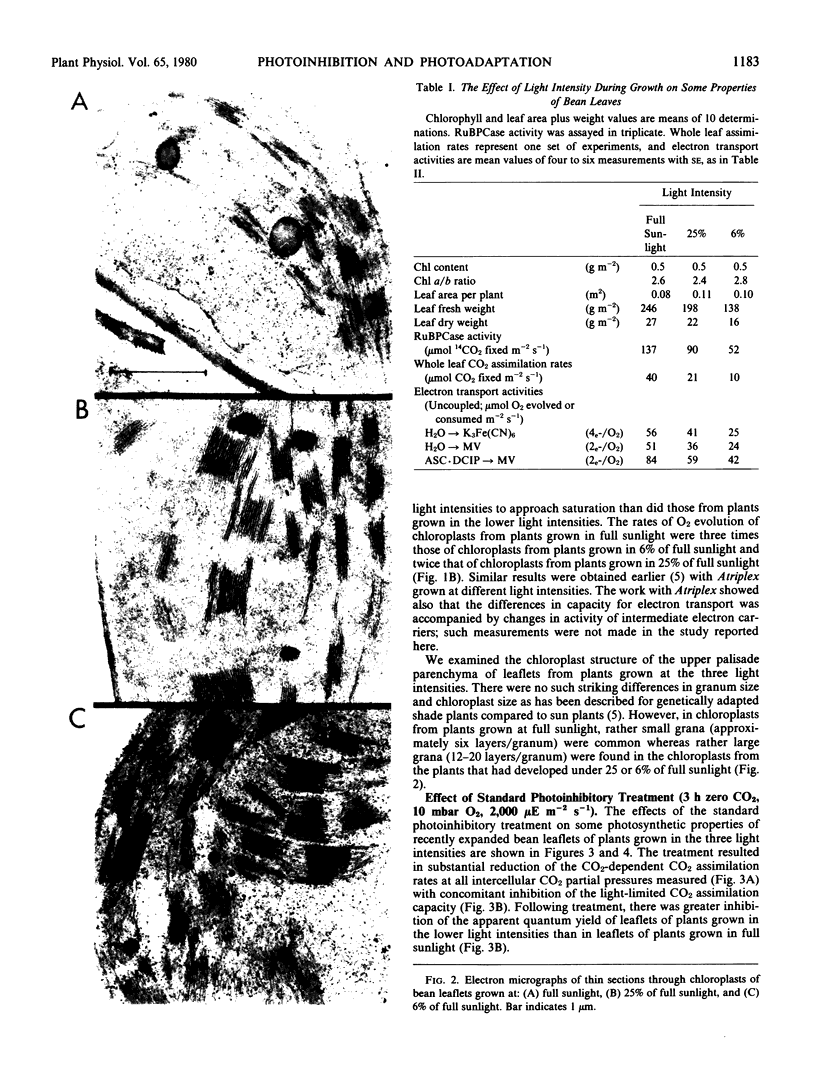
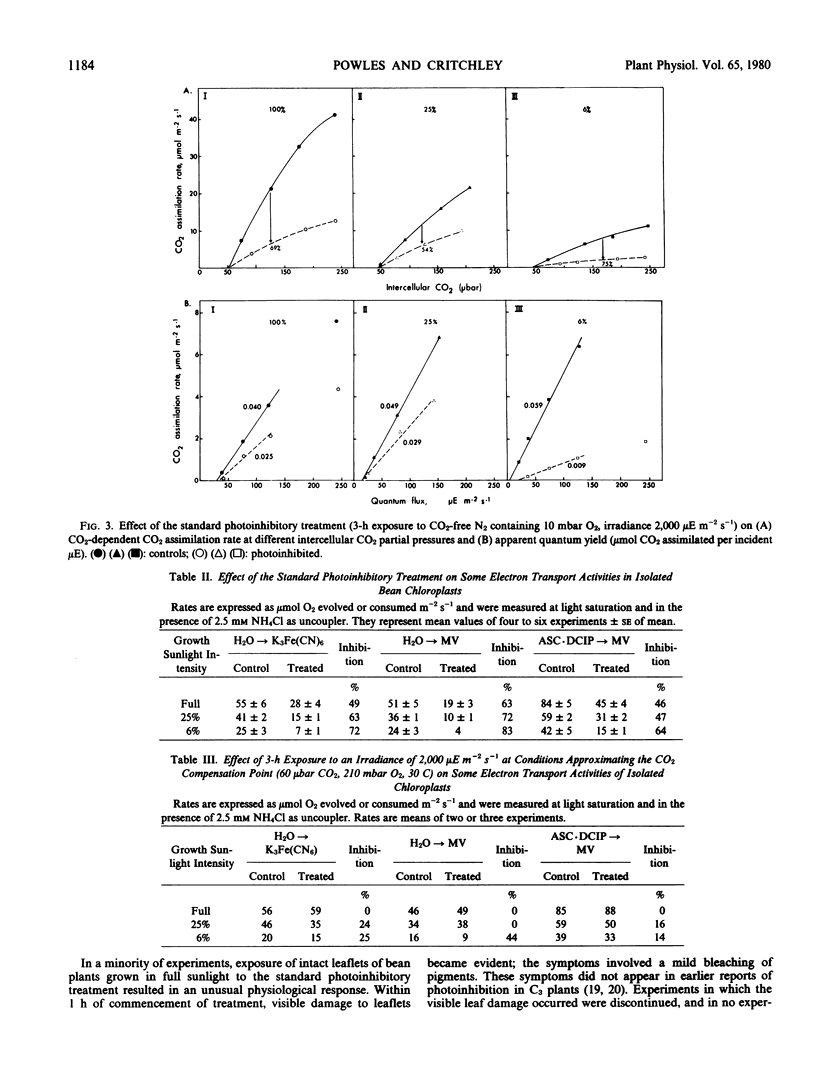

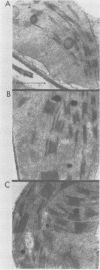
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntzen C. J., Armond P. A., Briantais J. M., Burke J. J., Novitzky W. P. Dynamic interactions among structural components of the chloroplast membrane. Brookhaven Symp Biol. 1976 Jun 7;(28):316–337. [PubMed] [Google Scholar]
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol. 1966 Jun;41(6):1037–1043. doi: 10.1104/pp.41.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Gassner E. B., Rurainski H. J. Photoinhibition of chloroplast reactions. Photochem Photobiol. 1966 Mar;4(2):215–227. doi: 10.1111/j.1751-1097.1965.tb05739.x. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Powles S. B., Osmond C. B. Photoinhibition of intact attached leaves of c(3) plants illuminated in the absence of both carbon dioxide and of photorespiration. Plant Physiol. 1979 Dec;64(6):982–988. doi: 10.1104/pp.64.6.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger G., Wolff C. Further evidence for dissipative energy migration via triplet states in photosynthesis. The protective mechanism of carotenoids in Rhodopseudomonas spheroides chromatophores. Biochim Biophys Acta. 1977 Apr 11;460(1):47–57. doi: 10.1016/0005-2728(77)90150-5. [DOI] [PubMed] [Google Scholar]