Abstract
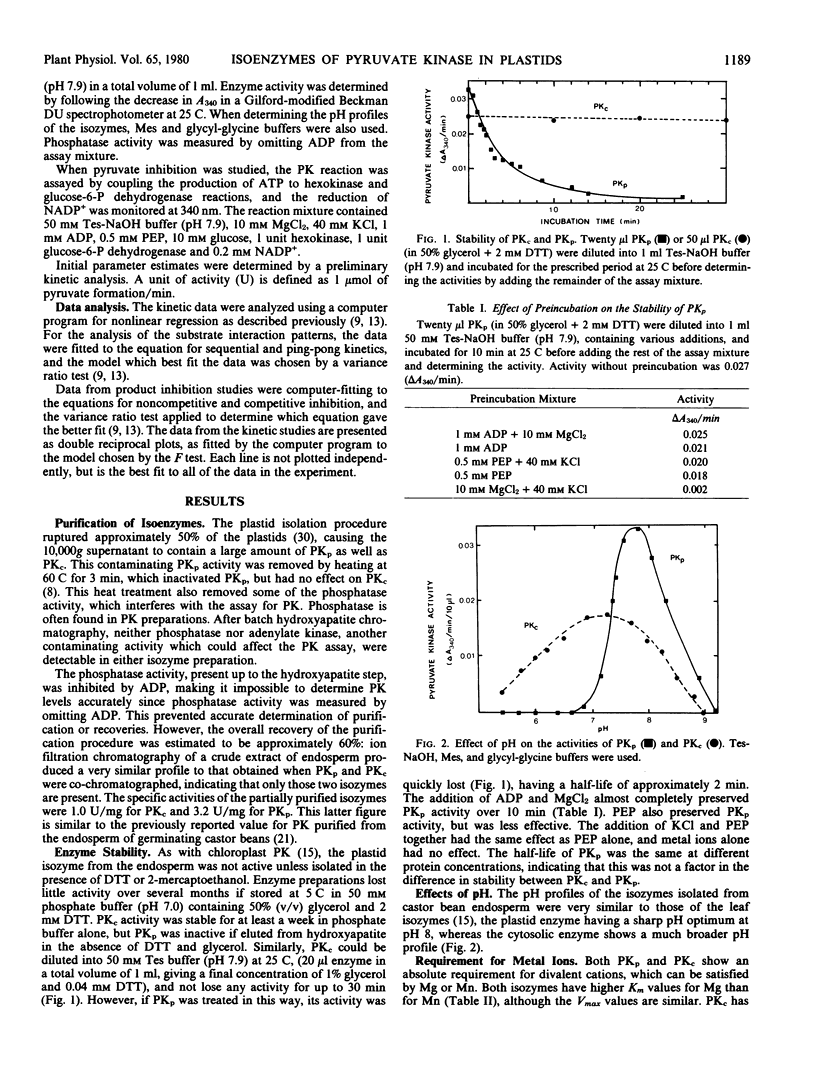
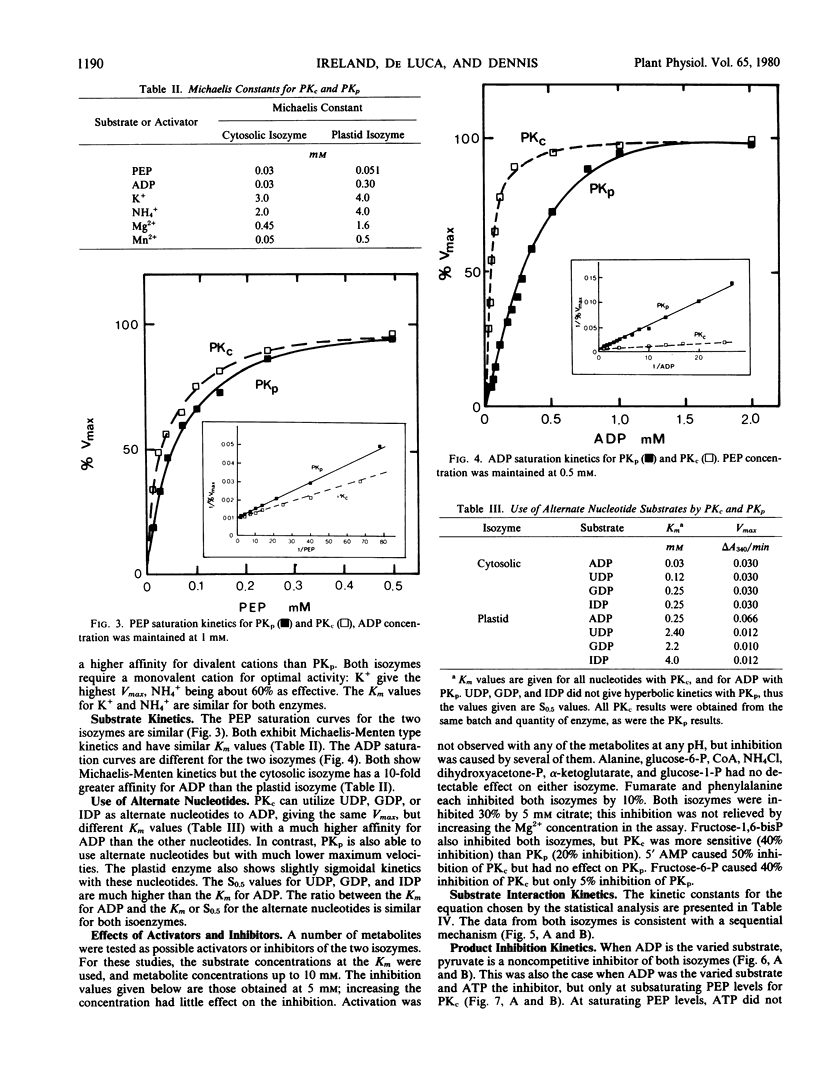
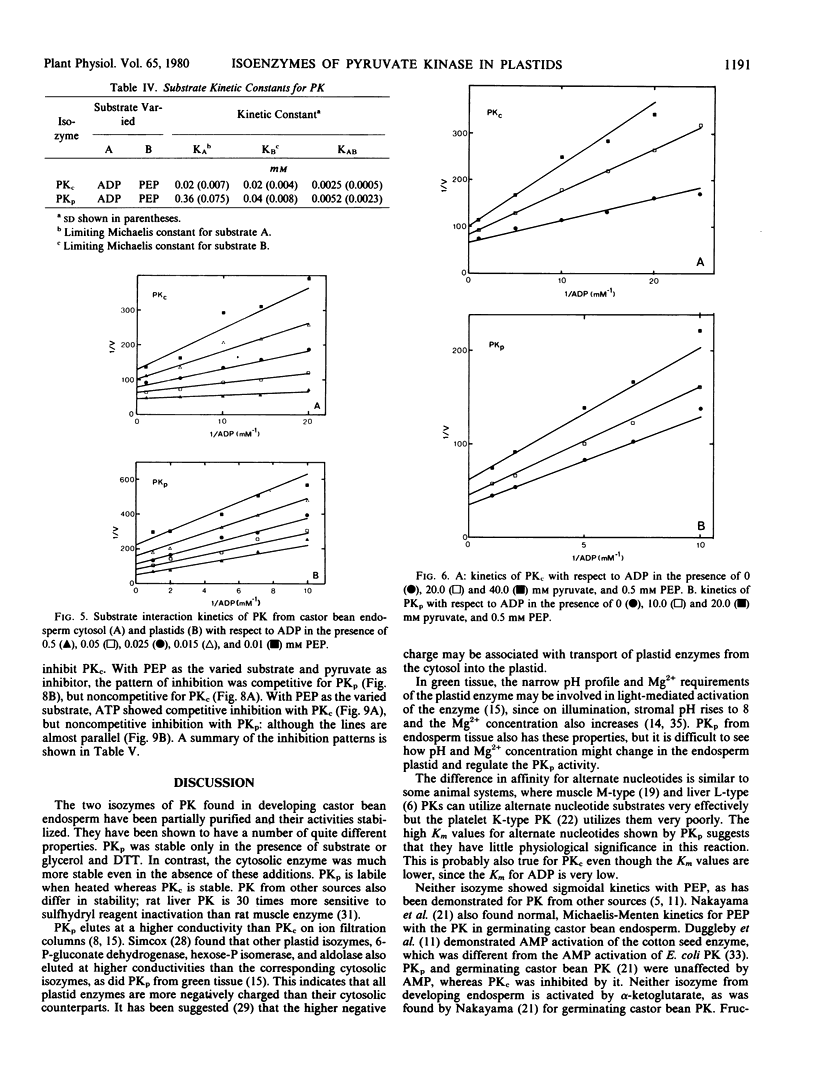
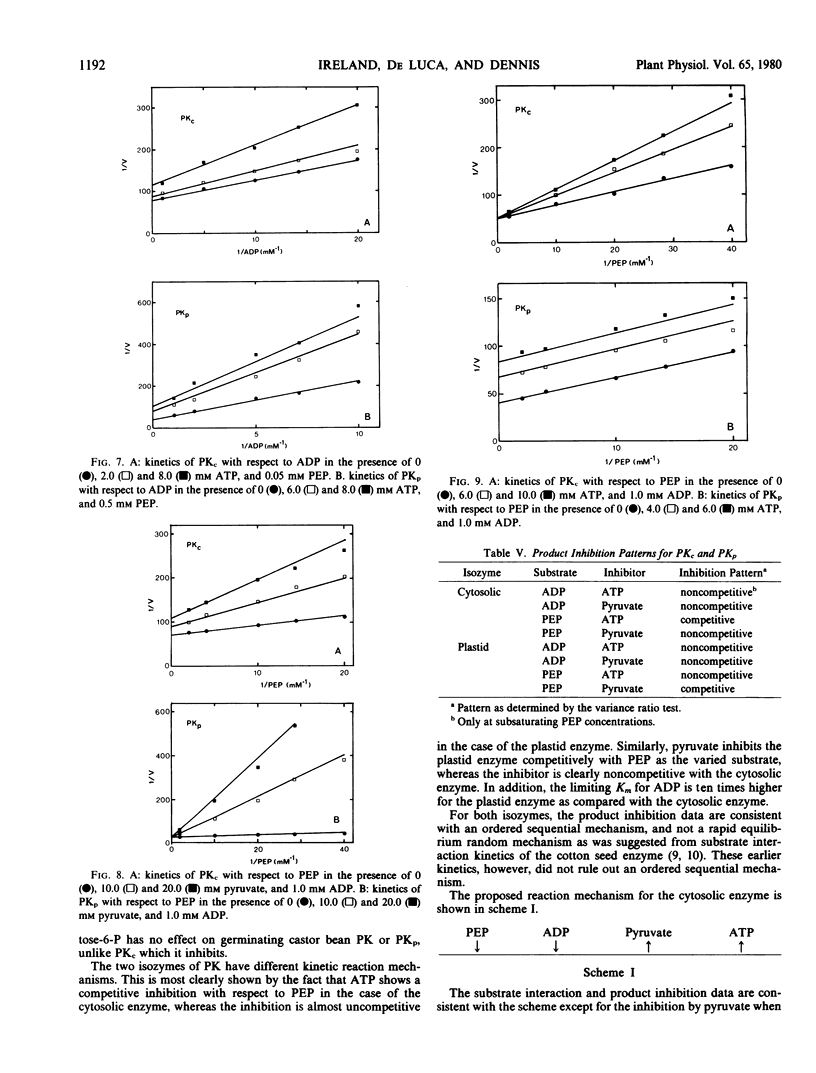
Isozymes of pyruvate kinase (PK) have been isolated from developing castor bean endosperm. One isozyme, PKc, is localized in the cytosol, and the other, PKp, is in the plastid. Both isozymes need monovalent and divalent cations for activity, requirements which can be filled by K+ and Mg2+. Both isozymes are inhibited by citrate, pyruvate, and ATP. PKc has a much broader pH profile than PKp and is also more stable. Both have the same Km (0.05 millimolar) for PEP, but PKp has a 10-fold higher Km (0.3 millimolar) for ADP than PKc (0.03 millimolar). PKc also has a higher affinity for alternate nucleotide substrates than PKp. The two isozymes have different kinetic mechanisms. Both have an ordered sequential mechanism and bind phosphoenolpyruvate before ADP. However, the plastid isozyme releases ATP first, whereas pyruvate is the first product released from the cytosolic enzyme. The properties of the two isozymes are similar to those of their counterparts in green tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Advani V. R. Chloroplast and cytoplasmic enzymes: three distinct isoenzymes associated with the reductive pentose phosphate cycle. Plant Physiol. 1970 May;45(5):583–585. doi: 10.1104/pp.45.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. 3. Pea leaf ribose 5-phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):245–249. doi: 10.1016/0005-2744(71)90052-0. [DOI] [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):237–244. doi: 10.1016/0005-2744(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Pacold I. Chloroplast and Cytoplasmic Enzymes: IV. Pea Leaf Fructose 1,6-Diphosphate Aldolases. Plant Physiol. 1972 Mar;49(3):393–397. doi: 10.1104/pp.49.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Carbonell J., Felíu J. E., Marco R., Sols A. Pyruvate kinase. Classes of regulatory isoenzymes in mammalian tissues. Eur J Biochem. 1973 Aug 1;37(1):148–156. doi: 10.1111/j.1432-1033.1973.tb02969.x. [DOI] [PubMed] [Google Scholar]
- De Luca V., Dennis D. T. Isoenzyme of pyruvate kinase in proplastids from developing castor bean endosperm. Plant Physiol. 1978 Jun;61(6):1037–1039. doi: 10.1104/pp.61.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G., Dennis D. T. Pyruvate kinase, a possible regulatory enzyme in higher plants. Plant Physiol. 1973 Oct;52(4):312–317. doi: 10.1104/pp.52.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G., Dennis D. T. The characterization and regulatory properties of pyruvate kinase from cotton seeds. Arch Biochem Biophys. 1973 Apr;155(2):270–277. doi: 10.1016/0003-9861(73)90115-x. [DOI] [PubMed] [Google Scholar]
- Garland W. J., Dennis D. T. Steady-state kinetics of glutamate dehydrogenase from Pisum sativum L. mitochondria. Arch Biochem Biophys. 1977 Aug;182(2):614–625. doi: 10.1016/0003-9861(77)90542-2. [DOI] [PubMed] [Google Scholar]
- Hind G., Nakatani H. Y., Izawa S. Light-dependent redistribution of ions in suspensions of chloroplast thylakoid membranes. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1484–1488. doi: 10.1073/pnas.71.4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland R. J., Deluca V., Dennis D. T. Isoenzymes of pyruvate kinase in etioplasts and chloroplasts. Plant Physiol. 1979 May;63(5):903–907. doi: 10.1104/pp.63.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Latzko E. Chloroplast phosphofructokinase: I. Proof of phosphofructokinase activity in chloroplasts. Plant Physiol. 1977 Aug;60(2):290–294. doi: 10.1104/pp.60.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard L. H. Gradient sievorptive chromatography. A focusing system for the separation of cellular components. Biochemistry. 1973 Sep 11;12(19):3627–3632. doi: 10.1021/bi00743a009. [DOI] [PubMed] [Google Scholar]
- McQUATE J. T., UTTER M. F. Equilibrium and kinetic studies of the pyruvic kinase reaction. J Biol Chem. 1959 Aug;234(8):2151–2157. [PubMed] [Google Scholar]
- Nishimura J., Hamasaki N., Minakami S. Platelet pyruvate kinase. Two interconvertible forms of the enzyme. Biochim Biophys Acta. 1978 Jan 12;522(1):104–112. doi: 10.1016/0005-2744(78)90326-1. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Shiio I. Regulation of the TCA and glyoxylate cycles in Brevibacterium flavum. II. Regulation of phosphoenolpyruvate carboxylase and pyruvate kinase. J Biochem. 1969 Sep;66(3):297–311. doi: 10.1093/oxfordjournals.jbchem.a129148. [DOI] [PubMed] [Google Scholar]
- Peterson J. B., Evans H. J. Properties of pyruvate kinase from soybean nodule cytosol. Plant Physiol. 1978 Jun;61(6):909–914. doi: 10.1104/pp.61.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman K. M., Krall A. R. A kinetic study of nucleotide interactions with pyruvate kinase. Biochemistry. 1965 Dec;4(12):2809–2814. doi: 10.1021/bi00888a035. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch Biochem Biophys. 1973 Jan;154(1):438–448. doi: 10.1016/0003-9861(73)90077-5. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A. Two isoenzymes of glucosephosphate isomerase from spinach leaves and their intracellular compartmentation. Eur J Biochem. 1974 Jun 1;45(1):77–82. doi: 10.1111/j.1432-1033.1974.tb03531.x. [DOI] [PubMed] [Google Scholar]
- Simcox P. D., Dennis D. T. 6-Phosphogluconate Dehydrogenase Isoenzymes from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1978 Aug;62(2):287–290. doi: 10.1104/pp.62.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox P. D., Dennis D. T. Isoenzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinis communis L. Plant Physiol. 1978 Jun;61(6):871–877. doi: 10.1104/pp.61.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox P. D., Reid E. E., Canvin D. T., Dennis D. T. Enzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1977 Jun;59(6):1128–1132. doi: 10.1104/pp.59.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Sue F., Morimura H. Crystallization, characterization and metabolic regulation of two types of pyruvate kinase isolated from rat tissues. J Biochem. 1967 Jul;62(1):71–91. doi: 10.1093/oxfordjournals.jbchem.a128639. [DOI] [PubMed] [Google Scholar]
- Tomlinson J. D., Turner J. F. Pyruvate kinase of higher plants. Biochim Biophys Acta. 1973 Nov 2;329(1):128–139. doi: 10.1016/0304-4165(73)90015-9. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Mort J. S., Sanwal B. D. The control of pyruvate kinase of Escherichia coli. Binding of substrate and allosteric effectors to the enzyme activated by fructose 1,6-bisphosphate. Biochemistry. 1976 Jan 27;15(2):277–282. doi: 10.1021/bi00647a006. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Rayman M. K., Sanwal B. D. The control of pyruvate kinases of Escherichia coli. II. Effectors and regulatory properties of the enzyme activated by ribose 5-phosphate. Can J Biochem. 1975 Apr;53(4):444–454. doi: 10.1139/o75-061. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]



