Abstract
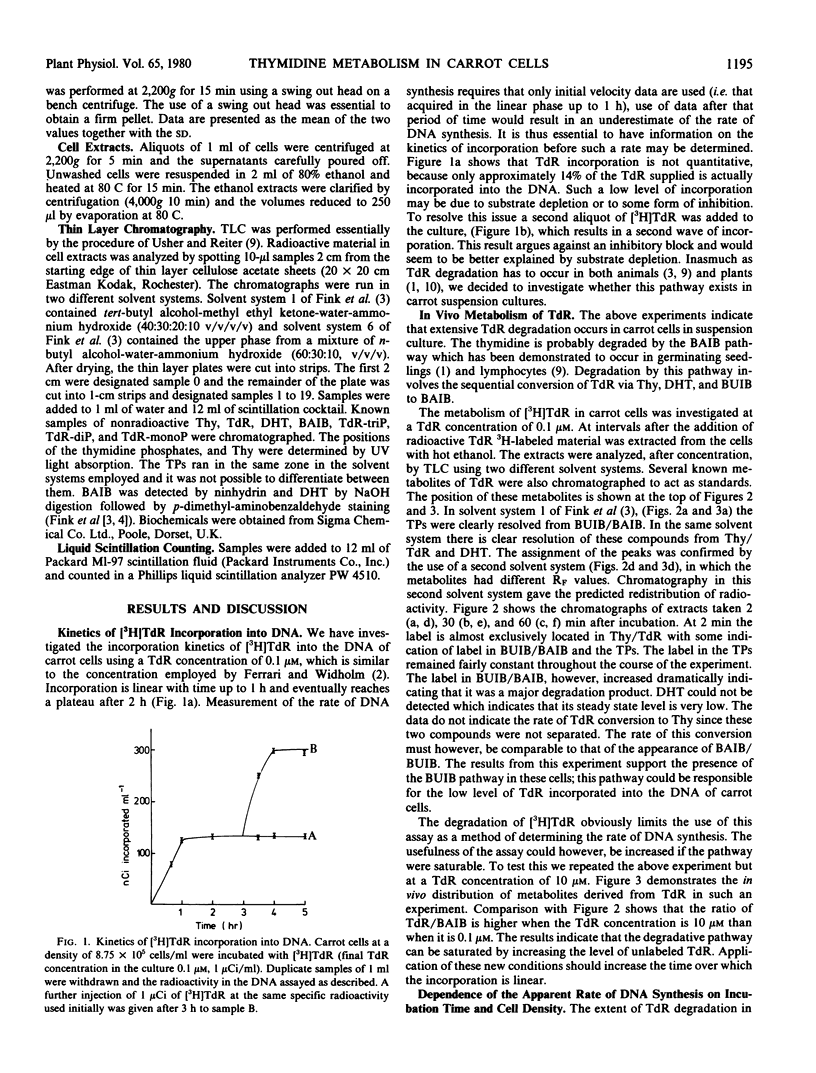
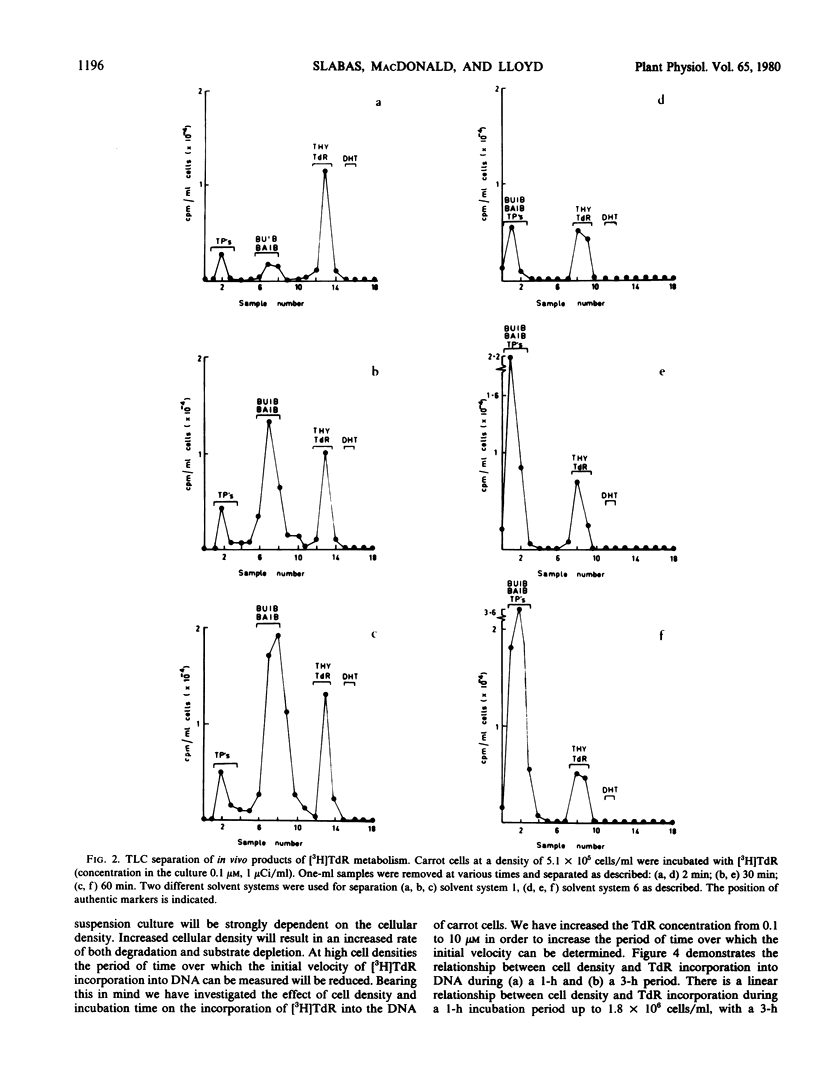
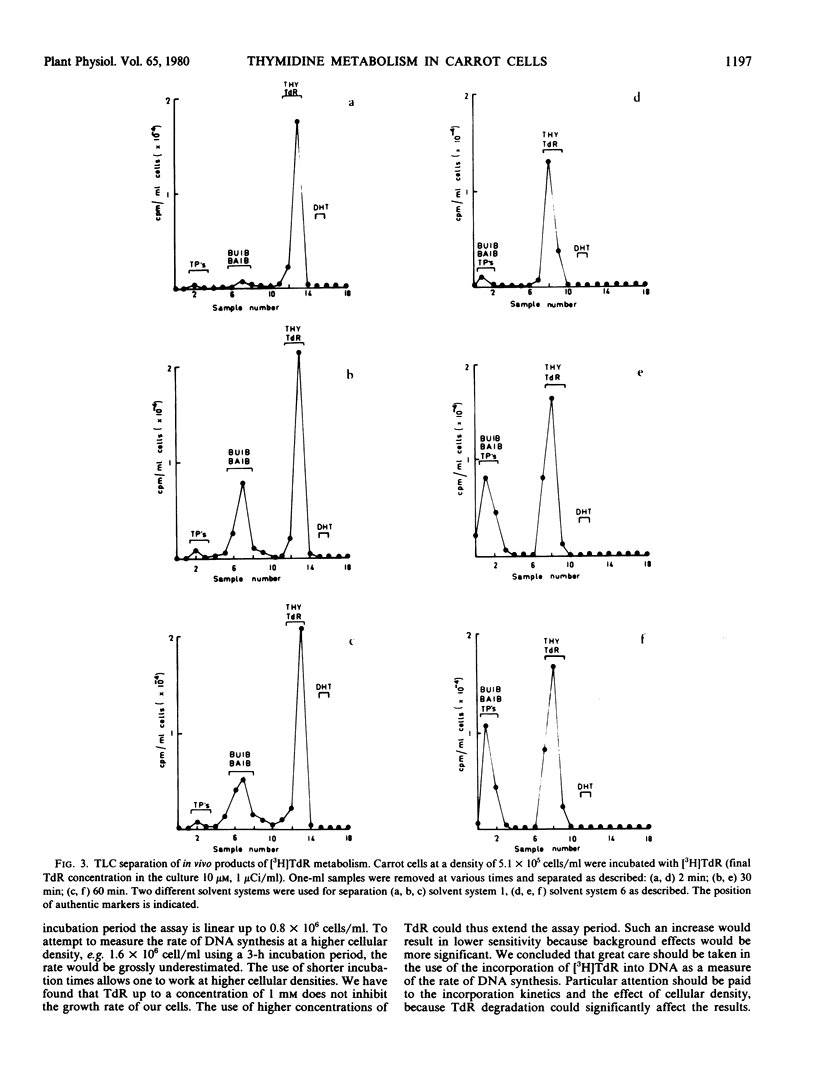
The kinetics of [3H]thymidine incorporation into the DNA of carrot suspension cultures were investigated. At a thymidine concentration of 0.1 micromolar, incorporation into DNA is not quantitative but ceases after only 14% of the thymidine has been incorporated. Thymidine incorporation into DNA is resumed following addition of a second aliquot of thymidine, which is consistent with substrate depletion. In vivo tracer experiments indicate that this may be due to a catabolic route for converting thymidine to β-aminoisobutyric acid. Bearing these observations in mind, conditions for determining the rate of DNA synthesis using [3H]thymidine incorporation have been investigated. It is concluded that by increasing the thymidine concentration to 10 micromolar the assay period may be increased, by reducing the influence of the degradative pathway, and that cell density and incubation time are critical factors in establishing a valid measure of the rate of DNA synthesis using this method.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans W. R., Axelrod B. Pyrimidine metabolism in germinating seedlings. Plant Physiol. 1961 Jan;36(1):9–13. doi: 10.1104/pp.36.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK K., CLINE R. E., HENDERSON R. B., FINK R. M. Metabolism of thymine (methyl-C14 or -2-C14) by rat liver in vitro. J Biol Chem. 1956 Jul;221(1):425–433. [PubMed] [Google Scholar]
- FINK R. M., MCGAUGHEY C., CLINE R. E., FINK K. Metabolism of intermediate pyrimidine reduction products in vitro. J Biol Chem. 1956 Jan;218(1):1–7. [PubMed] [Google Scholar]
- Ferrari T. E., Widholm J. M. A simple, rapid, and sensitive method for estimation of DNA, RNA, and protein synthesis in carrot cell cultures. Anal Biochem. 1973 Dec;56(2):346–352. doi: 10.1016/0003-2697(73)90200-5. [DOI] [PubMed] [Google Scholar]
- Harland J., Jackson J. F., Yeoman M. M. Changes in some enzymes involved in DNA biosynthesis following induction of division in cultured plant cells. J Cell Sci. 1973 Jul;13(1):121–138. doi: 10.1242/jcs.13.1.121. [DOI] [PubMed] [Google Scholar]
- Sung Z. R. Turbidimetric measurement of plant cell culture growth. Plant Physiol. 1976 Mar;57(3):460–462. doi: 10.1104/pp.57.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher D. C., Reiter H. Catabolism of thymidine during the lymphocyte cell cycle. Cell. 1977 Oct;12(2):365–370. doi: 10.1016/0092-8674(77)90112-x. [DOI] [PubMed] [Google Scholar]



