Abstract
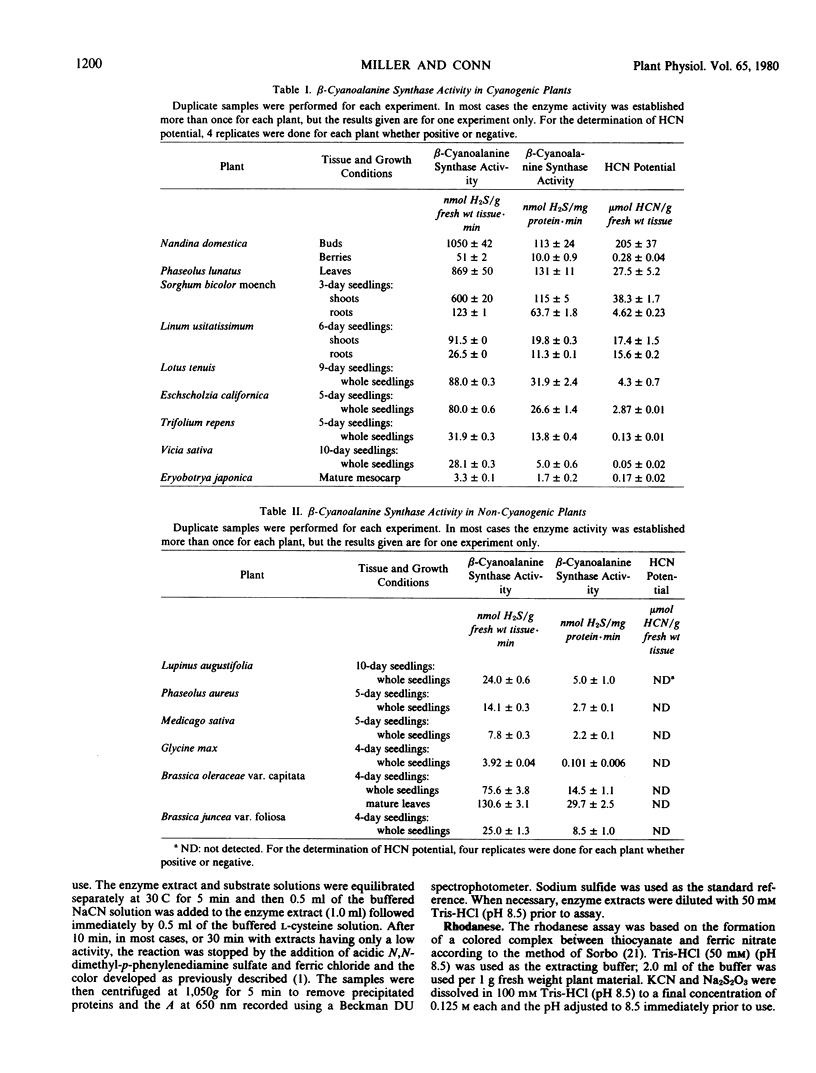
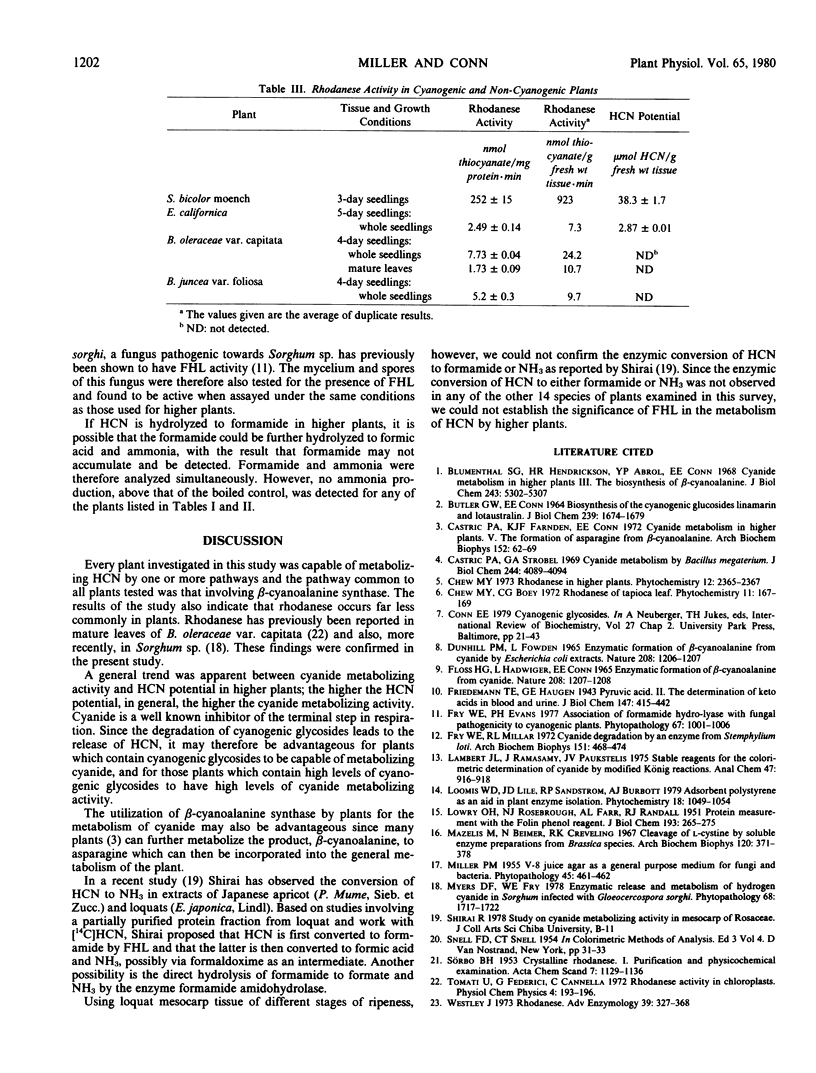
A survey has been made of the occurrence and distribution of three enzymes which metabolize cyanide in a variety of higher plants including both cyanogenic and non-cyanogenic species. The enzymes investigated were β-cyanoalanine synthase, rhodanese and formamide hydrolyase. β-Cyanoalanine synthase was found to be present in every higher plant tested whereas rhodanese was found to occur far less commonly in plants. Formamide hydrolyase activity was not detected in any of the higher plants tested.
In addition, quantitative analyses have been made of the potential hydrogen cyanide content of each plant investigated. A general trend was apparent between the hydrogen cyanide potential and cyanide metabolizing activity, in that the higher the hydrogen cyanide potential, in general, the higher the cyanide metabolizing activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER G. W., CONN E. E. BIOSYNTHESIS OF THE CYANOGENIC GLUCOSIDES LINAMARIN AND LOTAUSTRALIN. I. LABELING STUDIES IN VIVO WITH LINUM USITATISSIMUM. J Biol Chem. 1964 Jun;239:1674–1679. [PubMed] [Google Scholar]
- Blumenthal S. G., Hendrickson H. R., Abrol Y. P., Conn E. E. Cyanide metabolism in higher plants. 3. The biosynthesis of beta-cyanolanine. J Biol Chem. 1968 Oct 25;243(20):5302–5307. [PubMed] [Google Scholar]
- Castric P. A., Farnden K. J., Conn E. E. Cyanide metabolism in higher plants. V. The formation of asparagine from -cyanoalanine. Arch Biochem Biophys. 1972 Sep;152(1):62–69. doi: 10.1016/0003-9861(72)90193-2. [DOI] [PubMed] [Google Scholar]
- Castric P. A., Strobel G. A. Cyanide metabolism by Bacillus megaterium. J Biol Chem. 1969 Aug 10;244(15):4089–4094. [PubMed] [Google Scholar]
- Dunnill P. M., Fowden L. Enzymatic formation of beta-cyanoalanine from cyanide by Escherichia coli extracts. Nature. 1965 Dec 18;208(5016):1206–1207. doi: 10.1038/2081206a0. [DOI] [PubMed] [Google Scholar]
- Floss H. G., Hadwiger L., Conn E. E. Enzymatic formation of beta-cyanoalanine from cyanide. Nature. 1965 Dec 18;208(5016):1207–1208. doi: 10.1038/2081207a0. [DOI] [PubMed] [Google Scholar]
- Fry W. E., Millar R. L. Cyanide degradion by an enzyme from Stemphylium loti. Arch Biochem Biophys. 1972 Aug;151(2):468–474. doi: 10.1016/0003-9861(72)90523-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mazelis M., Beimer N., Creveling R. K. Cleavage of L-cystine by soluble enzyme preparations from Brassica species. Arch Biochem Biophys. 1967 May;120(2):371–378. doi: 10.1016/0003-9861(67)90253-6. [DOI] [PubMed] [Google Scholar]
- Tomati U., Federici G., Cannella C. Rhodanese activity in chloroplasts. Physiol Chem Phys. 1972;4(2):193–196. [PubMed] [Google Scholar]
- Westley J. Rhodanese. Adv Enzymol Relat Areas Mol Biol. 1973;39:327–368. doi: 10.1002/9780470122846.ch5. [DOI] [PubMed] [Google Scholar]



