Abstract
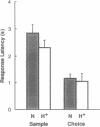
Normal monkeys and monkeys with lesions of the hippocampal formation and adjacent cortex (the H+ lesion) were trained on the delayed nonmatching to sample (DNMS) task with a delay of 0.5 s between the sample and the choice. The animals with H+ lesions learned the task normally at this short delay and also exhibited the same pattern of response latencies as normal monkeys. This finding contrasts with previous observations that initial learning of the DNMS task with delays of 8-10 s is impaired after H+ lesions. The absence of an impairment at a delay of 0.5 s indicates that the H+ lesion does not affect short-term memory. In contrast, when monkeys with H+ lesions were tested at longer delays (> 30 s), an impairment was observed. This selective impairment occurred when the delays were presented sequentially (from 0.5 s to 10 min) and also when delays were presented in a mixed order (1 s, 1 min, and 10 min). The data indicate that the H+ lesion produces a selective impairment in long-term memory, in the absence of a detectable deficit in short-term memory or perception. Accordingly, the findings confirm the long-standing idea, based primarily on studies of humans, that short-term memory is independent of medial temporal lobe function. The findings thereby establish an important parallel between memory impairment in monkeys and humans and provide additional support for the validity of the animal model of human amnesia in the monkey.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Royo P., Zola-Morgan S., Squire L. R. Impairment of long-term memory and sparing of short-term memory in monkeys with medial temporal lobe lesions: a response to Ringo. Behav Brain Res. 1992 Nov 30;52(1):1–5. doi: 10.1016/s0166-4328(05)80319-5. [DOI] [PubMed] [Google Scholar]
- Cave C. B., Squire L. R. Intact and long-lasting repetition priming in amnesia. J Exp Psychol Learn Mem Cogn. 1992 May;18(3):509–520. doi: 10.1037//0278-7393.18.3.509. [DOI] [PubMed] [Google Scholar]
- Cho Y. H., Beracochea D., Jaffard R. Extended temporal gradient for the retrograde and anterograde amnesia produced by ibotenate entorhinal cortex lesions in mice. J Neurosci. 1993 Apr;13(4):1759–1766. doi: 10.1523/JNEUROSCI.13-04-01759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A. R. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989 Nov;33(1-2):25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Hartley L. H., Rodger R., Nicolosi R. J., Hartley T. Blood pressure values in Macaca fascicularis. J Med Primatol. 1984;13(4):183–189. [PubMed] [Google Scholar]
- Kesner R. P., Novak J. M. Serial position curve in rats: role of the dorsal hippocampus. Science. 1982 Oct 8;218(4568):173–175. doi: 10.1126/science.7123228. [DOI] [PubMed] [Google Scholar]
- Kim J. J., Fanselow M. S. Modality-specific retrograde amnesia of fear. Science. 1992 May 1;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Milner P. M. A cell assembly theory of hippocampal amnesia. Neuropsychologia. 1989;27(1):23–30. doi: 10.1016/0028-3932(89)90087-0. [DOI] [PubMed] [Google Scholar]
- Murray E. A., Gaffan D., Mishkin M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J Neurosci. 1993 Oct;13(10):4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E. A., Mishkin M. Severe tactual as well as visual memory deficits follow combined removal of the amygdala and hippocampus in monkeys. J Neurosci. 1984 Oct;4(10):2565–2580. doi: 10.1523/JNEUROSCI.04-10-02565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara A. H., McGaugh J. L. Muscimol infused into the medial septal area impairs long-term memory but not short-term memory in inhibitory avoidance, water maze place learning and rewarded alternation tasks. Brain Res. 1992 Sep 18;591(1):54–61. doi: 10.1016/0006-8993(92)90977-h. [DOI] [PubMed] [Google Scholar]
- Overman W. H., Ormsby G., Mishkin M. Picture recognition vs. picture discrimination learning in monkeys with medial temporal removals. Exp Brain Res. 1990;79(1):18–24. doi: 10.1007/BF00228870. [DOI] [PubMed] [Google Scholar]
- Ringo J. L. Memory decays at the same rate in macaques with and without brain lesions when expressed in d' or arcsine terms. Behav Brain Res. 1991 Feb 28;42(2):123–134. doi: 10.1016/s0166-4328(05)80003-8. [DOI] [PubMed] [Google Scholar]
- SCOVILLE W. B., MILNER B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957 Feb;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L. R., Zola-Morgan S. The medial temporal lobe memory system. Science. 1991 Sep 20;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Suzuki W. A., Zola-Morgan S., Squire L. R., Amaral D. G. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. J Neurosci. 1993 Jun;13(6):2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo J., Cowan W. M. A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis). J Comp Neurol. 1984 Jan 10;222(2):265–300. doi: 10.1002/cne.902220208. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., DiScenna P. The hippocampal memory indexing theory. Behav Neurosci. 1986 Apr;100(2):147–154. doi: 10.1037//0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- Winocur G. Anterograde and retrograde amnesia in rats with dorsal hippocampal or dorsomedial thalamic lesions. Behav Brain Res. 1990 May 7;38(2):145–154. doi: 10.1016/0166-4328(90)90012-4. [DOI] [PubMed] [Google Scholar]
- Winocur G. The hippocampus and thalamus: their roles in short- and long-term memory and the effects of interference. Behav Brain Res. 1985 Aug;16(2-3):135–152. doi: 10.1016/0166-4328(85)90088-9. [DOI] [PubMed] [Google Scholar]
- Wright A. A., Santiago H. C., Sands S. F., Kendrick D. F., Cook R. G. Memory processing of serial lists by pigeons, monkeys, and people. Science. 1985 Jul 19;229(4710):287–289. doi: 10.1126/science.9304205. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S. M., Squire L. R. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science. 1990 Oct 12;250(4978):288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G. Lesions of the hippocampal formation but not lesions of the fornix or the mammillary nuclei produce long-lasting memory impairment in monkeys. J Neurosci. 1989 Mar;9(3):898–913. doi: 10.1523/JNEUROSCI.09-03-00898.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G., Suzuki W. A. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989 Dec;9(12):4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R. The neuropsychology of memory. Parallel findings in humans and nonhuman primates. Ann N Y Acad Sci. 1990;608:434–456. doi: 10.1111/j.1749-6632.1990.tb48905.x. [DOI] [PubMed] [Google Scholar]