Abstract
The IL-1 receptor antagonist (IL-1ra), encoded by the Il1rn gene, is an endogenous antagonist of the IL-1 receptor. Studies ofIl1rn knockout (KO) and wild type (WT) mice identified differences in several ethanol-related behaviors, some of which may be mediated by GABAergic transmission in the central nucleus of the amygdala (CeA). In this study we examined phasic (both evoked and spontaneous) and tonic GABAergic transmission in the CeA of Il1rn KO and WT mice and the ethanol sensitivity of these GABAergic synapses. The mean amplitude of baseline evoked GABAA-inhibitory postsynaptic potentials (IPSPs), and the baseline frequency of spontaneous GABAA-inhibitory postsynaptic currents (sIPSCs), but not the frequency of miniature GABAA-IPSCs (mIPSCs), were significantly increased in KO compared to WT mice, indicating enhanced presynaptic action potential-dependent GABA release in the CeA of KO mice. In KO mice, we also found a cell-type specific switch in the ongoing tonic GABAA receptor conductance such that the tonic conductance in low threshold bursting (LTB) neurons is lost and a tonic conductance in late spiking (LS) neurons appears. Notably, the ethanol-induced facilitation of evoked and spontaneous GABA release was lost in most of the CeA neurons from KO compared to WT mice. Ethanol superfusion increased the sIPSC rise and decay times in both KO and WT mice, suggesting ethanol-induced postsynaptic effects. The pretreatment of CeA slices with exogenous IL-1ra (Kineret; 100 ng/ml) returned sIPSC frequency in KO mice to the levels found in WT. Importantly, Kineret also restored ethanol-induced potentiation of the sIPSC frequency in the KO mice. These results show that IL-1ra regulates baseline GABAergic transmission in the CeA and is critical for the ethanol effects at these synapses.
Keywords: Il1rn knockout mice, CeA, GABAA, IPSCs, Kineret
1. Introduction
The interleukin 1 (IL-1) family is a group of 11 cytokines, that induce a complex network of cytokines to initiate and regulate inflammatory responses (Dinarello, 2011). The proinflammatory activities of cytokines IL-1α and IL-1β are regulated by an endogenous antagonist (IL-1ra), an IL-1 receptor type 1 (IL-1R1), and a decoy receptor (IL-1R2). Specifically, IL-1ra competes with IL-1 for binding sites on IL-1R1 and thus prevents activation of downstream signaling (Garlanda et al., 2013; Krumm et al., 2014).
Initiating intracellular signaling via the IL-1R1 system is a multistep process involving: IL-1α or IL-1β binding to the extracellular domain of IL-1R1, recruitment of accessory proteins (e.g., the co-receptor IL-1R1 accessory protein (IL-1RAcP)), formation of a receptor heterodimeric complex (comprised of IL-1α or IL-1β, IL-1RI, and IL-1RAcP), and assemblage of intracellular adaptor proteins. This leads to the activation of many intracellular signaling pathways and transcription factors, such as NF-κB, c-Jun N-terminal kinase, and p38 MAPK (Cohen, 2014).
Gene expression analyses showed alterations in immune/inflammatory response pathways, including the IL-1/IL-1R system, that were associated with a genetic predisposition to high alcohol consumption in mice (Mulligan et al., 2006). Behavioral studies also suggest the involvement of some of these genes in alcohol drinking and preference (Blednov et al., 2012). Modulation of GABAA receptors has been shown to alter many ethanol behaviors (Blednov et al., 2013; Blednov et al., 2003; Boehm et al., 2004), and GABAergic transmission in the CeA plays a critical role in a variety of alcohol-related behaviors elicited by acute and chronic ethanol (Koob and Volkow, 2010; Roberto et al., 2012). Therefore, we hypothesized that disruption of IL-1R1 signaling, by deletion of its negative regulator Il1rn, leads to a perturbation of GABAergic neurotransmission in brain regions involved in alcohol responses. In this study, we investigated electro physiologically basal GABAergic transmission and ethanol sensitivity inIl1rn KO and WT mice in the central nucleus of the amygdala (CeA), a brain region critical for alcohol-related behaviors and neuroadaptative mechanisms associated with alcohol dependence(Roberto et al., 2012). Here we show that both evoked and spontaneous GABAergic transmission are significantly increased in Il1rnKO compared to WT mice, and there is a difference in the cell-type specific tonic GABAA receptor conductance. Acute ethanol sensitivity of GABAergic responses is also altered in Il1rnKO compared to WT mice, and some of the Il1rnKO cellular phenotypes are rescued by application of exogenous IL-1ra(Kineret).
2. Materials and methods
2.1. Animal treatment
We obtained male Il1rn KO (n=32) and wild type (WT; n=31) mice from the Animal Resources Center at The University of Texas at Austin (see companion paper by Blednov et al. for details), and housed them in a temperature- and humidity-controlled room on a 12-hour light/dark cycle (lights on at 6:00 a.m.) with food and water available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the Institutional Animal Care and Use Committee policies of The Scripps Research Institute and The University of Texas at Austin.
2.2. Slice preparation
We anesthetized mice (3–6 months; weight: WT, 27.3 ± 0.6 g; Il1rn KO, 23.0 ± 0.4 g) with 3–5% isoflurane and decapitated and quickly removed the brains and placed them in ice-cold oxygenated high-sucrose cutting solution gassed with 95% O2 and 5% CO2. Coronal slices (300 μM) containing the CeA were made using a Leica 1000S vibrotome cutter (Campden, Lafayette, IN). The slices were then incubated in a gassed N-methyl-D-glucamine (NMDG)-containing recovery solution (in mM: 93 NMDG, 93 HCl, 2.5 KCl, 1.2 NaH2PO4,30 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10 MgSO4.7H2O, 0.5 CaCl2.2H2O) for 15 minutes at 31°C followed by incubation in gassed artificial cerebrospinal fluid (aCSF, in mM: 130 NaCl, 3.5 KCl, 1.25 NaH2PO4.H2O, 1.5 MgSO4.7H2O, 2.0 CaCl2.2H2O, 24 NaHCO3, and 10 glucose) for 1 hour at room temperature. We applied ethanol (44 mM in aCSF) directly onto the slice, and took measurements before ethanol (baseline), during its superfusion (10–15 minutes), and following washout (20–30 minutes). Since Kineret’s ability to pass the blood-brain barrier is limited (Gutierrez et al., 1994), we pretreated the CeA slices with Kineret (100 ng/ml in aCSF; (Clark et al., 2008))for 30–90 minutes prior to electrophysiological recordings(Fox et al., 2010; Galea et al., 2011).
2.3. Intracellular recording of evoked responses
We recorded from the medial subdivision of the CeA with sharp micropipettes filled with 3M KCl using bridge current-clamp mode (Cruz et al., 2012; Haubensak et al., 2010; Roberto et al., 2004). Most neurons were held near their resting membrane potential (RMP). We acquired data with an Axoclamp-2A preamplifier (Molecular Devices, Foster City, CA) and stored them for later analysis using Clampfit software (Molecular Devices). We evoked pharmacologically-isolated GABAA receptor-mediated inhibitory postsynaptic potentials (IPSPs) by stimulating locally within the CeA through a bipolar stimulating electrode while superfusing the slices with the glutamate receptor blockers, 6,7-dinitroquinoxaline-2, 3-dione (DNQX; 20 μM) and DL-2-amino-5-phosphonovalerate (DL-AP5; 30 μM), and the GABAB receptor antagonist (CGP 55845A; 1 μM). At the end of some recordings, we superfused either 30μM bicuculline or 50μM picrotoxin to confirm the GABAA receptor specificity of the IPSPs. To determine the synaptic response parameters for each cell, we followed an input-output (I-O) protocol (Roberto et al., 2003; Roberto et al., 2004) consisting of a range of five current stimulations (50–250 μA; 0.125 Hz), starting at the threshold current required to elicit an IPSP and ending with the strength required to elicit maximum amplitude. These stimulus strengths were maintained for all I-O protocols throughout the entire duration of the experiment. To determine changes in synaptic response, we calculated the IPSP amplitude with the stimulus strength adjusted such that the amplitude of the IPSP was 50% of maximal determined from the I-O relationship. Paired pulse facilitation (PPF) in each neuron was examined by using paired stimuli at 50- and 100-msec inter-stimulus intervals(Roberto et al., 2004). The PPF ratio was calculated as the second IPSP amplitude divided by the first IPSP amplitude.
2.4. Whole-cell patch-clamp recording
We performed whole-cell patch-clamp recording in the voltage clamp mode as described previously (Bajo et al., 2011; Herman et al., 2013). We used infrared/DIC visualization (Dodt and Zieglgansberger, 1990), followed by digitization and image enhancement, via an upright, fixed-stage Olympus microscope and EXi Blue camera (QImaging software)to facilitate visualization of the CeA neurons. We recorded pharmacologically-isolated GABAA receptor-mediated spontaneous inhibitory postsynaptic currents (sIPSCs) by applying blockers of glutamatergic (20 μMDNQX, 30 μM DL-AP5) and GABAB (1 μM CGP 55845A)receptors, and we recorded miniature GABAA-mediated IPSCs (mIPSCs) by adding0.5μM tetrodotoxin (TTX) to the bath. We used pipettes with input resistance 3–7 MΩ (access resistance <18 MΩ) filled with an internal solution (containing in mM: 145 KCl, 10 HEPES, 2 MgCl2, 5 EGTA, 2Na-ATP, and 0.2 Na-GTP, the latter two added fresh on the day of recording; pH 7.3–7.4, osmolarity 275–290 mOsm). For data acquisition, we used Multiclamp 700B and pClamp 10.2 software (Molecular Devices, Foster City, CA).
2.5. Data analysis and statistics
To analyze data acquired from intracellular and whole-cell recordings, we used Clampfit 10.2 (Molecular Devices) and MiniAnalysis 5.1 software (Synaptosoft Inc., Leonia, NJ), respectively. All 143 cells were clamped at −60 mV for the duration of the recording. In all experiments, series resistance (<10 M′Ω) was continuously monitored with a 10 mV hyperpolarizing pulse, and experiments with >20% change in series resistance were rejected in the final analysis. Frequency, amplitude, and decay of IPSCs were analyzed and visually confirmed using a semi-automated threshold based detection software (Mini Analysis, Synaptosoft Inc.). We determined averages of IPSC characteristics from baseline and experimental drug conditions containing a minimum of 60 spontaneous events. In voltage clamp recordings, we determined tonic currents using Clampfit 10.2 (Molecular Devices) and a previously described method (Glykys and Mody, 2007)in which the mean holding current (i.e., the current required to maintain the −60 mV membrane potential) was obtained by a Gaussian fit to an all-points histogram over a 5-sec interval. The all-points histogram was constrained to eliminate the contribution of IPSCs to the holding current. We quantified responses as the difference in holding current between baseline and experimental conditions. We used GraphPad Prism 5.0 (GraphPad Software, San Diego, CA) and Sigma Plot software for all statistical analysis of results obtained by whole-cell recordings. We accepted statistical significance at the p < 0.05 level using one- and two-way ANOVA and Student’s t-test.
2.6. Drugs
We purchased CGP 55845A, DNQX, and DL-AP5 from Tocris Biosciences (Ellisville, MI); GBZ (gabazine), THIP (gaboxadol), and TTX were from Sigma (St. Louis, MO). Human IL-1ra (Kineret, Amgen Inc., Thousand Oaks, CA) was from The University of Texas at Austin Pharmacy (Austin, TX).
3. Results
3.1. Baseline evoked CeA GABAergic responses are elevated and ethanol-induced increases in these responses are absent in neurons fromIl1rn KO compared to WT mice
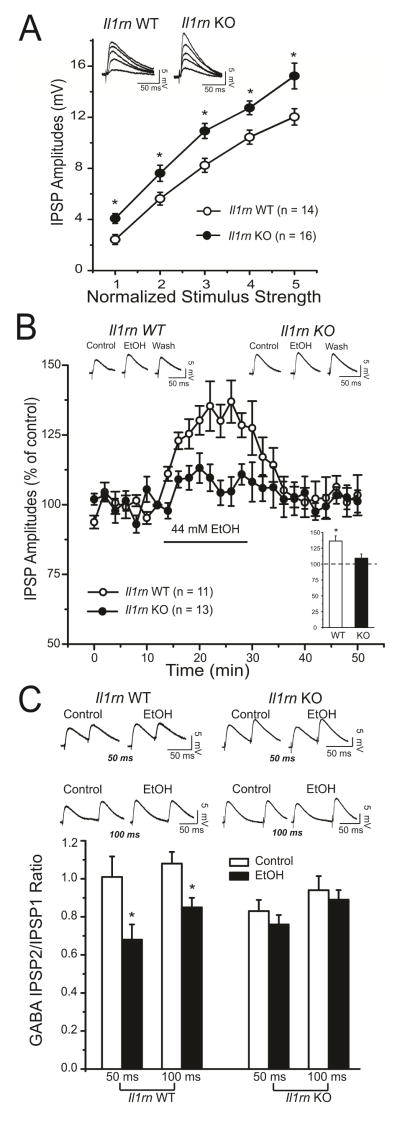
We first examined if there were any differences in baseline evoked GABA transmission in CeA neurons from Il1rn KO and WT mice. We recorded intracellularly with sharp pipettes from 30 neurons. There were no significant differences in voltage–current relationships (data not shown) or membrane properties between KO and WT neurons; thus we combined the data from the two groups, resulting in a mean resting membrane potential (RMP) of −79.8 ± 0.8 mV and a mean input resistance of 140.2 ± 6.5 MΩ. We pharmacologically isolated GABAA IPSPs and stimulated locally within the medial subdivision of the CeA. Baseline IPSP input–output curves generated by equivalent stimulus intensities were significantly higher in Il1rn KO compared to WT (Figure 1A), suggesting increased GABAergic transmission in the CeA of KO mice.
Figure 1. Basal evoked GABAergic transmission is enhanced in the CeA of Il1rn KO compared to wildtype (WT) mice.
A) Input-output curves of mean GABAAIPSP amplitudes are significantly increased over all stimulus strengths in Il1rn KO (n=16) compared with WT (n=14) neurons (t144=2.189; *p < 0.05). Top insert: Representative evoked IPSPs from WT and Il1rn KO mice. B) Time course depicting changes in evoked IPSP amplitude (at 50% maximal amplitude determined from the input-output relationship) upon 44 mM ethanol application and washout in Il1rn KO (n=13) and WT (n=11) neurons. Acute application of ethanol significantly increases evoked GABAergic transmission in WT, but not KO, with recovery upon washout of ethanol. Top insert: Representative evoked CeA IPSPs recorded before, during, and after ethanol washout in WT and Ilrn KO mice. Bottom insert: Histograms representing maximal percent increase in mean (± SEM) evoked IPSP amplitude(at half maximal amplitude) with ethanol application; *p<0.05. C) Top insert: Representative recordings of evoked PPF (at 50- and 100-msec interstimulus intervals) of IPSPs in CeA neurons from WT and Il1rn KO mice. Bottom insert: Histograms plotting the PPF ratios of IPSPs before and during ethanol in WT and Ilrn KO mice. Acute ethanol significantly (*p < 0.05) decreases the PPF ratio of IPSPs from WT, but not Il1rn KO mice.
We next studied the effect of acute superfusion (10–15 minutes) of 44 mM ethanol on evoked GABAergic transmission in CeA neurons from WT and Il1rn KO mice. We used 44 mM ethanol, as we have shown that this concentration provided a maximum ethanol-induced potentiation of the GABAergic transmission in the CeA (Nie et al., 2004; Roberto et al., 2003; Roberto et al., 2004). Ethanol significantly increased the peak amplitude by 30% in WT (Figure 1B; histogram insert), but there was no significant ethanol effectin KO neurons (Figure 1B). As depicted in the time course of Figure 1B, the maximum ethanol-induced facilitation of GABA responses (evoked using half-maximal strength) occurred within 10–12 minutes with recovery upon washout. We examined paired-pulse facilitation (PPF) of the IPSPs at 50- and 100-msec interstimulus intervals to assess pre- versus post-synaptic mechanisms. The ethanol-induced facilitation in CeA of WT mice, but not KO mice, was associated with a significant decrease in the PPF ratios of IPSPs (Figure 1C), suggesting increased GABA release upon ethanol application only in the WT neurons.
3.2. Basal spontaneous GABA release is elevated in the CeA of Il1rn KO compared to WT mice, and Kineret pretreatment normalizes this enhanced release
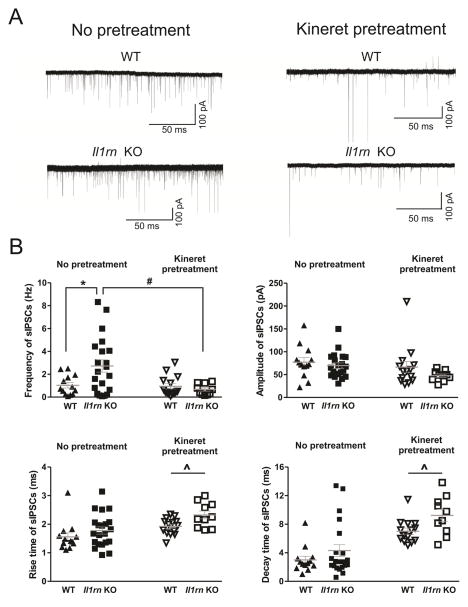
We studied baseline spontaneous inhibitory postsynaptic currents (sIPSCs) mediated by GABAA receptors in CeA of WT and Il1rn KO mice. There was a significantly higher sIPSC frequency in KO (2.7 ± 0.5 Hz) compared to WT (1.0 ± 0.2 Hz) mice, but no significant differences by genotype in the sIPSC amplitude, rise time, or decay time (Figure 2A left panels and 2B). The increased sIPSC frequency suggests enhanced action potential-dependent GABA release in CeA neurons of Il1rn KO compared to WT mice.
Figure 2. The enhancement of basal GABA release in the CeA of Il11rn KO mice is reversed by Kineret pretreatment.
A) Representative sIPSC recordings from CeA neurons from WT and Il1rn KO mice. Top panel: sIPSC traces from an untreated (left) and Kineret-pretreated neuron (right) from WT mice. Bottom panel: Left, the sIPSC frequency in an untreated neuron from a KO mouse is higher than in the WT mouse; Right, sIPSC trace from a Kineret pretreated neuron from an Il1rn KO mouse. B) The scatter graphs of sIPSC frequencies, amplitudes, and rise and decay times of individual CeA neurons from WT and Il1rn KO mice. The mean sIPSC frequency was higher (t32=2.36, p < 0.05)in KO (2.7 ± 0.5 Hz, n=21) compared to WT mice (1.0 ± 0.2 Hz, n=13), but there were no significant differences by genotype in the amplitude (WT: 77.2 ± 9.9 mV; KO: 69.6 ± 6 mV; t32=0.70, p > 0.05), rise time (WT: 1.6 ± 0.1 ms; KO: 1.8 ± 0.1 ms; t32=1.13, p > 0.05), or decay time (WT: 3.0 ± 0.5 ms; KO: 4.3 ± 0.8 ms; t32=1.16, p > 0.05). Kineret (100 ng/ml) pretreatment normalized the higher sIPSC frequency in KO and increased the rise and decay times of the basal spontaneous GABAA-mediated transmission in both strains. There were main effects of Kineret (F(1,54)=5.80, p < 0.05) and strain x Kineret interaction (F(1,54)=4.86, p < 0.05), with no main effect of strain on frequency. We also found main effects of Kineret (F(1,54) = 11.93, p < 0.01) and strain (F(1,54)=5.23, p < 0.05), without strain x Kineret interaction on the rise time in WT (untreated: 1.6 ± 0.1 ms vs. Kineret pretreated: 1.9 ± 0.1 ms) and KO mice (untreated: 1.8 ± 0.1 ms and Kineret pretreated: 2.3 ± 0.1 ms). Finally, there were main effects of Kineret (F(1,54)=34.24, p < 0.01) and strain (F(1,54)=5.31, p < 0.05), without interaction on the decay time (untreated WT: 3.0 ± 0.5 ms vs. Kineret pretreated WT: 7.1 ± 0.4 ms; untreated KO: 4.3 ± 0.8 ms vs. Kineret pretreated KO: 9.3 ± 0.9 ms).* indicates t-test comparison of WT vs. Il1rn KO; # indicates comparison of untreated to Kineret pretreated in Il1rn KO; ^ indicates main effect of Kineret pretreatment (two-way ANOVA followed by Bonferroni post hoc test).
Next, we tested the hypothesis that acute application of an exogenous IL-1R antagonist (Kineret), would reduce the differences in sIPSC frequency between the two strains. We pretreated CeA slices with 100 ng/ml Kineret for 30–90 minutes prior to whole-cell recordings and compared the baseline sIPSCs from untreated and pretreated slices (Figure 2 right panels and 2B). There was a significant main effect of Kineret pretreatment and a significant strain x Kineret interaction, with no main effect of strain. Kineret pretreatment significantly reduced baseline sIPSC frequency in KO mice (from 2.7 ± 0.5 in untreated to 0.7 ± 0.2 Hz in Kineret pretreated CeA neurons)to the levels found in WT, without affecting baseline frequency in WT neurons (1.0 ± 0.2 Hz for both untreated and Kineret pretreated; n=13–14). Although Kineret pretreatment had no effect on the baseline amplitude in either WT or KO mice, it significantly increased sIPSC kinetics in both strains. Specifically, we found significant main effects of Kineret and strain, but no interaction, on the rise time of sIPSCs in WT (1.6 ± 0.1 and 1.9 ± 0.1 ms for untreated and Kineret pretreated, respectively) and KO mice (1.8 ± 0.1 and 2.3 ± 0.1 ms for untreated and Kineret pretreated, respectively). We also found significant main effects of Kineret and strain, but no interaction, on the decay time in WT (3.0 ± 0.5 and 7.1 ± 0.4 ms for untreated and Kineret pretreated, respectively) and KO mice (4.3 ± 0.8 and 9.3 ± 0.9 ms in untreated and Kineret pretreated, respectively). Our results indicate that exogenous Kineret can reverse the alteration in basal CeA GABAergic transmission elicited by deletion of the endogenous IL-1ra. However, the changes in sIPSC decay time following Kineret pretreatment in both WT and KO mice also suggest that Kineret may modulate postsynaptic GABAA receptors.
3.3. Deletion of Il1rn decreases the ethanol-induced facilitation of spontaneous GABAergic transmission in the CeA
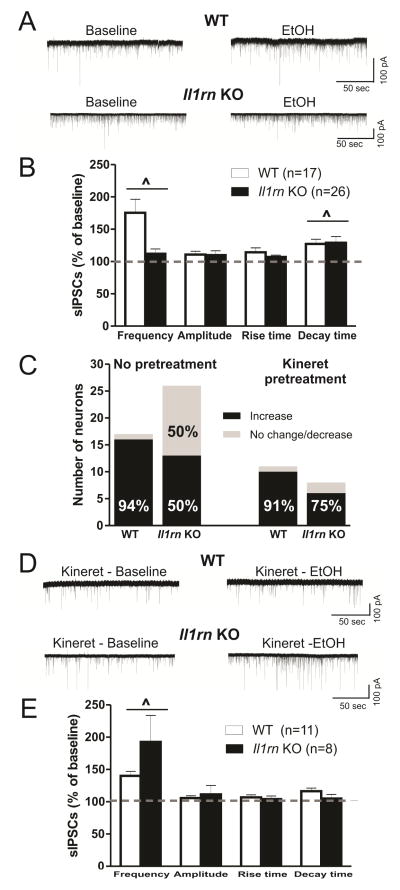
We next examined the role of IL-1ra in ethanol facilitation of GABAergic transmission. Ethanol increased sIPSC frequency in CeA of both strains (WT: 175.5 ± 20.8% of baseline, n=17; KO: 112.1 ± 7.4% of baseline, n=26); there was a significant main effect of ethanol, but no strain or ethanol x strain interaction on sIPSC frequency (Figure 3A and B). Although the overall ethanol-induced increase in frequency was not dependent on genotype, we did observe a genotype difference in the proportion of CeA cells showing increased sIPSC frequency upon ethanol application. In comparison to WT, where 16 of 17 (94%) neurons demonstrated an ethanol-induced increase (> 115% of baseline) in frequency, fewer neurons (13 of 26 or 50%) from Il1rnKO mice demonstrated the ethanol-induced increase (p < 0.05, Chi-square test; Figure 3C). Acute ethanol increased the mean decay time of sIPSCs in most WT (127.2 ± 7.4% of baseline; p < 0.05) and KO (129 ± 9.6% of baseline; p < 0.05) neurons (Figure 3B). There were no effects of ethanol on amplitude and rise time of sIPSCs, suggesting that IL-1rais important in ethanol-induced modulation of GABAergic transmission in some CeA neurons, mediated predominantly via presynaptic mechanisms.
Figure 3. Deletion of Il1rn reversibly changes the responsiveness of CeA neurons to ethanol.
A) Top panel, superfusion of 44 mM EtOH increases sIPSCs in a WT neuron. Bottom panel, EtOH (44 mM) superfusion slightly increases IPSCs in a KO neuron. B) Summary of ethanol effects on the mean sIPSC frequency, amplitude, and rise and decay times in CeA neurons from WT and Il1rn KO mice. Ethanol had a significant main increase in the mean frequency (F(1,41)=5.37, p <0.05)and decay time of sIPSCs (^ p < 0.05, two-way ANOVA). C) The proportion of neurons responding to ethanol with an increase in sIPSC frequencies is significantly lower in Il1rn KO compared to WT mice, but there is no difference between the two strains after Kineret pretreatment for 30–90 minutes (p < 0.05, Chi-square test). D) Representative recordings of CeA neurons after Kineret pretreatment. Top panel: sIPSC frequencies following application of 44 mM EtOH were still enhanced after Kineret pretreatment in a WT neuron. Bottom panel, in the presence of Kineret, 44 mM ethanol superfusion significantly increases sIPSCs in anIl1rn KO neuron. E) In the presence of Kineret, ethanol significantly increases the mean sIPSC frequency in both WT and Il1rn KO mice. Ethanol does not increase the decay time of the sIPSCs in neurons pretreated with Kineret. (^p < 0.05, two-way ANOVA with Bonferroni post hoc test).
Since Kineret decreased sIPSC frequencies of CeA neurons from Il1rnKO mice to a level similar to that found in WT, we also examined the effect of Kineret pretreatment on the ethanol-induced facilitation of GABAergic transmission (Figure 3D and E). Kineret pretreatment increased the proportion of neurons in KO mice that respond to ethanol with changes in sIPSC frequencies (p < 0.05; Figure 3C). Specifically, in the KO mice there were 6 of 8 (75%) CeA neurons showing an ethanol-induced increase (192.7 ± 40.9%) in sIPSC frequencies after Kineret pretreatment compared to 50% of untreated cells. In WT mice, Kineret had no effect on the proportion of CeA neurons responding to ethanol with increased (140.0 ± 7.0%) sIPSC frequencies; thus, 10 of 11 or 91% showed increased frequencies elicited by acute ethanol. Ethanol did not alter sIPSC amplitude, rise time, or decay time (Figure 3E). These data suggest that exogenous application of IL-1ra can restore ethanol’s enhancement of GABAergic transmission in KO mice, whereas IL-1ra has limited effects in WT mice.
3.4. Basal vesicular GABA release in the CeA of Il1rn KO is similar to that in WT mice
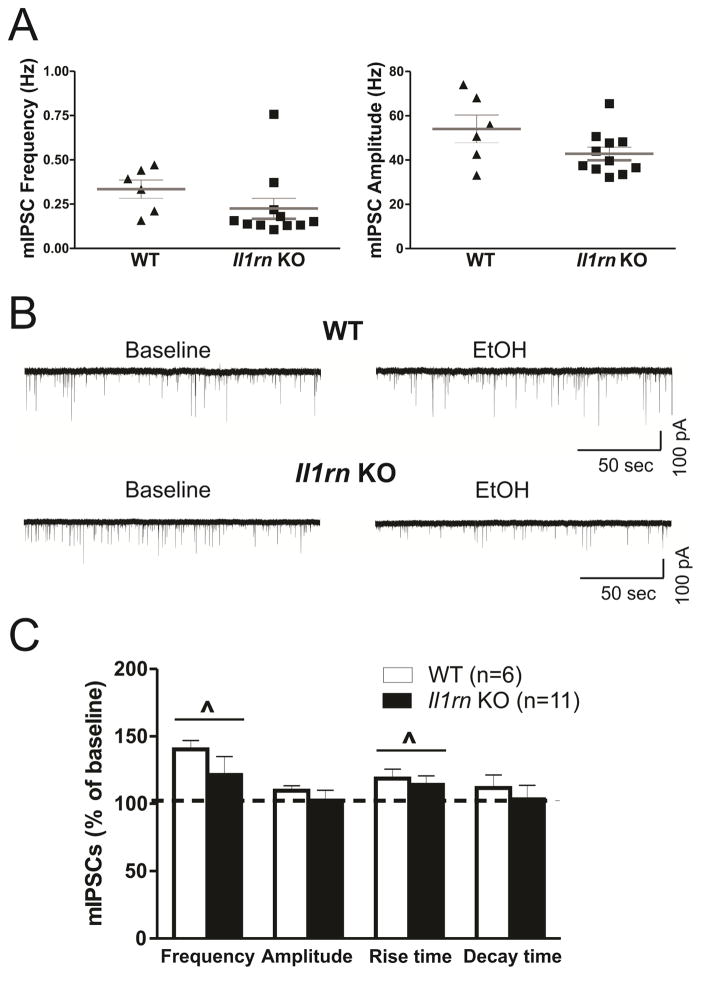
We also examined a role for IL-1ra in action potential-independent (vesicular) GABAergic transmission (as measured by miniature IPSCs, mIPSCs, in the presence of TTX) but found no significant differences in baseline mIPSCs between WT and Il1rn KO mice (Figure 4A and B left panel). Ethanol significantly increased the frequency of mIPSCs in both genotypes (WT: 140.4 ± 6.5% of baseline, n=6; KO: 121.5 ± 13.6% of baseline, n=11) (Figure 4B and C). Similar to effects on the sIPSCs, ethanol increased mIPSC frequencies (>115% of baseline) in all of the WT neurons (6 of 6), compared to only 6 of 11 (55%) KO neurons (p < 0.05, Chi-square test; data not shown). In contrast to the sIPSCs, we observed a significant effect of ethanol on mIPSC rise time (WT: 118.8 ± 7.1% of baseline; KO: 114.1 ± 6.7% of baseline), whereas there were no ethanol effects on mIPSC decay time in WT or Il1rn KO mice (Figure 4C). These data further support a role for IL-1ra in the ethanol-induced facilitation of presynaptic vesicular GABA release in CeA.
Figure 4. Deletion of Il1rn alters ethanol effects on action potential-independent GABA transmission in CeA.
A) Scatter graphs of mIPSC frequencies and amplitudes of individual CeA neurons from WT (n=6) and Il1rn KO (n=11) mice. B) Representative recordings of mIPSCs from neurons following acute ethanol application. Top panel: traces of mIPSCs from a WT neuron before (left) and during 44 mM ethanol application (right). Bottom panel: traces of mIPSCs from a KO neuron showing ethanol-induced decrease in the mIPSC frequency. C) The comparison of ethanol effects on the mean mIPSC frequency, amplitude, and rise and decay times between CeA neurons from WT and Il1rn KO mice. Two-way ANOVA showed a significant main effect of ethanol (F(1,15)=6.48; ^p < 0.05) on the mean mIPSC frequencies in WT and Il1rn KO mice and a significant effect of ethanol on mIPSC rise time (F(1,15)=5.68, p < 0.05).
3.5. Cell type-specific changes in tonic GABAergic signaling in CeA neurons of Il1rn KO compared to WT mice
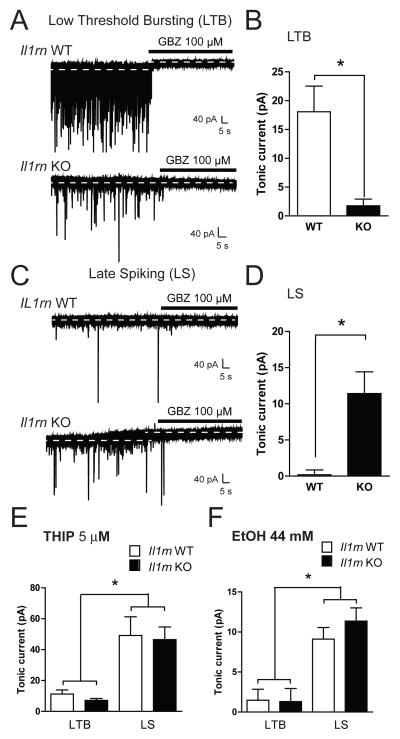
As our results suggested that IL-1ra is critical in action potential-dependent GABAergic transmission, we assessed ongoing tonic GABAergic signaling because it can dynamically regulate overall inhibitory network activity (Krook-Magnuson and Huntsman, 2005; Semyanov et al., 2004). We examined the tonic conductance using whole-cell voltage-clamp recordings from 27 Il1rn KO and 29 WT neurons. Cell typing was based on spike characteristics, as previously described (Chieng et al., 2006; Dumont et al., 2002). CeA neurons are primarily composed of three main cell types: low-threshold bursting (LTB), late spiking (LS), and regular spiking (RS)(Chieng et al., 2006; Dumont et al., 2002). A GABAA receptor-mediated tonic current was defined as the difference in holding current (i.e., the current required to maintain the neuron at −60mV) before and after application of the GABAA receptor antagonist gabazine. Focal application of gabazine (GBZ, 100 μM) produced a significant reduction in holding current in LTB neurons from WT mice (18.1 ± 4.5 pA; Figure 5A, upper trace and 5B) that was not observed in LTB neurons from KO mice (1.8 ± 1.2 pA; Figure 5A, lower trace and 5B). In contrast, 100 μMGBZ had no effect on holding current in LS neurons from WT mice (0.2 ± 0.7 pA, n = 6; Figure 5C, upper trace and 5D), but did produce a significant reduction in LS neurons from KO mice (11.4 ± 3.0 pA; Figure 5C, lower trace and 5D). Focal application of gabazine onto RS neurons produced variable effects on holding currents (5.5 ± 2.0 and 7.9 ± 2.9 pA in WT and KO mice, respectively). This variability is in line with previous reports on the inconsistent nature of tonic currents in this cell population (Herman et al., 2013).
Figure 5. Tonic GABAergic signaling is altered in specific CeA cell types fromIl1rn KO compared to WT mice.
A) Representative recordings from a Low Threshold Bursting (LTB) neuron from a WT (upper trace) and Il1rn KO mouse (lower trace) during focal application of gabazine (GBZ, 100 μM). Dashed lines indicate average holding current. B) Summary of the tonic current in LTB neurons from WT (white bar) and KO mice (black bar) revealed by application of GBZ (n=7for both genotypes; *p<0.05, unpaired t-test). C) Recordings from a Late Spiking (LS) neuron from a WT (upper trace) and Il1rn KO mouse (lower trace) during application of GBZ. Dashed lines indicate average holding current. D) Summary of the tonic current in LS neurons from WT (white bar) and Il1rn KO mice (black bar) revealed by application of GBZ (n=6 (WT), n=5 (Il1rn KO); *p<0.05, unpaired t-test). E)Summary of the tonic current in LTB and LS neurons from WT (white bars) and Il1rn KO mice (black bars) revealed by focal application of 5 μM THIP (n=6–7; *p<0.05, one-way ANOVA with Bonferroni post hoc comparison). F)Summary of the tonic current in LTB and LS neurons from WT (white bars) and Il1rn KO mice (black bars) revealed by focal application of 44 mM EtOH(n=5–6; *p<0.05, one-way ANOVA with Bonferroni post hoc comparison).
As there were altered tonic conductances in two CeA cell populations from Il1rn KO mice, we determined the role of the δ subunit of the GABAA receptor in these conductances (see (Herman et al., 2013). In WT mice, LTB neurons displayed a significantly higher baseline sIPSC frequency compared to LS neurons (Figure 5A and C); however, this effect was not observed in Il1rn KO mice, suggesting that the absence of phasic inhibitory tone may contribute to the decrease in tonic conductance observed in LTB neurons from KO mice. We observed no cell type-specific change in baseline sIPSC frequency between LS or RS neurons from either genotype. We used the δ subunit-preferring GABAA agonist, gaboxadol (THIP), to determine the contribution of this subunit to the changes in tonic signaling. Focal application of 5 μM THIP induced a significantly greater increase in holding current in LS (49.2 ± 12.1 pA) compared to LTB (11.2 ± 2.6 pA) neurons from WT mice (Figure 5E). THIP also increased tonic conductance in LS (46.5 ± 8.2) compared to LTB (7.2 ± 1.2 pA) neurons from Il1rn KO mice (Figure 5E), demonstrating cell type differences but no genotype differences. These data suggest that there are no differences in expression or function of the δ subunit-containing GABAA receptors between neurons from WT and Il1rn KO mice.
We next asked if the acute effects of ethanol on tonic signaling differed between cell types or genotypes. Consistent with THIP effects, focal application of EtOH (44 mM) elicited a greater increase in holding current in LS compared to LTB neurons from both WT (9.1 ± 1.5 vs. 1.5 ± 1.4 pA; p < 0.05; Figure 5F) and Il1rn KO mice (11.4 ± 1.7 vs. 1.3 ± 1.6 pA; p < 0.05; Figure 5F), indicating that there was no effect of genotype on the cell type-specific effects of acute ethanol on tonic conductance. Collectively, these data suggest that genetic deletion of Il1rn produces functional changes in tonic inhibition, which can significantly impact CeA activity. Table 1 provides an overall summary of the effects of ethanol and Kineret on the genotypes and cell types used in our study.
Table 1.
Summary of the effects of ethanol and Kineret in CeA neurons from Il1rn WT and KO mice. Ethanol (44 mM) was applied by superfusion for 10–15 minutes. Kineret (100 ng/ml) was superfused for 30–90 minutes prior to recordings.
| Recordings | Parameters/Pharmacology | Baseline | Ethanol | Kineret Baseline | Kineret + Ethanol | |||
|---|---|---|---|---|---|---|---|---|
| KO vs. WT | WT | KO | WT | KO | WT | KO | ||
| eIPSPs | Amplitude | ↑ | ↑ | = | n.a. | n.a. | n.a. | n.a. |
| PPF | = | ↓ | = | n.a. | n.a. | n.a. | n.a. | |
| sIPSCs | Frequency | ↑ | ↑ | ↑# | = | = | ↑ | ↑ |
| Amplitude | = | = | = | = | = | = | = | |
| Rise time | = | = | = | ↑ | ↑ | = | = | |
| Decay time | = | ↑ | ↑ | ↑ | ↑ | = | = | |
| mIPSCs | Frequency | = | ↑ | ↑ | n.a. | n.a. | n.a. | n.a. |
| Amplitude | = | = | = | n.a. | n.a. | n.a. | n.a. | |
| Rise time | = | ↑ | ↑ | n.a. | n.a. | n.a. | n.a. | |
| Decay | = | = | = | n.a. | n.a. | n.a. | n.a. | |
| Tonic | GBZ |
KO: LS+/LTB− WT: LS−/LTB+ |
↑ | ↑ | n.a. | n.a. | n.a. | n.a. |
| THIP | = | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | |
Abbreviations: GBZ (gabazine; 100 μM), KO (Il1rn KO mice), LS (late spiking neuron), LTB (low-threshold bursting neuron), THIP (gaboxadol; 5 μM), WT (Il1rn WT mice).
Symbols: ↑ increase; # decrease in number of cells responding to ethanol compared to WT mice; = no change; ↓ decrease; n.a. not applicable; + presence of tonic GABA conductance; − lack of tonic GABA conductance.
4. Discussion
In our study, we found that deletion of Il1rn alters CeA GABAergic transmission and its sensitivity to acute ethanol. Our results show significant alterations in basal phasic and tonic GABAergic transmission, and a lack of ethanol-induced facilitation of phasic GABAergic transmission in a significant portion of CeA neurons from Il1rn KO mice. Importantly, the changes in the phasic currents and ethanol responsiveness are reversed or restored by exogenous IL-1ra (Kineret). Thus, both genetic and pharmacological manipulations provide convergent evidence for a role of IL-1ra in GABAergic responses and ethanol potentiation in CeA neurons. We speculate that the alterations of GABA responses observed in Il1rn KO mice are not isolated to amygdala and likely extend to other brain regions, considering that global knockout of Il1rn affects multiple central and peripheral targets.
4.1. IL-1ra is involved in the regulation of GABAergic transmission in the central amygdala
Increased basal transmission, characterized by increased amplitudes of evoked GABAergic responses and sIPSC frequencies, suggests a role for IL-1ra in the modulation of action potential-dependent GABA release in the CeA. In contrast, we observed no changes in the basal mIPSC frequencies between WT and Il1rn KO mice, suggesting that the total number of presynaptic axon terminals and the vesicular release at individual synapses are likely unchanged by Il1rn deletion. We speculate that IL-1ra may be involved in the modulation of neuronal or axonal firing rates. Notably, pretreatment with Kineret reversed the increased sIPSC frequency observed in Il1rn KO mice, further supporting the importance of IL-1ra in the regulation of action potential-dependent GABA release. IL-1ra inhibits signaling by binding to the IL-1R1 and preventing the recruitment of IL-1RAcP, which is critical for the initiation of IL-1R1 signaling (Garlanda et al., 2013; Krumm et al., 2014). We hypothesize that deletion of Il1rn induces a tonic activation of IL-1R1 signaling in presynaptic axon terminals, mediating the observed increase in action potential-dependent GABA release. Although there were no differences in basal sIPSC kinetics and amplitude between WT and Il1rnKO mice, Kineret altered the kinetics in both groups, suggesting that IL-1ra may also act post synaptically in modulating GABAergic transmission and that postsynaptic IL-1R1s may be regulated differently compared to presynaptic IL-1R1s. We propose that the IL-1ra modulation of GABAA transmission may depend on synaptic localization of IL-1R1 and intracellular signaling.
4.2. IL-1ra plays a critical role in the regulation of tonic GABA conductances
Deletion of Il1rn leads to significant enhancement of phasic action potential-dependent GABAergic transmission, which may result from disruptions in local synaptic activity and network function. Tonic inhibition is a dynamic regulator of overall network activity and plays a significant role in the fine-tuning of local inhibitory circuitry that regulates network function(Krook-Magnuson and Huntsman, 2005; Semyanov et al., 2004). In the CeA, distinct types of tonic inhibition in specific cell populations provide multiple levels of inhibitory control that can regulate overall output. We previously showed that the tonic GABA conductance in CeA is celltype-specific and mediated by two populations of GABAA receptors with different subunit compositions(primarily δ or α1 subunits) and distinct functional properties (Herman et al., 2013). In the present study, we found a cell type-specific shift in tonic conductance in neurons from Il1rn KO compared to WT mice. In the KO mice, the ongoing tonic conductance in LTB neurons is absent and an ongoing tonic conductance in LS neurons appears. Interestingly, the functional ‘switch’ is similar to what we observed in rats chronically treated with ethanol (Herman et al., 2013; Herman and Roberto, 2014). This switch may represent a compensatory response associated with an increase in phasic GABA release and/or disturbance of IL-1R1 signaling.
The mechanisms mediating the switch in tonic conductances between LTB and LS neurons in Il1rn KO mice may include altered expression of IL-1R1 and GABAA receptors, trafficking of the receptor subunits to the membrane, and/or receptor assembly. Previously, we reported atonic GABA conductance that is not active in the basal state and is mediated primarily by δ subunits(Herman et al., 2013). It issensitive to physiological conditions in which local GABA concentrations are elevated (e.g., blockade of GABA re-uptake, ethanol, etc.). Here we report that, despite the loss of ongoing tonic inhibition in LTB neurons and the presence of ongoing tonic inhibition in LS neurons in KO mice, the ability of a δ subunit-preferring agonist (THIP) and acute ethanol to further stimulate tonic inhibition remains unchanged, suggesting that there is no change in function or membrane expression of the δ subunit in either cell population or genotype. It is possible that there is an alteration of expression/function of other GABAA receptor subunits mediating tonic GABA currents, such as α1 and α5 subunits. The α1 subunit has been shown to mediate the ongoing tonic conductance driven by action potential-dependent GABA release in CeA LTB neurons (Herman et al., 2013). The lack of this current in LTB neurons and its presence in LS neurons raises the possibility of a switch in the expression and/or function of α-containing GABA receptors in these neurons. Whereas GABA receptors containing both α1 and δ subunits are found in the hippocampus (Glykys et al., 2007), such receptors seem to be missing or are present in very limited numbers in CeA neurons(Herman et al., 2013).
Previously, we showed no significant role for the α5 subunit in the tonic GABA conductance in the CeA, although immunohistochemical analysis confirms expression of the α5 subunit, especially in LS neurons (Herman et al., 2013). In this regard, an IL1β-induced increase in the surface expression of α5-containing GABAA receptors and α5-mediated tonic GABA currents in hippocampal neurons (Wang et al., 2012) suggest that α5 subunits may play a role in the tonic GABA conductance in Il1rn KO mice. Assessment of a role for different α subunits in the tonic GABA conductance in CeA from Il1rn KO mice will be the subject of future studies.
4.3. Kineret restores ethanol’s facilitation of GABAergic transmission in Ilrn1 KO mice
Acute ethanol increases GABAergic transmission predominantly via presynaptic mechanisms, resulting in increased GABA release in the CeA of mice(Bajo et al., 2008; Herman et al., 2013; Nie et al., 2004) and rats(Roberto et al., 2010; Roberto et al., 2003; Roberto et al., 2004). In addition, ethanol potentiates tonic GABA conductance and modulates firing rates in a cell type-specific manner in the murine CeA (Herman et al., 2013). In WT mice, we verified previous findings that ethanol enhances both evoked and spontaneous GABAergic transmission in CeA by increasing GABA release. This facilitatory effect of ethanol on the evoked IPSP and sIPSC frequency is lost in some Il1rn KO neurons, indicating that IL-1ra may play an important role in mediating ethanol potentiation of activity-dependent GABA release.
Application of Kineret restores the basal phasic GABAergic transmission and ethanol effects in Il1rn KO mice, and it also modulates the kinetics of phasic GABAergic transmission in both WT and KO mice. Furthermore, we demonstrate that Kineret effectively penetrates the slices to alter CeA GABA transmission and ethanol responses.
5. Conclusion
We demonstrate significant increases in action potential-mediated GABA release and changes in the tonic GABA conductance of specific neurons in Il1rn KO mice that may profoundly impact overall CeA activity and its GABAergic projection afferents, thus impacting alcohol-related behaviors. In summary, the IL-1ra system plays a critical role in basal and ethanol-induced GABAergic transmission in the CeA. We speculate that an undisturbed IL-1R signaling system, properly regulated by IL-1ra, modulates GABAergic transmission in a cell type-specific manner. The subpopulations of CeA neurons involved and the neurocircuitry responsible for mediating relevant alcohol-related behaviors will be the subject of future studies. Our electrophysiological analysis provides evidence that changes mediated by the IL-1/IL-1R system are critical for alcohol responses at the neuronal level in mice.
Highlights.
Deletion of Il1rn in mice results in:
alterations in phasic and tonic GABAergic transmission in central amygdala
a decrease in the number of CeA neurons responding to acute ethanol
rescue of this phenotype by Kineret
Acknowledgments
We thank Dr. George R. Siggins for his critical review and comments on the manuscript, George Luu for technical assistance, Dr. Marian L. Logrip for help with statistical analyses, and Dr. Jody Mayfield for many helpful comments, critical review, and editing of the manuscript.. The Scripps Research Institute’s manuscript number for this paper is 24053.
Supported by NIH/NIAAA INIA West Consortium U01-AA013498 to M.R.AA013520 to Y.A.B. and R.A.H.AA013517, AA006420, AA015566 and AA021491 to M.R.
Footnotes
Author contributions
M.B. and M.R. designed and performed experiments, analyzed data, prepared figures, and wrote the manuscript. M.A.H., F.P.V., S.G.M. and C.S.O. performed experiments, analyzed data, prepared figures, and contributed to writing the manuscript; R.A.H. and Y.A.B. provided the mice and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Roberto M, Madamba SG, Siggins GR. Neuroadaptation of GABAergic transmission in the central amygdala during chronic morphine treatment. Addict Biol. 2011;16:551–564. doi: 10.1111/j.1369-1600.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Chandra D, Homanics GE, Rudolph U, Harris RA. Linking GABA(A) receptor subunits to alcohol-induced conditioned taste aversion and recovery from acute alcohol intoxication. Neuropharmacology. 2013;67:46–56. doi: 10.1016/j.neuropharm.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Jung S, Alva H, Wallace D, Rosahl T, Whiting PJ, Harris RA. Deletion of the alpha1 or beta2 subunit of GABAA receptors reduces actions of alcohol and other drugs. J Pharmacol Exp Ther. 2003;304:30–36. doi: 10.1124/jpet.102.042960. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, Garrett EM, Blednov YA, Harris RA. gamma-Aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochem Pharmacol. 2004;68:1581–1602. doi: 10.1016/j.bcp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Chieng BC, Christie MJ, Osborne PB. Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol. 2006;497:910–927. doi: 10.1002/cne.21025. [DOI] [PubMed] [Google Scholar]
- Clark SR, McMahon CJ, Gueorguieva I, Rowland M, Scarth S, Georgiou R, Tyrrell PJ, Hopkins SJ, Rothwell NJ. Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J Cereb Blood Flow Metab. 2008;28:387–394. doi: 10.1038/sj.jcbfm.9600537. [DOI] [PubMed] [Google Scholar]
- Cohen P. The TLR and IL-1 signalling network at a glance. Journal of cell science. 2014;127:2383–2390. doi: 10.1242/jcs.149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biological psychiatry. 2012;71:666–676. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt HU, Zieglgansberger W. Visualizing unstained neurons in living brain slices by infrared DIC-video microscopy. Brain Research. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Martina M, Samson RD, Drolet G, Pare D. Physiological properties of central amygdala neurons: species differences. Eur J Neurosci. 2002;15:545–552. doi: 10.1046/j.0953-816x.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- Fox E, Jayaprakash N, Pham TH, Rowley A, McCully CL, Pucino F, Goldbach-Mansky R. The serum and cerebrospinal fluid pharmacokinetics of anakinra after intravenous administration to non-human primates. J Neuroimmunol. 2010;223:138–140. doi: 10.1016/j.jneuroim.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea J, Ogungbenro K, Hulme S, Greenhalgh A, Aarons L, Scarth S, Hutchinson P, Grainger S, King A, Hopkins SJ, Rothwell N, Tyrrell P. Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: results of a dose-ranging study. J Cereb Blood Flow Metab. 2011;31:439–447. doi: 10.1038/jcbfm.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Gutierrez EG, Banks WA, Kastin AJ. Blood-borne interleukin-1 receptor antagonist crosses the blood-brain barrier. J Neuroimmunol. 1994;55:153–160. doi: 10.1016/0165-5728(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M. Novel Subunit-Specific Tonic GABA Currents and Differential Effects of Ethanol in the Central Amygdala of CRF Receptor-1 Reporter Mice. J Neurosci. 2013;33:3284–3298. doi: 10.1523/JNEUROSCI.2490-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Roberto M. Cell-type-specific tonic GABA signaling in the rat central amygdala is selectively altered by acute and chronic ethanol. Addict Biol. 2014 doi: 10.1111/adb.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson EI, Huntsman MM. Excitability of cortical neurons depends upon a powerful tonic conductance in inhibitory networks. Thalamus & related systems. 2005;3:115–120. doi: 10.1017/S1472928807000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumm B, Xiang Y, Deng J. Structural biology of the IL-1 superfamily: key cytokines in the regulation of immune and inflammatory responses. Protein science: a publication of the Protein Society. 2014;23:526–538. doi: 10.1002/pro.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, Siggins GR. The Central Amygdala and Alcohol: Role of gamma-Aminobutyric Acid, Glutamate, and Neuropeptides. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Wang DS, Zurek AA, Lecker I, Yu J, Abramian AM, Avramescu S, Davies PA, Moss SJ, Lu WY, Orser BA. Memory deficits induced by inflammation are regulated by alpha5-subunit-containing GABAA receptors. Cell reports. 2012;2:488–496. doi: 10.1016/j.celrep.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]