Abstract
Structural and metabolic abnormalities in fronto-striatal structures have been reported in children with prenatal methamphetamine (MA) exposure. The current study was designed to quantify functional alterations to the fronto-striatal circuit in children with prenatal MA exposure using functional magnetic resonance imaging (fMRI). Because many women who use MA during pregnancy also use alcohol, a known teratogen, we examined 50 children (age range 7–15), 19 with prenatal MA exposure, 15 of whom had concomitant prenatal alcohol exposure (the MAA group), 13 with heavy prenatal alcohol but no MA exposure (ALC group), and 18 unexposed controls (CON group). We hypothesized that MA exposed children would demonstrate abnormal brain activation during a visuospatial working memory (WM) “N-Back” task. As predicted, the MAA group showed less activation than the CON group in many brain areas, including the striatum and frontal lobe in the left hemisphere. The ALC group showed less activation than the MAA group in several regions, including the right striatum. We found an inverse correlation between performance and activity in the striatum in both the CON and MAA groups. However, this relationship was significant in the caudate of the CON group but not the MAA group, and in the putamen of the MAA group but not the CON group. These findings suggest that structural damage in the fronto-striatal circuit after prenatal MA exposure leads to decreased recruitment of this circuit during a WM challenge, and raise the possibility that a rewiring of cortico-striatal networks may occur in children with prenatal MA exposure.
INTRODUCTION
Methamphetamine (MA) abuse is a continuing public health problem worldwide, and recent data indicated 16–17 million Americans over the age of 12 have used methamphetamine, including approximately 19,000 pregnant women (Colliver et al., 2006). Until recently, little was known about the effects of prenatal MA exposure on the developing brain, but new reports have demonstrated a variety of detrimental effects on behavior, cognition, brain structure, and brain function in children exposed to MA in utero. A recent and large prospective study reported restricted fetal growth in newborn infants with known prenatal MA exposure, along with poorer neurobehavioral outcomes, such as increased stress and depressed arousal and movement scores (Smith et al., 2006; Smith et al., 2008). Another study documented lower verbal memory, spatial memory, attention, and visual-motor integration scores in children with prenatal MA exposure relative to unexposed controls (Chang et al., 2004). Several neuroimaging studies reported that children exposed to MA in utero exhibit brain abnormalities. Structural and metabolic brain abnormalities, especially in the dopamine-rich prefrontal-striatal circuitry, have been detected (as reviewed in Derauf et al., 2009). Cellular and molecular mechanisms explaining the damage to monoaminergic neurons, as well as the resulting effects on developing neural circuitry, have been proposed (as reviewed in Frost & Cadet, 2000). Further, a recent study by our group reported that MA exposed children exhibited abnormal fMRI activity patterns during a verbal learning paradigm (Lu et al., 2009). Taken together, these data strongly suggest that prenatal MA exposure negatively impacts brain development. However, conclusions about the specific effects of prenatal MA in humans are limited because of the high rates of concomitant alcohol use by MA abusing mothers during pregnancy. A recent study showed that nearly half of MA using pregnant women also drink alcohol (Smith et al., 2006), and alcohol is a known teratogen, frequently resulting in various brain and cognitive abnormalities (as reviewed in Riley & McGee, 2005).
Here, we aimed to evaluate alterations to visuo-spatial working memory (WM) neural circuitry in children with prenatal MA exposure versus typically developing unexposed controls. In order to explore the specific effects of prenatal MA exposure, we attempted to account for the effects of concomitant alcohol exposure by including an additional contrast group of children with heavy prenatal alcohol but no MA exposure, and included alcohol exposure clinical severity as a parameterized between-group covariate in our analyses. One important circuit affected by MA is the fronto-striatal loop. The output of the basal ganglia exerts a gating function, filtering out noise and distractions and enhancing select memories through disinhibition of the prefrontal cortex (Gruber et al., 2006). This modulation of mnemonic processes is thought to occur via dopaminergic projections to the prefrontal cortex (Graham & Goldman-Rakic, 1995; Gruber et al, 2006). MA has known dopaminergic neurotoxicity (as reviewed in Frost & Cadet, 2000). Further, structural and metabolic abnormalities in the striatum have been reported in children with prenatal MA exposure (Smith et al., 2001; Chang et al., 2004; Sowell et al., 2010). Additionally, our group recently found that children with prenatal MA exposure have left hemispheric white-matter abnormalities, in tracts connecting frontal and striatal structures (Colby et al, submitted). Thus, we hypothesized that MA exposed children would have functional deficits in frontal and striatal regions due to specific effects of MA and not comorbid alcohol exposure. More specifically, because the frontostriatal loop is important for performing WM tasks (Lewis et al., 2004; Gruber et al., 2006; McNab & Klingberg, 2008), we predicted that MA exposed children would show abnormal frontal and striatal activity relative to both CON and ALC groups while performing a WM task.
METHODS
Participants
After prospective participants were screened for every type of exclusion criteria detailed below, fifty subjects, ranging from 7 to 15 years of age, were retained and included in the analyses presented in this report. Each participant was classified into one of three groups based on prenatal exposure histories: a methamphetamine-exposed group (MAA, n=19, 15 with concomitant alcohol exposure, age range 7–13), an alcohol-exposed group (ALC, n=13, age range 7–15), and a non-exposed control group (CON, n=18, age range 7–15). Exposure status was established by extensive interviews administered to the parents or adult guardians of participants. Additionally, social, medical and/or legal records were used when available to confirm exposure histories.
Participants were included in the MAA group if prenatal exposure to methamphetamine was confirmed by parental or guardian report, or by maternal or infant medical records. Fifteen of the 19 children in the MAA group were also exposed to alcohol prenatally. Children in the MAA group were recruited from three sources: 1) older children of mothers who were in an MA rehabilitation program and had infants born positive for MA, 2) a social skills training group for children with fetal alcohol spectrum disorders (FASDs) at UCLA, and 3) self-referral in response to advertisements and word-of-mouth. Participants in the alcohol-exposed group (ALC) had exposure to four or more drinks per occasion at least once per week or 14 drinks or more per week and were not exposed to methamphetamine during gestation (n=13). Most ALC subjects were recruited from the same social skills training group as the MAA subjects. Typically-developing controls (CON) were excluded from the study if they had exposure to illicit drugs or more than two alcoholic drinks on any occasion or an average of one drink or more per week during gestation. CON subjects (n=18) were recruited from the same Los Angeles communities as the exposed groups via advertisement, and effort was made to recruit from similar socioeconomic strata.
Details of diagnostic procedures for fetal alcohol spectrum disorders used to classify ALC and MAA subjects are described in another report (O'Connor et al., 2006). Briefly, an experienced clinician examined alcohol-exposed children using the Diagnostic Guide for Fetal Alcohol Syndrome (FAS) and Related Conditions (Astley, 2004). This system uses a 4-digit diagnostic code reflecting the magnitude of expression of four key diagnostic features of FAS: 1) growth deficiency, 2) the FAS facial phenotype, including short palpebral fissures, flat philtrum, and thin upper lip, 3) central nervous system dysfunction, and 4) gestational alcohol exposure. This classification method has been shown to correlate with brain function and structure (Astley & Clarren, 2001). Using these criteria, children with alcohol exposure (with or without concomitant MA exposure) were diagnosed with fetal alcohol syndrome (FAS), partial FAS, sentinel features, or alcohol-related neurodevelopmental disorder (ARND). Table 1 illustrates the clinical severity of alcohol exposure in each group.
Table 1.
Alcohol Exposure Clinical Severity by Group
| MAA (n = 19) | ALC (n=13) | CON (n=18) | |
|---|---|---|---|
| No Alcohol | 4 | 0 | 18 |
| Exposed (least severe) | 1 | 0 | 0 |
| ARND | 9 | 4 | 0 |
| Sentinel | 2 | 2 | 0 |
| PFAS | 2 | 3 | 0 |
| FAS (most severe) | 1 | 4 | 0 |
ARND = Alcohol-related neurodevelopmental disorder, Sentinel = Shows mild facial dysmorphology, PFAS = Partial FAS, FAS = Fetal Alcohol Syndrome
Other exclusion criteria precluding participation in the study for subjects in all three groups included: 1) prenatal exposure to cocaine or opiates, 2) age younger than 7 years, 3) IQ less than 70, 4) head injury with loss of consciousness for more than 20 minutes, 5) a physical (e.g., hemiparesis), psychiatric, or developmental (e.g., autism) disability that would preclude participation, 6) other potential known causes of mental deficiency (e.g., chromosomal disorders), 7) significant maternal illness with increased risk for fetal hypoxia (e.g., sickle cell disease), 8) presence of metallic implants in the body which posed a risk for MRI. Additionally, subjects were excluded from the study if they performed below 1.5 standard deviations from the mean performance of their group on the N-Back task (n=7), or due poor fMRI data quality (n=8). After the exclusion of all unsuitable subjects, the fifty remaining participants were included in the analyses described below.
Procedures
Following a complete description of the study protocol, all participants and their parents gave informed assent/consent according to procedures approved by the UCLA Institutional Review Board.
Image Acquisition
Functional magnetic resonance imaging data were collected on a 3 Tesla Siemens Allegra head-only magnet. Multislice echo-planar imaging was used with a gradient echo echo-planar imaging sequence. We used TR = 3 seconds, TE = 25 ms, 3mm slice thickness with 1 mm skip, 36 slices, 64 X 64 pixels yielding 3.1 mm in-plane resolution with whole-brain acquisition. A high-resolution T2-weighted echo-planar imaging volume was collected in the anterior commissure-posterior commissure plane, coplanar with the functional scan to facilitate the subsequent spatial registration of each subject’s data into the Montreal Neurological Institute (MNI)-152 standard coordinate space (TR = 5 seconds, TE = 33 ms, flip angle = 90°, 3mm slice thickness with 1 mm skip, 36 axial slices covering the entire brain, matrix size = 128 X 128 with 1.6 X 1.6 mm in-plane resolution).
Neurocognitive Evaluations
Children underwent extensive neuropsychological testing. Included among tests administered was an abbreviated version of the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV, Wechsler, 2003). A prorated full-scale intelligence quotient (FSIQ) was derived from the Verbal Comprehension Index (VCI) and Perceptual Reasoning Index (PRI). This method is described in the WISC-IV manual. FSIQ was not available for 1 of the ALC subjects.
Functional Imaging Task: Visuo-spatial N-Back
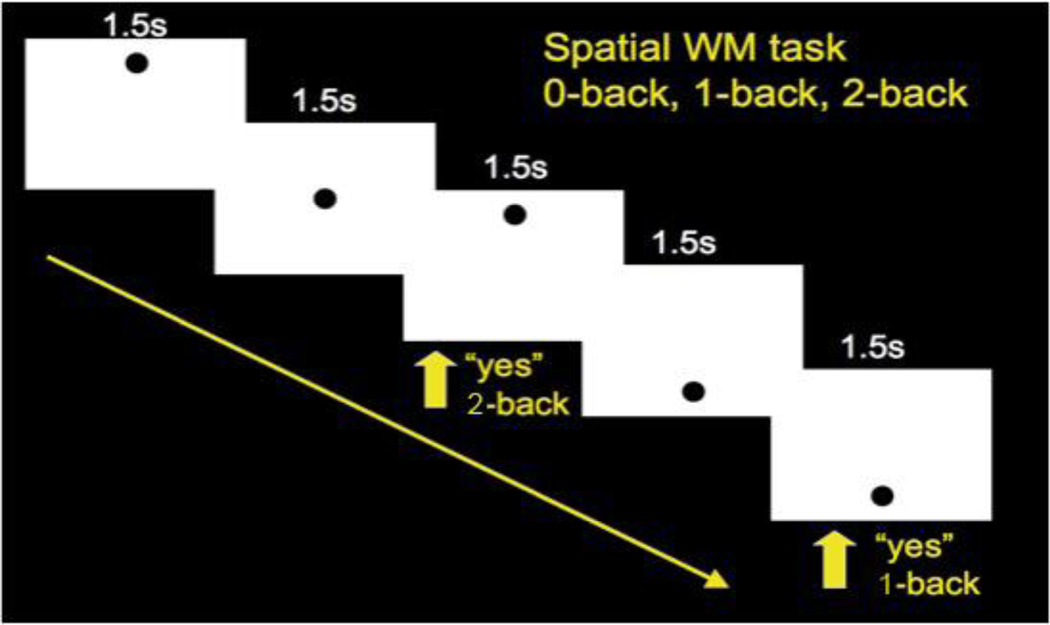
Figure 1 depicts our visuo-spatial N-Back task. The task consisted of rest and experimental blocks, with 3 rest blocks (30 seconds each, during which subjects stared at a blank screen) and 12 experimental blocks with 4 each of 0, 1 and 2-Back blocked trials randomly interspersed. The 0, 1 and 2-back blocks started with a display of the instructions “Push for Center”, “Push for 1-Back”, and “Push for 2-Back”, respectively. Each experimental block consisted of 16 stimuli presented for 500 msec each, with a 1,500 msec inter-stimulus interval. The stimulus “O” was presented in one of 9 distinct visuo-spatial locations. In the 1-back task, participants were asked to respond if the stimulus was in the same location as the previous stimulus, and in the 2-back task they were asked to respond if the stimulus was in the same location 2 steps back. All subjects were able to perform the task during pre-scan training. The entire task lasted 8.2 minutes. Accuracy was recorded and brain activation between groups was compared.
Figure 1. Parametric Visuo-spatial Working Memory “N-Back” Task.
Zero, one, and two item loads were presented in separate blocks. Subjects were required to maintain and update the location of a black circle and make a button press according to the target location within each block.
Image Analysis
Preprocessing
FMRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl, Smith et al., 2004). Unless otherwise noted, all of the individual tools drew from that library. Prior to analysis, each image was partially processed and assessed for image quality using the following preprocessing methods: they were motion corrected using MCFLIRT (Motion Correction FMRIB’s Linear Image Registration Tool, Jenkinson et al., 2002) but not smoothed, and visually inspected for artifact. Five subjects were excluded due to visible slice dropout on more than 10 volumes and three participants were excluded because of excessive field distortion, caused by movement outside of the field of view over the course of the scanning session. In the remaining participants, the following .pre-statistics processing was applied: non-brain removal on structural and motion corrected functional images using FSL’s Brain Extraction Tool (BET) (Smith, 2002), spatial smoothing using a Gaussian kernel of FWHM 6.0mm, grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 30.0 s).
Single Subject Statistical Analyses
FMRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Time-series statistical analysis was carried out using FMRIB’S Improved Linear Model (FILM), with local autocorrelation correction done to estimate and correct the temporal autocorrelation, improving parameter estimates (Woolrich et al, 2001). FMRI data were analyzed using standard methods within the FMRIB’s fMRI Expert Analysis Tool (FEAT version 5.98). Registration to both high resolution, T2-weighted structural and the Montreal Neurological Institute (MNI)-152 standard space template images was carried out with FLIRT (FMRIB’s Linear Image Registration Tool, Jenkinson & Smith, 2001, Jenkison et al., 2002), using 6 and 12 degrees of freedom respectively. Significance of Z (Gaussianised T/F) statistic images was determined by using a voxelwise threshold of Z >1.7 and a (corrected) cluster significance threshold of P=0.05 (Friston et al., 1994; Worsley et al., 1996; Worsley, 2001).
Group Analyses
For fMRI signal detection and localization, a hemodynamic response model was created based on the convolution of the stimulus timing with a double gamma model of the nominal hemodynamic “impulse” response (Woolrich et al., 2001). The temporal derivative was also modeled, compensating for the spread in acquisition time of the slices across the TR period and allowing robust detection of activation slightly out of phase with the stimulus. Each condition was contrasted with rest on a single-subject level, leading to estimates of beta weights and variance at each voxel location. To account for residual motion related intensity changes after motion correction, 6 motion parameters outputted by MCFLIRT (Motion Correction FMRIB’s Linear Image Registration Tool) and their temporal derivatives were modeled: the former accounted for slow changes and the latter for sudden changes in head position. Working-memory activation was derived by contrasting activation during the 2-Back with activation during the 0-Back task. The 2-Back task was used in the contrast because this condition involves greater cognitive demands than the 1-Back task, and using a challenging task facilitates the detection of possible group differences in brain activation.
In order to explore the specific effects of prenatal MA exposure, we conducted whole-brain pair-wise group analyses (i.e., MAA vs. ALC, ALC vs. CON, MAA vs. CON) and included alcohol exposure clinical severity as a parameterized between-group covariate (with a score of 0 indicating no prenatal alcohol exposure, 1 indicating some alcohol exposure or a diagnosis of ARND, 2 indicating a sentinel diagnosis, 3 representing a diagnosis of PFAS, and 4 representing an FAS diagnosis). In addition, follow-up analyses were performed in order to address the possible confounding effects of group differences in age and N-back task performance. In an analysis comparing ALC and MAA participants, subject age was included as a between-group covariate in order to remove variance in brain activation due to age differences between groups. Additionally, in an analysis comparing the CON and MAA groups, subject accuracy on the 2-Back task was included as a between-group covariate in order to model variance in brain activation due to group differences in performance on the task. Significance for Z (Gaussianised T/F) statistic images resulting from group analyses was determined by using a voxelwise threshold of Z >1.7 and a (corrected) cluster significance threshold of P=0.05 (Friston et al., 1994; Worsley et al., 1996; Worsley, 2001).
Brain Activation-Performance Correlations
In order to explore the correlations between performance on the 2-Back task and functional brain activation during working memory (2-Back – 0-Back contrast) in each of the 3 groups, we conducted whole-brain analyses in which demeaned accuracy scores were included as a within-group covariate.
Statistical Analysis of Demographics and Performance Data
Statistical analyses were conducted using SPSS Statistics 17.0. Raw scores were evaluated for accuracy on the visuo-spatial N-Back task. Group differences for integer variables (e.g., age) were evaluated with analysis of variance (ANOVA). Group differences in categorical data (e.g., gender) were assessed with a Pearson Chi-square test.
RESULTS
Demographics
Demographic descriptors and behavioral performance on the N-Back task and on FSIQ measures are reported in Table 2. Groups did not differ from each other in gender distribution. The groups differed in FSIQ [F (2,48) = 10.62], with the CON group scoring significantly higher than both the ALC (p < 0.001) and MAA groups (p = 0.017), but the MAA and ALC groups did not differ from each other. The groups also differed in age [F (2,49) = 3.93] with the ALC group being significantly older than the MAA group (p = 0.023) but not the CON group, and the CON and MAA group did not differ from each other. The groups differed in overall accuracy on the N-Back task [F (2,49) = 6.47, p = 0.002] and in accuracy on the 2-Back condition of the task [F (2,49) = 4.50, p = 0.019]. In both cases, the CON group scored significantly higher than the MAA group but not the ALC group, and the 2 exposed groups did not differ from each other.
Table 2.
Demographics and Performance for Each Group (Mean and Standard Deviation)
| MAA (n=19) | ALC (n=13) | CON (n=18) | Group Differences | |
|---|---|---|---|---|
| Age | 9.16 (1.83) | 11.46 (2.44) | 10.28 (2.61) | ALC>MAA [F (2,49) = 3.93, p=.023] |
| Female/Male | 8 / 11 | 4 / 9 | 9 / 9 | None |
| Accuracy (Total) | .84 (.08) | .88 (.07) | .92 (.04) | CON>MAA [F (2,49) = 6.47, p=.002] |
| Accuracy (2-Back) | .71 (.11) | .78 (.10) | .80 (.09) | CON>MAA [F (2,49) = 4.50, p=.019] |
| FSIQ | 97.47 (14.08) | 86.67 (16.49) | 111.67(14.61) | CON>ALC [F (2,48) = 10.62, p<.001]; CON> MAA [F (2, 48) =10.62, p=.017] |
Values expressed as mean (SD)
Group Averages and Group Differences in Brain Activation
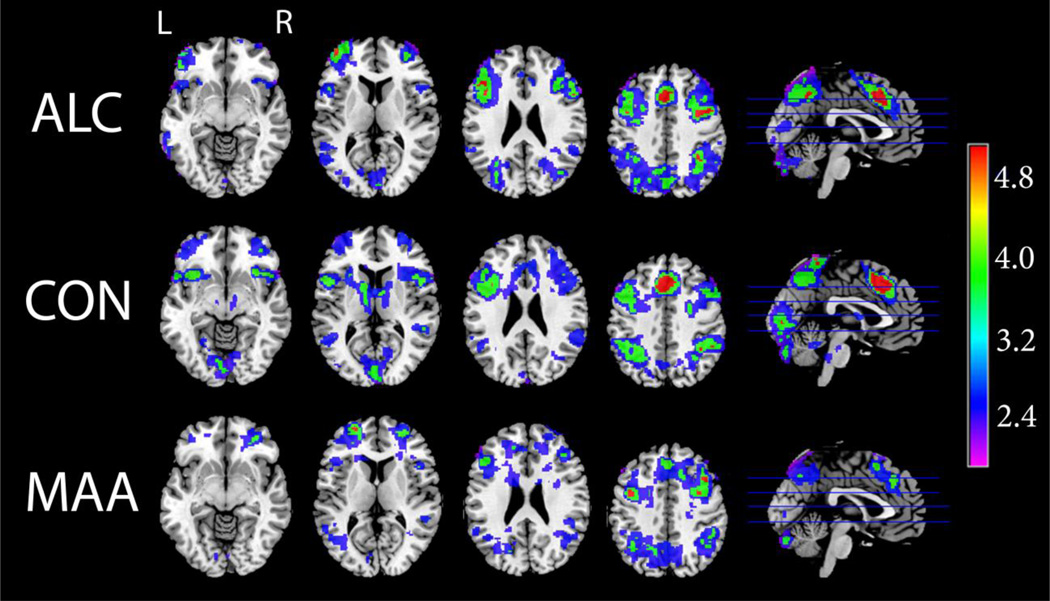
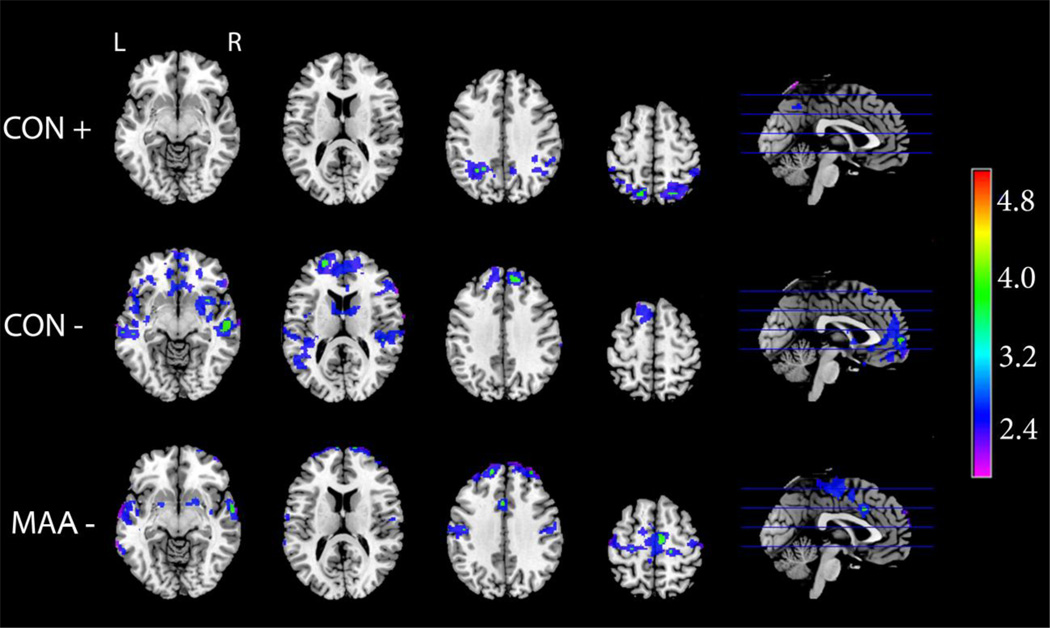
Figure 2 depicts group average activation maps during the visuo-spatial working memory task (2-Back – 0-Back contrast), showing activation in the canonical frontal-parietal-cerebellar WM network for the MAA, ALC, and CON groups. Qualitative comparisons suggest some discrepancies between groups, primarily in two areas involved in dopaminergically-mediated executive processing. Activation in the basal ganglia was apparent in the CON group, but not the exposed groups. Further, ALC subjects seemed to show greater intensity of activation than the other two groups in dorsal frontal regions.
Figure 2. Visuo-spatial N-Back: Group Average Activation Maps.
Axial and sagittal sections displaying group average activation maps during the visuo-spatial n-back working memory task. Shown here are regions of significantly (Z>1.7 and a corrected cluster significance threshold of p=0.05) greater activation during working memory (2-back – 0-Back contrast). Colors on the maps correspond to the color bar on the right.
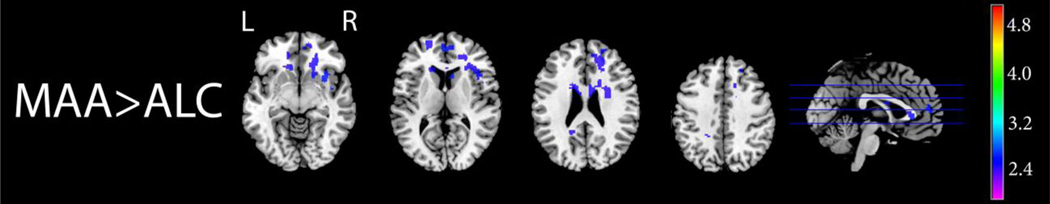
After controlling for age, the MAA group showed increased activation relative to the ALC group in several areas, including in the anterior cingulate bilaterally, in the right insula, right caudate and putamen, right frontal orbital cortex, and right frontal pole, and in the left precuneous in the 2-Back – 0-Back contrast. Figure 3 illustrates these group differences. Initial group comparison analyses suggested that the ALC group had increased activation relative to MAA subjects in various brain regions, notably in the right cerebellum and lateral occipital cortex, and in the left dorsolateral prefrontal cortex (data not shown). However, these differences where no longer significant in a follow-up analysis which included subject age as a between-group covariate in order to statistically control for the significant group difference in age. This suggests that these differences in brain activation reflected age differences between the ALC and MAA group, rather than differences which could be attributed to prenatal exposure to different teratogens.
Figure 3. Visuo-spatial N-Back: MAA>ALC.
Axial and sagittal sections displaying group differences in brain activation during the visuo-spatial N-Back working memory task. Shown here are regions of significantly (Z>1.7 and a corrected cluster significance threshold of p=0.05) greater activation during working memory (2-Back – 0-Back contrast) in the MAA than the ALC group with age included as a covariate. Colors on the maps correspond to the color bar on the right.
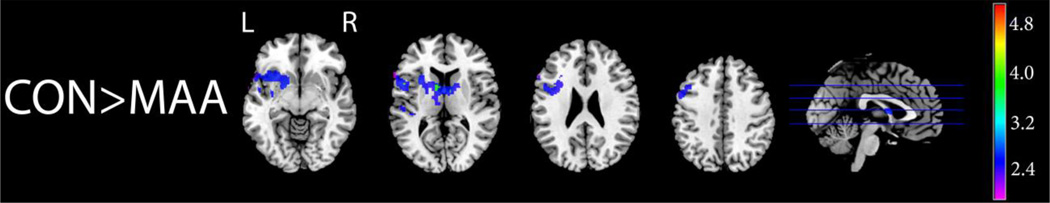
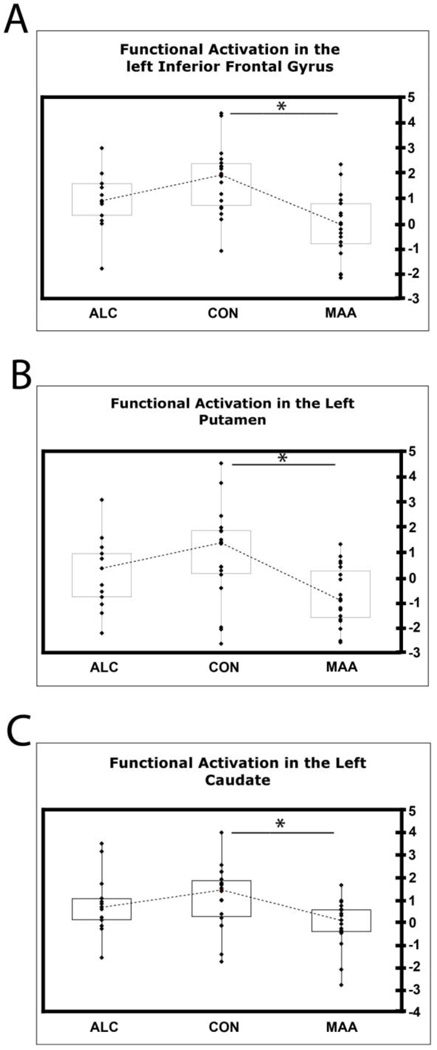
Figure 4 illustrates that the MAA group had decreased activation relative to the CON group in many brain areas, including, as predicted, frontal and basal ganglia regions in the left hemisphere during working memory (2-Back) versus rest (0-Back). This group difference was most prominent in the left caudate, left putamen, and left inferior frontal gyrus, around Broca’s area. In these three regions (Table 3), plots of the functional activation (t-values) for each subject in all 3 groups revealed that ALC individuals showed a level of functional activation intermediate between that of CON and MAA participants (Fig. 5).
Figure 4. Visuo-spatial N-Back: CON>MAA.
Axial and sagittal sections displaying group differences in brain activation during the visuo-spatial N-Back working memory task. Shown here are regions of significantly (Z>1.7 and a corrected cluster significance threshold of p=0.05) greater activation during working memory (2-Back – 0-Back contrast) in the CON than MAA group. Colors on the maps correspond to the color bar on the right.
Table 3.
Regions of interest: anatomical name, MNI coordinates, hemisphere, and location in figures.
| Anatomical Structure | MNI x, y, z coordinates | Hemisphere | Location |
|---|---|---|---|
| Inferior Frontal Gyrus | −50, 8, 16 | Left | Figure 5 A |
| Putamen | −22, 10, 4 | Left | Figure 5 B |
| Caudate | −10, 4, 10 | Left | Figure 5 C |
| Putamen | −20, 2, −8 | Left | Figure 7 |
Figure 5. Group Differences in activation in the Left Inferior Frontal Gyrus, Left Putamen, and Left Caudate.
In order to investigate how individuals’ activation during WM vs. rest contributed to group differences in activation, we plotted t-values (x-axis) for each subject from regions of interest that were significant in the group comparison map (see Fig. 4) in the left inferior frontal gyrus (A), in the left putamen (B), and in the left caudate (C). The medians are joined by interrupted lines. A star represents a significant difference (p < 0.05) in functional activation between groups.
Other brain regions in the left hemisphere where the MAA group showed decreased activation relative to the CON group included the middle frontal gyrus, the precentral gyrus, the frontal orbital cortex, the superior and middle temporal gyri, the temporal pole, the planum temporale, and the insula. Additionally, MAA subjects recruited both left and right thalamus less than CON subjects during working memory versus rest (Fig 4). A follow-up analysis in which accuracy on the 2-Back task was included as a between-group covariate in order to control for the variance in functional activation due to the performance difference between the CON and the MAA groups revealed very similar results (data not shown). This suggests that activation differences between the CON and MAA groups illustrated in Figure 4 were not explained by group differences in accuracy on the task.
Brain Activation-Performance Correlations
To better understand what activation patterns are associated with better visuospatial WM performance, we performed whole-brain analyses of the correlations between activation during working memory (2-Back - 0-Back contrast) and accuracy on the 2-Back task, in each of the 3 groups. Results showed that, in the ALC group, correlations between brain activation and performance did not reach significance in any brain regions. In the CON group, activation in some superior and posterior brain regions was positively correlated with task accuracy such that increased activation was associated with better performance. These regions encompassed the supramarginal and angular gyri, the superior parietal lobule, and the precuneous, bilaterally (Fig. 6, top panel).
Figure 6. Correlations Between Performance on the 2-Back Task and Functional Brain Activation in the CON and MAA groups.
Axial and sagittal sections displaying regions showing a significant (Z>1.7 and a corrected cluster significance threshold of p = 0.05) correlation between accuracy on the 2-Back task and functional brain activation during working memory (2-Back – 0-Back contrast). The top panel represents regions whose activation is positively correlated with performance in the CON group. The middle panel represents regions whose activation is negatively correlated with performance in the CON group. The bottom panel represents regions whose activation is negatively correlated with performance in the MAA group. Colors on the maps correspond to the color bar on the right.
There was a negative correlation between performance and activation in the inferior temporal gyrus and temporal pole, the middle temporal gyrus, the anterior cingulate and paracingulate gyri, the frontal orbital cortex and frontal pole bilaterally, and the left superior frontal gyrus, in both the CON and MAA groups (Fig. 6, middle and bottom panels). In addition, activation in the right inferior frontal gyrus and insula, and in the planum temporale and caudate nucleus bilaterally was negatively correlated with task accuracy in the CON group only (Fig. 6, middle panel). In all these regions, individuals with less activation performed better on the task. Specific to the MAA group was a negative correlation between performance and activation in the left parahippocampal gyrus, and bilaterally in the pre- and post-central gyri, superior temporal gyrus, and putamen (Fig. 6, bottom panel).
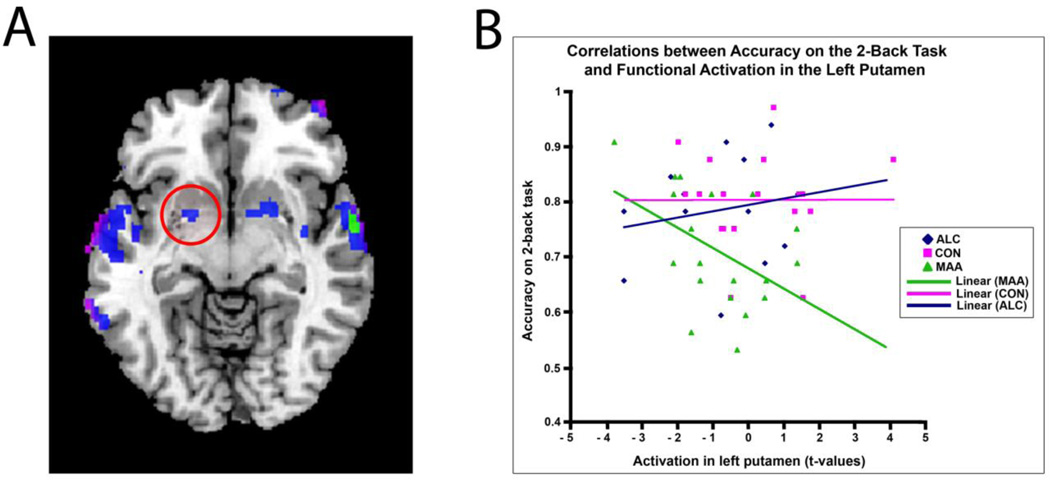
The left putamen was both an area in which MAA individuals showed decreased activation relative to controls (Fig. 4 and Fig. 5B), and a region whose activation was negatively correlated with performance in the MAA group (Fig. 6 bottom panel and Fig. 7A). In this region (Table 3), plotting the functional activation (t-values) in sample voxels for each subject in all three groups revealed that the negative correlation between performance and activation in the left putamen (r = −0.46) may be specific to MAA subjects. In the ALC and CON groups, there was no significant correlation between activation in this region and accuracy on the 2-Back task (r = 0.18 and r = 0.002, respectively, Fig. 7B). However, the group-by-score interaction did not reach statistical significance, so caution should be taken in interpreting the non-significant effects in the ALC and CON groups.
Figure 7. Correlations Between Performance on the 2-Back Task and Functional Brain Activation in the Left Putamen.
(A) Axial section displaying regions showing a significant (Z>1.7 and a corrected cluster significance threshold of p = 0.05) negative correlation between accuracy on the 2-Back task and functional brain activation during working memory (2-Back – 0-Back contrast) in the MAA group. Circled in red is the left putamen. (B) Regression plots for correlations between functional activation in the left putamen (t-values) and accuracy on the 2-Back task in all 3 groups. Pink, CON group; Blue, ALC group; Green, MAA group.
DISCUSSION
We observed overall regional activation in the neuronal network known to be involved in WM: in frontal and parietal regions, including the dorsolateral prefrontal cortex (DLPFC), middle frontal gyrus, posterior parietal lobe (Goldman-Rakic, 1988; Courtney et al., 1996; Smith & Jonides, 1996; Braver et al., 1997; Klingberg et al., 1997; Belger et al., 1998; Carlson et al., 1998; Casey et al., 1998; Jonides et al., 1998; Callicot et al., 1999; Rypma et al., 1999, Braver et al., 2001) and in the cerebellum (Desmond et al., 1997; Kirschen et al., 2005). All of these brain areas were activated to some extent in the MAA, ALC, and CON groups. As predicted, we found that the MAA group had decreased activation relative to the CON group in striatal and frontal regions in the left hemisphere during working memory (2-Back - 0-Back contrast). Various human and animal studies have established that the basal ganglia plays a crucial role in WM, which is thought to be mediated by dopaminergic projections from the striatum to the prefrontal cortex (Goldman-Rakic, 1995; Graham & Goldman-Rakic, 1995; Gruber et al., 2006; McNab & Klingberg, 2008). Structural and metabolic abnormalities of the striatum and fronto-striatal connections have been demonstrated in subjects with prenatal exposure to MA, specifically reduced striatal volumes (Chang et al., 2004), abnormalities in the white matter fibers connecting the cortex and the striatum (Colby et al., submitted), and increased creatine and phosphocreatine in the striatum, suggesting abnormal energy metabolism in this region (Smith et al., 2001). Our finding suggests that damage to this fronto-striatal circuit in subjects with prenatal exposure to MA may be related to suppression of activation in the brain regions involved in this circuit while performing a WM task. Additional brain regions in which we reported decreased activation in MAA relative to the CON group, such as the left inferior frontal gyrus and lateral temporal lobe, and the thalamus bilaterally, are also known to show structural and/or metabolic abnormalities in subjects with prenatal MA exposure (Chang et al., 2009; Sowell et al., 2010).
Structural, metabolic, and functional abnormalities in the striatum have also been reported in subjects with severe prenatal alcohol exposure. Several studies have shown reduced striatal volumes in children with FAS (Mattson et al., 1996) and FASDs (Archibald et al, 2001; Cortese et al., 2006). Metabolic abnormalities in striatal regions as a result of severe prenatal exposure to alcohol have been reported both in human (Clark et al., 2000) and animal (Schneider et al., 2005; Schneider et al., 2008) studies. In addition, a functional imaging study showed decreased caudate activation during a response inhibition task in subjects with FASDs, suggesting a functional impairment of the fronto-striatal circuitry in this population (Fryer et al., 2007). In the present study, we found that ALC individuals showed a level of functional activation intermediate between that of CON and MAA participants in the left caudate and putamen, and in another study with an overlapping sample of subjects, direct comparisons of the ALC and MAA groups revealed that, although both exposed groups showed striatal volume reductions compared to controls, MAA individuals were more severely affected (Sowell et al., 2010). Although we must use caution in interpreting this finding, because the difference in functional activation between the ALC group and either the CON or MAA group did not reach statistical significance in the left striatum, this observation suggests that in children with prenatal exposure to drugs of abuse, greater damage (i.e., greater volume deficits) to the fronto-striatal circuit is associated with suppression of activation in the brain regions important for WM, relative to unexposed controls.
Correlation analyses revealed activation involving basal ganglia circuits are related to WM performance. Our findings of negative correlations between activation in the caudate bilaterally and task accuracy in the control group corroborates a previous report that, despite the important role of the striatum in WM, basal ganglia activation during WM decreases over the course of development (Scherf et al., 2006). An unexpected but intriguing finding was that, in the MAA group, there was no correlation between performance and activation in the caudate, but in this group, we found a negative correlation between accuracy on the 2-Back task and activation in the putamen bilaterally. There are five distinct basal ganglia-thalamocortical circuits (or loops) organized in parallel, with each circuit engaging specific regions of the cerebral cortex, striatum, pallidum, substantia nigra, and thalamus, and each circuit serving distinct motor, cognitive, or emotional functions (Alexander et al., 1986; Cummings, 1993). The dorsolateral prefrontal loop, which is involved in working memory, engages the dorsolateral caudate (Cummings, 1993; Lewis et al., 2004) whereas the motor loop engages the putamen (Alexander et al., 1986; Cummings, 1993). It has been shown that striatal dopamine depletion leads to changes in cortico-striatal network properties, leading to a remapping of cerebral connectivity that reduces spatial segregation and causes increased interaction between different cortico-striatal loops (Helmich et al., 2010). This has been demonstrated to occur as a result of a reduction in striatal dopamine in Parkinson’s disease (Helmich et al., 2010). One possible interpretation for the fact that, in the MAA group, we found a negative correlation between performance and activation in the putamen, rather than the expected negative correlation between performance and activation in the caudate, is that a mechanism similar to the one occurring in Parkinson’s disease may take place in children with prenatal exposure to MA. It is plausible that damage to dopamine terminals in the striatum during ontogeny may affect the development of neural circuits and lead to a comparable remapping phenomenon. Thus, in MAA individuals, the dorsolateral prefrontal loop activated during working memory tasks may preferentially engage the putamen, rather than the dorsolateral caudate, perhaps because of decreased spatial segregation between the motor loop and the dorsolateral prefrontal loop.
This interpretation is consistent with a related observation in the striatum. We found that activation in the left putamen was negatively correlated with performance in the MAA group, but not in the ALC and CON groups. Because the dorsolateral prefrontal loop involved in working memory engages the caudate and not the putamen (Cummings, 1993; Lewis et al., 2004), we would not expect to see any correlation between performance and activation in the putamen in the CON or ALC groups. The putamen is normally a part of the motor loop (Alexander et al., 1986; Cummings, 1993), but if such remapping of cerebral connectivity occurred in people with prenatal MA exposure, resulting in preferential recruitment of the putamen during WM tasks, we would expect that the MAA group would be the only group showing a negative correlation between performance and activation in the putamen. The interpretation that a rewiring of cortico-striatal networks may occur as a result of prenatal MA exposure may explain our findings, but future studies should aim at directly measuring and quantifying functional connectivity across different networks in order to test this hypothesis. Our data does not provide insight as to whether this possible remapping phenomenon represents a detrimental effect of methamphetamine teratogenicity or a favorable compensatory mechanism in response to damage to specific parts of the networks.
After controlling for age, direct statistical comparisons between the ALC and MAA groups suggested that ALC individuals showed decreased activation relative to MAA subjects in several areas, located mostly in the right hemisphere, in the 2-Back – 0-Back contrast. These areas included the right caudate and putamen. As noted above, the striatum is a region involved in WM function and known to be a target of the teratogenic effects of both alcohol (Mattson et al., 1996; Clark et al., 2000; Archibald et al., 2001; Schneider et al., 2005; Cortese et al., 2006) and MA (Smith et al., 2001; Chang et al., 2004; Colby et al., submitted). Some evidence suggests that this brain structure may be more damaged in the right hemisphere in persons prenatally exposed to alcohol, whereas striatal abnormalities may be more pronounced in the left hemisphere in subjects with prenatal MA exposure. To our knowledge, the only human report of physiological abnormalities in the striatum of subjects with FASDs showed decreases in relative regional metabolic rates in the caudate head bilaterally, but only in the right caudate body and right putamen (Clark et al., 2000). In addition, an fMRI study of prenatal alcohol exposure reported decreased activation in the right caudate in ALC children and adolescents during a response-inhibition task, suggesting functional abnormalities in this region (Fryer et al., 2007). In contrast, our group recently reported white-matter abnormalities in corticostriate projections restricted to the left hemisphere in an overlapping sample of subjects with prenatal MA exposure (Colby et al., submitted), partially replicating a previous report (Cloak, 2009). Taken together with the finding that the MAA group had decreased activation relative to the CON group in the left striatum, the observation that the ALC group had decreased activation relative to MAA subjects in the right striatum provides additional support for the idea that in exposed children, damage to the fronto-striatal circuit is associated with suppression of activation in the brain regions involved in this circuit, while performing a WM task. While we did not have strong predictions about laterality in activation, and although the etiology of these apparent left-right structural asymmetries is unknown, these findings are intriguing and suggest that alcohol and methamphetamine exposure during gestation may have specific effects on brain structure and function. The differences reported here might be due to distinct mechanisms of teratogenic action for the two drugs, or interactions between them which may differentially impact the two hemispheres.
There are some important limitations to this study. Because maternal smoking records were unavailable in a large portion of our current sample, we were unable to add prenatal nicotine exposure as a covariate in our analyses. This limitation is particularly concerning, given that nicotine has been shown to induce lasting abnormalities in neurogenesis in animal models (Slotkin, 1998). We cannot exclude the possibility that nicotine exposure could contribute partly to the observed differences between exposed groups and control subjects. However, well-controlled animal studies of prenatal exposure to alcohol and methamphetamine have shown that each of these substances is sufficient to induce lasting structural, metabolic, and behavioral changes, in the absence of any concurrent exposure to nicotine or other drugs of abuse (as reviewed in Thompson et al., 2009). We also expect that exposed groups would have similarly matched nicotine exposure rates and that the possible nicotine main effect would have only minimal influence on the MAA vs. ALC contrast. However, future studies should examine the possibility of second-order nicotine-MA or nicotine-ALC interactive effects. Given that polydrug exposure is apparently the norm, at least in Southern California from where our sample was drawn, it is not clear that samples with “pure” single drug exposures would be as relevant to the populations we ultimately hope to serve.
Precise exposure histories and dosages were generally unavailable given that many of the subjects in our exposed groups had been adopted. This is true of most retrospective human studies of prenatal drug exposure given that quantities and frequencies of drug exposure are difficult to accurately recall years after the drug use, and may be compounded by the stigma of admitting to drug use during pregnancy. Further, women who receive methamphetamine from their partners may not actually know the dosage they ingest. Potential underreporting by biological mothers is of sufficient concern that reports from adoptive mothers (based on observation of biological mother’s behavior or from social services reports) may have similar levels of validity to that of biological mothers.
Finally, the majority of our MA-exposed participants also had concomitant alcohol exposure, and although we included alcohol exposure clinical severity as a parameterized between- group covariate in our analyses in hopes of enhanced specificity for detecting MA effects, it is possible that higher-order interaction effects between alcohol and MA may account for some of our observations pertaining to the MAA group. Besides, it is not clear that we accounted for all the variance due to possible differences in quantity and frequency of alcohol exposure between our ALC and MAA groups. Nonetheless, given that we did detect group differences supporting our a priori hypotheses, and given that some of our hypotheses are supported by animal studies with well-documented single drug exposures, it is likely that the results we report here have more to do with MA exposure, or the combination of MA and alcohol exposure, than with alcohol exposure alone.
Despite these limitations, this study offers important contributions to the study of prenatal exposure to MA. By providing the first report of abnormal brain activation during working memory in children and adolescents with prenatal MA exposure, by evaluating these effects of prenatal MA exposure in the context of an alcohol-exposed contrast group, and by raising the possibility that a rewiring of cortico-striatal networks may occur in these subjects, this study provides support to the idea that methamphetamine exposure leads to unique patterns of functional activation and perhaps functional connectivity within the developing brain. Future studies should focus on the integration of observations from different brain imaging modalities in order to precisely characterize the structural, metabolic, and functional brain abnormalities resulting from prenatal methamphetamine exposure. This will enable clinicians to develop appropriate behavioral, educational, and occupational interventions in order to address the specific needs of this population.
Acknowledgments
Grant support:
This work was supported by NIDA grants R21 DA15878 and R01 DA017831, the March of Dimes (6FY2008-50) and the NIAAA (U01 AA017122) awarded to ERS. Additional support was provided by the National Center on Research Resources, General Clinical Research Center (3 M01 RR00425) awarded to LMS and National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 RR021813 entitled Center for Computational Biology (CCB; http://nihroadmap.nih.gov/bioinformatics).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Ms. Roussotte reports no competing interests.
Dr. Bramen reports no competing interests.
Dr. Nunez reports no competing interests.
Ms. Quandt reports no competing interests.
Dr. Smith reports no competing interests.
Dr. O’Connor reports no competing interests.
Dr. Bookheimer reports no competing interests.
Dr. Sowell reports no competing interests.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Measuring the facial phenotype of individuals with prenatal alcohol exposure: correlations with brain dysfunction. Alcohol & Alcoholism. 2001;36(2):147–159. doi: 10.1093/alcalc/36.2.147. [DOI] [PubMed] [Google Scholar]
- Astley SJ. Diagnostic guide for fetal alcohol spectrum disorders: the 4-digit diagnostic code. Seattle, WA: University of Washington; 2004. [Google Scholar]
- Baddeley A. Recent developments in working memory. Current Opinion in Neurobiology. 1998;8:234–238. doi: 10.1016/s0959-4388(98)80145-1. [DOI] [PubMed] [Google Scholar]
- Belger A, Puce A, Krystal JH, Gore JC, Goldman-Rakic P, MvVarthy G. Dissociation of mnemonic and perceptual processes during spatial and nonspatial working memory using fMRI. Hum Brain Mapp. 1998;6:14–32. doi: 10.1002/(SICI)1097-0193(1998)6:1<14::AID-HBM2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE. Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. NeuroImage. 2001;14:48–59. doi: 10.1006/nimg.2001.0791. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Carlson S, Martinkauppi S, Rama P, Salli E, Korvenoja A, Aronen HJ. Distribution of cortical activation during viusospatial n-back tasks as revealed by functional magnetic resonance imaging. Cereb Cortex. 1998;8:743–752. doi: 10.1093/cercor/8.8.743. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, O’Craven K, Davidson RJ, Irwin W, Nelson CA, Noll DC, Hu X, Lowe MJ, Rosen BR, Truwitt CL, Turski PA. Reproducibility of fMRI results across four institutions using a spatial working memory task. NeuroImage. 1998;8:249–261. doi: 10.1006/nimg.1998.0360. [DOI] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Research: Neuroimaging. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, Hedemark B, Smith LM, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. NeuroImage. 2009;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AM, Li D, Conry J, Conry R, Loock C. Structural and functional brain integrity of fetal alcohol syndrome in nonretarded cases. Pediatrics. 2000;105(5):1096–1099. doi: 10.1542/peds.105.5.1096. [DOI] [PubMed] [Google Scholar]
- Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72:2068–2075. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Bookheimer SY. Localization of brain function using magnetic resonance imaging. TINS. 1994;17(7):268–277. doi: 10.1016/0166-2236(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Colby JB, Smith L, O’Connor MJ, Bookheimer SY, Van Horn JD, Sowell ER. The effects of prenatal methamphetamine/polydrug exposure on white matter microstructure: A tract-based diffusion imaging study. (submitted) [Google Scholar]
- Colliver JD, Kroutil LA, Dai L, Gfroerer JC. Misuse of prescription drugs: Data from the 2002, 2003, and 2004 National Surveys on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2006. [Google Scholar]
- Cortese BM, Moore GJ, Bailey BA, Jacobson SW, Delaney-Balck V, Hannigan JH. Magnetic resonance and spectroscopic imaging in prenatal alcohol-exposed children: Preliminary findings in the caudate nucleus. Neurotoxicology and Teratology. 2006;28:597–606. doi: 10.1016/j.ntt.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JF. Object and spatial visual working memory activate separate neural systems in the human cortex. Cereb Cortex. 1996;6:39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Derauf C, Kekatpure M, Neyzi N, Lester B, Kosofsky B. Neuroimaging of children following prenatal drug exposure. Seminars in Cell and Developmental Biology. 2009;20:441–454. doi: 10.1016/j.semcdb.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcoholism: Clinical and Experimental Research. 2007;31(8):1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Frost DO, Cadet JL. Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: a hypothesis. Brain Research Reviews. 2000;34:103–118. doi: 10.1016/s0165-0173(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:1137–1156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Graham VW, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Dayan P, Gutkin BS, Solla SA. Dopamine modulation in the basal ganglia locks the gate to working memory. J Comput Neurosci. 2006;20:153–166. doi: 10.1007/s10827-005-5705-x. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb Cortex. 2010;20(5):1175–1186. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimization method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, Willis CR. The role of parietal cortex in verbal working memory. J Neurosci. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Schraedley-Desmond P, Desmond JE. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: An fMRI study. NeuroImage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Klingberg T, O’Sullivan BT, Roland PE. Bilateral activation of fronto-parietal networks by incrementing demand in a working memory task. Cereb Cortex. 1997;7:465–471. doi: 10.1093/cercor/7.5.465. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. European Journal of Neuroscience. 2004;19:775–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Lu LH, Johnson A, O’Hare ED, Bookheimer SY, Smith LM, O’Connor MJ, Sowell ER. Effects of prenatal methamphetamine exposure on verbal memory revealed with functional magnetic resonance imaging. J Dev Behav Pediatr. 2009;30:185–192. doi: 10.1097/DBP.0b013e3181a7ee6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisza KL, Allman AA, Schiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: A functional magnetic resonance imaging study. Pediatric Research. 2005;58(6):1150–1157. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20(6):1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- O'Connor MJ, Frankel F, Paley B, Schonfeld AM, Carpenter E, Laugeson EA, Marquardt R. A controlled social skills training for children with fetal alcohol spectrum disorders. J Consult Clin Psychol. 2006;74:639–648. doi: 10.1037/0022-006X.74.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Mattson SN, O'Connor MJ, Sowell ER. Altered frontal-parietal functioning during verbal working memory in children and adolescents with heavy prenatal alcohol exposure. Hum Brain Mapp. 2009;30:3200–3208. doi: 10.1002/hbm.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rympa B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependant roles of frontal brain regions in the maintenance of working memory. NeuroImage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of deveopmental change in visuospatial working memory. Journal of Cognitive Neuroscience. 2006;18(7):1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Barnhart TE, Larson JA, DeJesus OT, Mukherjee J, Nickles RJ, Converse AK, Roberts AD, Kraemer GW. Moderate-level prenatal alcohol exposure alters striatal dopamine system function in rhesus monkeys. Alcoholism: Clinical and Experimental Research. 2005;29(9):1685–1697. doi: 10.1097/01.alc.0000179409.80370.25. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Gajewski LL, Larson JA, Roberts AD, Converse AK, DeJesus OT. Sensory processing disorder in a primate model: Evidence from a longitudinal study of prenatal alcohol and prenatal stress effects. Child Dev. 2008;79(1):100–113. doi: 10.1111/j.1467-8624.2007.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Smith EE, Jonides J. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Grotta SD, Liu J, Lester BM. The infant development environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Grotta SD, Fallone M, Liu J, Lester BM. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O'Hare ED, McCourt ST, Mattson SN, O'Connor MJ, Bookheimer SY. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. Neuroreport. 2007;18(7):635–639. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Leow AD, Bookheimer SY, Smith LM, O'Connor MJ, Kan E, Rosso C, Houston S, Dinov ID, Thompson PM. Differentiating prenatal methamphetamine from alcohol exposure using tensor based brain morphometry and discriminant analysis. Journal of Neuroscience. 2010;30(11):3876–3885. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: effects on brain development and implications for policy and education. Nature Reviews Neuroscience. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children-Fourth Edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Jezzard P, Matthews PM, Smith SM, editors. Statistical analysis of activation images. Ch 14 in. Functional MRI: An Introduction to Methods. 2001 [Google Scholar]