Abstract
Circadian clocks of most organisms are synchronized with the 24-hour solar day by the changes of light and dark. In Drosophila, both the visual photoreceptors in the compound eyes as well as the blue-light photoreceptor Cryptochrome expressed within the brain clock neurons contribute to this clock synchronization. A specialized photoreceptive structure located between the retina and the optic lobes, the Hofbauer-Buchner (H-B) eyelet, projects to the clock neurons in the brain and also participates in light synchronization. The compound eye photoreceptors and the H-B eyelet contain Rhodopsin photopigments, which activate the canonical invertebrate phototransduction cascade after being excited by light. We show here that 2 of the photopigments present in these photoreceptors, Rhodopsin 5 (Rh5) and Rhodopsin 6 (Rh6), contribute to light synchronization in a mutant (norpAP41) that disrupts canonical phototransduction due to the absence of Phospholipase C-β (PLC-β). We reveal that norpAP41 is a true loss-of-function allele, resulting in a truncated PLC-β protein that lacks the catalytic domain. Light reception mediated by Rh5 and Rh6 must therefore utilize either a different (nonretinal) PLC-β enzyme or alternative signaling mechanisms, at least in terms of clock-relevant photoreception. This novel signaling mode may distinguish Rhodopsin-mediated irradiance detection from image-forming vision in Drosophila.
Keywords: Rhodopsin 5, Rhodopsin 6, Phospholipase C, Cryptochrome, Drosophila melanogaster, Hofbauer-Buchner eyelet
Light synchronization of circadian clocks in animals is mediated by both visual and dedicated “circadian” photoreceptors. One of the reasons for this complexity may be the importance of accurate twilight detection for correct light resetting of the clock. In mammals, it has been shown that the rods and cones as well as melanopsin-expressing retinal ganglion cells contribute to light synchronization and that the 2 systems are partly redundant (Panda et al., 2003). Similarly, in Drosophila, the compound eyes and the blue-light photoreceptor Cryptochrome (Cry) mediate clock synchronization (Rieger et al., 2003; Stanewsky et al., 1998). Although Cry is expressed in the photoreceptor cells of the compound eyes, rescue experiments showed that expression of Cry within clock neurons contributes to their light sensitivity and mediates clock synchronization (Emery et al., 2000; Fogle et al., 2011). Within the eyes, the outer R1–R6 photoreceptor cells express the major fly Rhodopsin 1 (Rh1), whereas the inner R7 and R8 cells express either Rh3 or Rh4 (R7) and Rh5 or Rh6 (R8). In addition, Rh6 is expressed in the 4 adult photoreceptor cells comprising the Hofbauer-Buchner (H-B) eyelet, which is a remnant of the larval photoreceptor and located between the retina and the lamina of the optic lobe (Hofbauer and Buchner, 1989; Sprecher and Desplan, 2008; Yasuyama and Meinertzhagen, 1999). The H-B eyelet projects to the ventral group of the clock neurons, and blocking potential signals from these connections interferes with light synchronization of the clock (Helfrich-Förster et al., 2002; Malpel et al., 2002; Veleri et al., 2007). Finally, the Rh2-expressing ocelli may also contribute to light input of the clock (Rieger et al., 2003).
Functional evidence for the contribution of Rho dopsins to circadian light reception is largely restricted to morphological mutants that remove the relevant opsin-expressing photoreceptors (Helfrich-Förster et al., 2001; Klarsfeld et al., 2004; Mealey-Ferrara et al., 2003; Rieger et al., 2003). In contrast, lack of a crucial fly visual phototransduction enzyme (PLC-β encoded by the norpA gene), or of the major Rh1, as well as interference with retinal syn thesis more directly argued for the involvement of opsin molecules, as each of the 3 situations increased the amount of light required for light synchronization (Ohata et al., 1998; Stanewsky et al., 1998). Combination of norpA and cry mutants led to severe decrements in light synchronization, arguing for a synergistic function of opsin and Cry-mediated photoreception (Emery et al., 2000; Mealey-Ferrara et al., 2003; Stanewsky et al., 1998).
The remaining light sensitivity of norpAP41 cryb double mutants was further decreased by expressing tetanustoxin (TNT) under control of the Rh5 promoter (Veleri et al., 2007). This promoter is active in 30% of the inner R8 photoreceptor cells and in the H-B eyelet (although the latter lacks the Rh5 protein) (Sprecher and Desplan, 2008) (Fig. 1). It followed that the further reduction of light sensitivity in the TNT-expressing norpAP41 cryb flies is independent of PLC-β. This finding suggested that the photopigment uncovered in these flies is either not opsin based or that the Rh5 and/or Rh6 opsins use alternative noncanonical modes of phototransduction (Veleri et al., 2007).
Figure 1.
Rh5 and Rh6 genes are active in the R8 and H-B eyelet photoreceptor cells. R8 photoreceptor cells are visualized by Rh5 promoter-driven lacZ expression (anti-βGAL staining, green) and antibody staining against the Rh6 protein (red). Whereas anti-Rh6 labels R8 rhabdomeres, anti-βGAL labels the R8 cell bodies. Note that each R8 cell expresses only one of the opsin genes (i.e., it is either red or green) but that in the H-B eyelet, visible as a round, drop-like structure in the upper and lower 3 images (arrows), both genes are active (yellow), even though the Rh5 protein is not detectable (Sprecher and Desplan, 2008). The arc-like processes emanating from the eyelet (arrowheads) project directly to the circadian clock neurons in the central brain. La = lamina; Me = medulla. Scale bars: 100 μm (top row) and 10 μm (middle and bottom rows).
Here, we investigated the involvement of Rh5 and Rh6 in light entrainment of the circadian clock by investigating loss-of-function mutants of both genes individually or in combination. By combining both opsin mutants with cryb and norpAP41 cryb double mutants, we reveal that both opsins contribute to clock resetting independent of PLC-β, suggesting that novel opsin-mediated phototransduction mechanisms contribute to circadian photoreception.
MATERIALS AND METHODS
Fly Stocks
All stocks were raised at 25 °C in standard medium. All flies tested behaviorally were in the same w1118 genetic background to avoid behavioral difference caused by genetic background and/or eye color. norpAP41 and cryb mutants have been described previously (Veleri et al., 2007), except that here a cryb version without the recessive marker ss1 was used (kindly provided by F. Rouyer). The Rh52 and Rh61 alleles harbor intragenic deletions of coding sequences described in Yamaguchi et al. (2008) and Cook et al. (2003), respectively. Because the Rh52 stock carried ls-tim and Rh61 mutants are linked to s-tim, differences in light sensitivity between the 2 mutants could be caused by the different versions of Tim present in these strains (the L-Tim protein expressed only in ls-tim flies is less light sensitive compared to S-Tim, which is expressed in both ls-tim and s-tim flies) (Sandrelli et al., 2007). We therefore crossed the ls-tim allele into the Rh61 stock, so that all flies analyzed here (including the controls) are ls-tim. For the generation of Rh5-tau-lacZ flies, see the supplementary online material (SOM).
Immunohistochemistry
Cephalic ganglia with attached retina of adult males were dissected in Drosophila Ringer solution and fixed in 4% paraformaldehyde overnight at 4 °C. Samples were incubated with primary antibodies against β Galactosidase (β-Gal) or Rh6, as described in detail in the SOM.
Behavioral Analysis
Individual 1- to 3-day-old adult male flies were placed in a glass tube with agar medium to monitor behavior using the DAM system (Drosophila Activity Monitoring System, TriKinetics, Waltham, MA) as described previously (Veleri et al., 2007). Initially, flies were kept for 4 days in 12-hour:12-hour light-dark cycles (LD) of white LED light (400 lux). This was followed by an additional 7 days where the flies were kept in a 6-hour delayed or advanced 12-hour:12-hour LD cycle (compared to the initial LD cycle). Light:dark transitions were not abrupt but performed by 2-hour linear ramping from 0 to 400 lux (see Results). Finally, flies were released for 4 to 5 days in constant dark (DD). Analysis of locomotor activity by plotting daily average histograms and actograms was perf ormed using the fly toolbox and MATLAB software (MathWorks, Natick, MA) as described previously (Levine et al., 2002). HLT Control V2.0 software in combination with an LED light box (Hoenig Lichttechnik, Bonstetten, Germany) was used to control illumination. For phase determination, activity data were transferred to a custom-made Excel macro (Microsoft, Redmond, WA). Briefly, evening activity peaks of individual flies from each genotype and each day were determined by the calculation of average phase values, which were then plotted in Figures 2 to 4 (for details, see Sehadova et al. [2009]).
Figure 2.
Rh5 and Rh6 contribute to light resetting. Flies of the indicated genotypes were exposed to 2 “ramped” LD cycles (4 days and 7 days, respectively) separated by a delay, where the lights stayed off for an additional 6 hours. Subsequently, flies were released into DD for at least 3 days. Left panels: double-plotted average actograms showing activity data for each genotype. Middle panels: Phase determination of evening activity peaks for better genotype comparison. Right panels: Daily average histograms (same n as indicated next to the average actograms, except for w, where n = 6) summarizing the daily activity during the first LD cycle (top) and after the 6-hour shift (bottom). Gray or black areas indicate darkness, and white areas indicate lights-on. Gradient shadings in the histograms indicate the linear ramping of light intensities for 2 hours in the morning and evening. See Materials and Methods and text for details.
Figure 4.
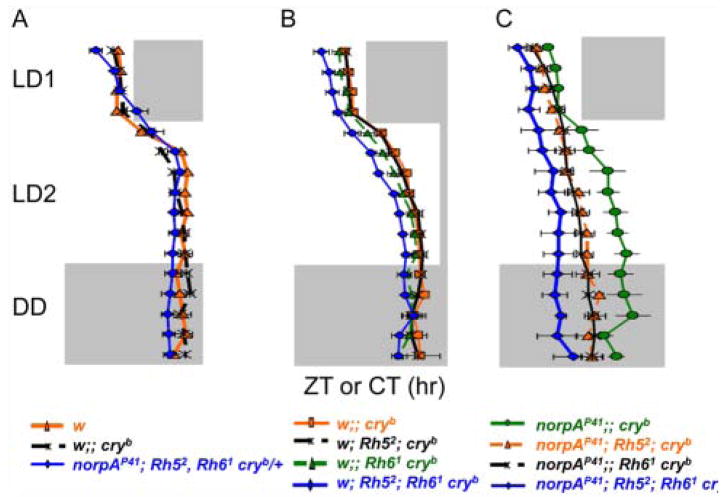
Rh5 and Rh6 function independent of norpA: Flies of the indicated genotypes were exposed to shifted LD cycles, followed by release into DD, exactly as described in the legend to Figure 2. Note that flies of the norpAP41; Rh52; Rh61 cryb/+ genotype (top row) served as norpA mutant cry+ controls. They quickly resynchronized to the new LD regime, as previously been reported for norpAP41 single mutants (Emery et al., 2000), despite being homozygous mutant for Rh52. Data were plotted and analyzed exactly as described in the legend to Figure 2 and in Materials and Methods. n for all data plots is indicated next to average actograms.
Sequencing of the norpAP41 Allele
Total RNA was isolated from norpAP41 and control flies with TRI Reagent followed by LiCl purification according to the manufacturer’s instructions (Ambion, Huntingdon, Cambridgeshire, UK). cDNA synthesis was performed with a RT-PCR kit following the manufacturer’s protocol (Applied Biosystems, Foster City, CA). Genomic DNA and cDNA were sequenced with oligonucleotides covering the entire coding sequence. One oligo pair (5′-norpA4a: ATACTGTGAT GACTTCTTCGG and 3′-norpA8a: ATTATACTCGAACTTGCCCTG) revealed that the norpAP41 contains a large internal deletion, removing 287-bp coding sequences from exon 8 and exon 9, corresponding to 351 bp of genomic DNA deleting intron 9 in addition to the coding sequences (Fig. 5).
Figure 5.
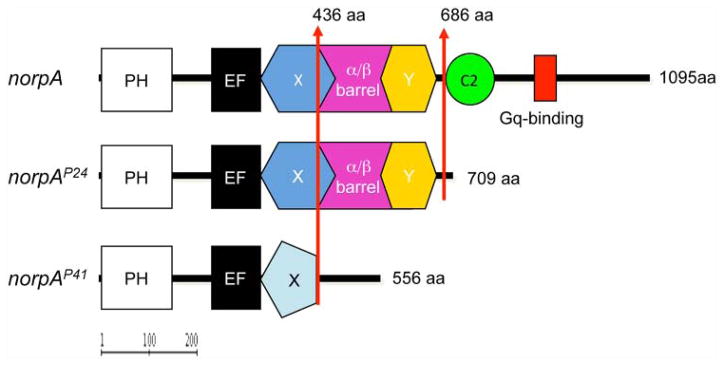
Functional domains of PLC-β in wild-type and norpA loss-of-function alleles. Upper panel shows the domain structure of Phospholipase C-β (PLC-β) encoded by norpA. In photoreceptors, PLC-β isdirectlyactivatedbytheheterotrimericGproteinGqtohydrolyzephosphatidylinositol4,5-bisphosphate into the second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). PH = Pleckstrin homology domain, involved in phosphoinositide and/or Gβγ binding EF = EF hand–like Ca2+ binding domain; X/Y = homologous domains found in all eukaryotic PLC enzymes involved in catalytic activity; α/β barrel = α/β barrel domain, catalytic domain, binding of Gβ; C2 = Ca2+ and lipid binding domain; C-terminal tail = Gq binding, membrane localization. Middle panel: Predicted protein encoded by norpAP24, which lacks C2 and the C-terminal tail but retains the catalytic domain. Lower panel: norpAP41-encoded protein, lacking the catalytic domain and all C-terminal sequences. Domain prediction was performed using SMART and InterPro Web tools accessible via Flybase (http://flybase.org).
RESULTS
Rhodopsin 5 and Rhodopsin 6 Contribute to Light Resetting
To investigate the involvement of Rh5 and Rh6 in light entrainment, we exposed flies to 2 consecutive 12-hour:12-hour light-dark (LD) cycles (lasting 4 and 7 days, respectively) that were separated by a 6-hour delay (i.e., the lights stayed “off” for 6 hours longer during the last day of the first LD cycle) (Fig. 2). Light intensity during both LD cycles was linearly ramped up from 0 to 400 lux for 2 hours, remained constant for 8 hours, and was linearly ramped down again to 0 lux for 2 hours. Subsequently, flies were exposed to DD (3–5 days). To determine the ability to resynchronize to the shifted LD cycle, we used 3 different ways of plotting or analyzing the data: 1) plotting of average actograms showing activity throughout the experiment (Fig. 2, left panel). The ability to resynchronize can be estimated by counting the number of days in the second LD cycle during which flies show transient delays of the evening activity peak before reaching a stable phase. If this phase is also the starting point of the evening activity in the subsequent DD, the clock of these flies has been stably resynchronized (Emery et al., 2000). 2) To aid the localization of the daily evening peak, we determined the peak activity phase for each day of the experiment (Fig. 2, middle panel). 3) Daily average histograms were calculated for the 4 days during the first LD cycle (before shift) and for the 7 days of the second LD cycle (after shift) (Fig. 2, right panel). Before the shift, these plots show the typical bimodal behavior of flies, with a morning and evening activity peak and periods of inactivity during the day and night. Resynchronization to the shifted LD cycle is indicated if the same pattern is observed in the “after-shift” histogram, whereas impaired resyn chronization leads to an evening peak that is shifted towards the middle of the day (Veleri et al., 2007).
As expected and previously reported (Emery et al., 2000; Hanai and Ishida, 2009; Helfrich-Förster et al., 2001), wild-type flies synchronized their evening activity rapidly (after 1 day) to the shifted LD regime, whereas cryb mutants needed about 3 to 5 days (Figs. 2 and 3A). We then tested cryb flies in combination with individual and double mutant loss-of-function alleles for Rh52 (Yamaguchi et al., 2008) and Rh61 (Cook et al., 2003) in the above assay. Rh52; cryb and Rh61 cryb double mutants resyn chronized to the shifted LD cycle with a similar speed as cryb single mutants (Fig. 2). Careful inspection and direct comparison of the quantified evening peak phases indicate that Rh61 cryb flies need more time to resynchronize compared to cryb and Rh52; cryb flies, but the difference is very subtle (Fig. 3B; compare last day of original LD cycle with days 1–4 after the shift).
Figure 3.
Direct comparison of evening activity phase during shifted LD cycles. Evening activity peak phase of related genotypes was plotted to allow comparison of the resynchronization kinetics. (A) Comparison of w; cryb and norpAP41; Rh52; Rh61 cryb/+ flies, showing quick resynchronization of cry+ compared to homozygous cryb genotypes. (B) Comparison of single and double Rh52 and Rh61 mutants combined with cryb compared to cryb single mutants, showing that the 2 Rhodopsin mutants combined slow down resynchronization. (C) Comparison of the same genotypes as shown in the middle panel, additionally carrying norpAP41. Rh52 and Rh61 further slow down resynchronization of norpAP41; cryb double mutants, demonstrating their norpA-independent function. n corresponds to those indicated in Figures 2 and 4. Gray or black areas indicate darkness, and white areas indicate lights-on.
The Rh52; Rh61 cryb triple mutants responded to the shifted LD regime by slowly delaying their evening activity. Even after 7 days in the new regime, we find that in most of the animals (n = 36), the evening activity has moved only to about the middle of the light phase (Fig. 2, bottom row, left and right panels). Determination of the evening peak activity phase of all tested Rh52; Rh61 cryb individuals (n = 66) also shows that the triple mutants resynchronize slower compared to the double mutants or to cryb single mutants (Fig. 3B; compare last day of original LD cycle with days 1–6 after the shift). These results show that Rh5 and Rh6 contribute to clock resetting by white light.
Rhodopsin 5 and Rhodopsin 6 Function Independently of norpA
Previously, we reported that the remaining resynchronization ability observed in norpAP41; cryb double mutants is aβολished by the additional expression of TNT under control of the Rh5 promoter (Veleri et al., 2007). This suggested that the photopigment acting in the TNT-blocked cells does not require PLC-β encoded by norpA and therefore may not rely on canonical Rhodopsin photo transduction. Because the Rh5 promoter targets both Rh5- and a few Rh6-expressing cells (Fig. 1), this raises the possibility that both opsins could function independent of PLC-β (Veleri et al., 2007). To directly test this possibility, we crossed the Rh52; cryb and Rh61 cryb double mutants as well as the Rh52; Rh61 cryb triple mutants into the norpAP41 mutant background and tested their ability to resynchronize to shifted LD cycles.
Control norpAP41; cryb double mutants slowly resynchronized to the shifted LD regime (Figs. 3C and 4). Comparable to previous reports, these double mutants required about 5 to 7 days before reaching a stable phase of their evening activity peak (Emery et al., 2000; Veleri et al., 2007). The addition of both the Rh52 or Rh61 single mutants increased the time required for synchronization further so that even after 7 days in the shifted LD regime, the “evening” activity peak occurred before or in the middle of the day (Fig. 4). Finally, flies lacking both Rhodopsins in the background of norpAP41 and cryb (quadruple mutants) showed only minimal tendencies (if any) to resynchronize their behavior. As a consequence, their “evening” activity peak occurred shortly after lights-on in the shifted light-dark regime (Fig. 4). Phase analysis confirmed the observations of the raw activity data and demonstrates that additional removal of Rh5 and/or Rh6 leads to further impairment of the ability to resynchronize to LD cycles (Figs. 3C and 4).
Next, we tested if the PLC-β–independent contribution of Rh5 and Rh6 is restricted to phase delays or if they also show defects in synchronization to phase-advanced LD cycles. For this, flies were subjected to similar conditions as described above, except that instead of a 6-hour delay, the LD cycle was advanced by 6 hours. Wild-type, norpAP41, and cryb mutants synchronized quickly to the new regime, whereas norpAP41; cryb double mutants needed several days to do so, indicated by “transients” during which activity also occurred after lights-off in the advanced LD regime (Suppl. Fig. S1). Introducing Rh52 or Rh61 into the double mutant background interfered more strongly with resynchronization compared to the norpAP41; cryb double mutant, and the norpAP41; Rh52; Rh61 cryb quadruple mutant showed again the strongest phenotype (Suppl. Fig. S1). Individuals from the latter 3 genotypes kept advancing their evening activity peaks during the advanced LD regime without reaching a stable phase relationship. As a consequence, their evening activity peak occurred well before lights-off at the last day of LD (indicated by arrows in Suppl. Fig. S1). Because the various Rhodopsin mutants show transient delays during delayed LD cycles (Figs. 2–4) and transient advances during advanced LD cycles (Suppl. Fig. S1), it is clear that this behavior represents slow resynchronization and is not caused by an altered endogenous period length compared to the controls. We conclude that Rh5 and Rh6 contribute to phase delays and advances in an additive and PLC-β–independent manner.
Molecular Characterization of the norpAP41 Allele
The results described above strongly imply that Rh5 and Rh6 do not depend on PLC-β–mediated phototransduction. It is therefore important to confirm if the norpAP41 (or norpA39 according to new nomenclature) allele applied here is indeed a true null allele, as previously suggested by electro physiological and behavioral tests (Lindsley and Zimm, 1992; Riesgo-Escovar et al., 1995). We therefore determined the norpA DNA sequence in norpAP41 mutant flies and revealed that the gene carries a 351-bp genomic deletion removing parts of exon 8, most of exon 9, and the entire intron 9, which corresponds to a 286-bp deletion in the norpA cDNA (nucleotides 1962–2248 according to Bloomquist et al. [1988]) (Fig. 5). The resulting protein that could potentially be encoded by norpAP41 consists of 436 N-terminal amino acids identical to the wild-type protein (1095 amino acids in total) followed by a frame shift, resulting in the substitution of 120 amino acids and a premature stop codon (Fig. 5). The predicted mutant protein therefore interrupts the catalytic domain (α/β-barrel, extending from residue 318–682) and lacks the C-terminal tail required for Gq protein interaction and localization of PLC-β to the Rhabdomere (Katan, 1998). In contrast, the other frequently used null allele norpAP24 (or norpA36) carries a 28-bp deletion within exon 10, which results in the absence of the same C-terminal domains, except for retaining the complete catalytic domain (Pearn et al., 1996) (Fig. 5). Although this truncated protein could theoretically retain enzymatic activity, norpAP24 behaves like a molecular and functional null allele. Due to the additional lack of the catalytic domain in norpAP41, any residual PLC- activity in flies carrying this allele is essentially ruled out (Fig. 5).
DISCUSSION
In Drosophila, it has been shown that multiple light input routes are used to synchronize the circadian clock (Emery et al., 2000; Helfrich-Förster et al., 2001; Klarsfeld et al., 2004; Mealey-Ferrara et al., 2003; Rieger et al., 2003; Stanewsky et al., 1998; Veleri et al., 2007). The circadian clock of fruit flies responds to moonlight levels of 0.03 lux, both at a molecular and behavioral level (Bachleitner et al., 2007). Wild-type flies robustly resynchronize to shifted LD cycles at light intensities of only 0.0011 lux (Stanewsky et al., 1998) and to even lower intensities of monochromatic blue light (Hirsh et al., 2010). This high sensitivity depends on retinal photoreceptors rather than Cry because it decreases in flies lacking the eyes, Rh1, PLC-β, or retinas but that retain Cry (Bachleitner et al., 2007; Ohata et al., 1998; Stanewsky et al., 1998). Consistent with this, it has recently been shown that Cry-mediated light excitation of clock neurons requires light intensities that are several magnitudes higher (2–19 mW/cm2 or about 100,000 lux) (Fogle et al., 2011).
Under natural conditions, light synchronization is a challenging task due to fluctuations of light intensities influenced by weather changes and also because of considerable light levels during the night (see above). Therefore, photoreceptors mediating circadian entrainment probably evolved to detect twilight as a reliable marker for the beginning and the end of the day. In addition to changes in light intensity, light quality also changes during dawn and dusk (Roenneberg and Foster, 1997). This may explain why photopigments with different light spectra, for example, Rh1 (λmax = 480 nm), Rh5 (λmax = 437 nm), and Rh6 (λmax = 508 nm) (Salcedo et al., 1999), have been recruited for clock resetting. In fact, previous studies suggest that these 3 opsins contribute to synchronization to green (472–603 nm, λmax = 519 nm) and yellow (475–724 nm, λmax = 580 nm) light (Hanai and Ishida, 2009) and Rh1 and Rh6 also to synchronization to red light (λmax = 634 nm) (Hanai et al., 2008). Our findings strongly support a role for both Rh5 and Rh6 for circadian entrainment because we show that flies lacking both opsins and Cry show enhanced defects in resynchronization to 400-lux white light compared to cryb single mutants (Figs. 2 and 3B). Although not analyzed in the current study, based on the discussion above, we predict that the effects of both opsins could also be revealed at much lower light intensities, perhaps even in the presence of Cry.
Rh5 and Rh6 Do Not Depend on PLC-β
Previous observations had suggested that light reception in Rh5-Gal4–positive cells (30% of the R8 cells and the H-B eyelet) (Fig. 1) does not depend on PLC-β encoded by norpA (Veleri et al., 2007). Prompted by this, we tested here if Rh5 (30% of R8 cells) and/or Rh6 (70% of R8 cells and H-B eyelet) depends on norpA. Strikingly, both opsin mutations lead to a further decrement of circadian light entrai nment in norpAP41 cryb double mutants, demon strating that Rh5 and Rh6 have PLC-β (norpA)–independent yet light-dependent activities (Figs. 3C and 4). This is very surprising because Rh5 and Rh6 are able to restore norpA-dependent ERG light responses in outer R1–R6 photoreceptors lacking Rh1 (Pearn et al., 1996; Salcedo et al., 1999). Moreover, norpA is being expressed in the H-B eyelet (Malpel et al., 2002). One possible explanation would have been that the norpA allele we applied still produces some functional PLC-β. In this scenario, the slow resynchronization seen in norpAP41 cryb double mutants could still be mediated by residual opsin-based phototransduction including that of Rh1, Rh5, and Rh6. Importantly, our sequence analysis of norpAP41 rules out this possibility: norpAP41 contains a large internal deletion within the norpA gene, resulting in a predicted truncated protein that lacks everything including and downstream of the catalytic domain (Fig. 5). Hence, even if this truncated protein is stable, it could not hydrolyze the membrane-bound phosphoinositide 4,5-biphosphate (PIP2) into the DAG and IP3 second-messenger molecules, a reaction normally catalyzed by the full-length PLC-β enzyme. In fact, norpAP41 is the only allele described so far containing such a severe alteration, resulting in a true functional null allele (Pearn et al., 1996). Therefore, Rh5 and Rh6 must either utilize a different signaling mechanism in addition to the canonical one or they activate other PLC-β enzymes. The only other PLC-β encoded by the Drosophila genome PLC-21C would potentially be a good candidate, as it is expressed at moderate levels in the eyes, head, and brain according to the FlyAtlas adult expression database (Chintapalli et al., 2007). Nevertheless, its function seems to be specific for olfactory signal transduction, and this PLC-β enzyme can be activated by a different Gqα isoform compared to the visual one (Kain et al., 2008).
We favor the idea that Rh5 and Rh6 can use alternative signaling pathways. One reason for this takes into account that it would be disadvantageous for circadian photoreceptors to show adaptation to light. Circadian photoreceptors can be seen as irradiance detectors, whose primary function is to collect light information over time, especially during dawn and dusk (Davies et al., 2010). They do not need to create sharp images of their environment, nor do they need to adapt to rapidly changing light intensities as visual photoreceptors do. In fact, these features of visual photoreceptors, which involve adaptation and termination mechanisms, would counteract the “light sampling” duty of circadian photoreceptors. Several adaptive termination mechanisms operate in the canonical phototransduction cascade, and one of them is coupled to PLC-β itself. In addition to being activated by an active (GTP-bound) Gqα subunit, PLC-β functions as a GTPase activating protein (GAP), leading to inactivation of Gqα (Cook et al., 2000). It would therefore be advantageous if Rh5 and Rh6 activity could somehow circumvent at least some of these termination mechanisms, perhaps by bypassing PLC-β signaling. This would not rule out additional canonical phototransduction by both opsins for image-forming vision. In fact, norpA-dependent opsin-mediated phototransduction does contribute to circadian light reception, as indicated by the phenotypes of single norpA and norpAP41 cryb double mutants (see above).
Phenotype and Remaining Light Sensitivity in norpAP41; Rh52; Rh61 cryb Mutants
The quadruple mutants are severely affected in resynchronization to delayed and advanced LD cycles and are only weakly synchronized in the original (preshift) LD cycles (Fig. 3C and Suppl. Fig. S1). Even though this shows that most light entrainment pathways are nonfunctional in these mutants, it is also clear that some light input remains. The cryb allele applied here is a severe hypomorph and may allow some residual Cry-dependent light input to the clock (Dolezelova et al., 2007; Stanewsky et al., 1998). Alternatively, yet unidentified circadian photoreceptive pathways may contribute to entrainment, most likely located in or close to the clock neurons in the dorsal brain (Chen et al., 2011; Veleri et al., 2003). To distinguish between these possibilities, the current genetic variants should be combined with true cry loss-of-function alleles, like cry01 or cryOUT (Dolezelova et al., 2007; Yoshii et al., 2008).
It is known that norpA mutations can lead to age-dependent photoreceptor cell degeneration (Meyertholen et al., 1987), and therefore, it is likely that all genotypes in the current study carrying the norpAP41 allele suffer from a certain degree of such degeneration. But because norpAP41 cryb double mutants retain the ability to synchronize to LD cycles, it is clear that this degeneration does not contribute to the phenotypes observed here. More over, norpA-induced retinal degeneration can be rescued by the additional removal of Rhodopsin expression because it depends on endocytosis of Arrestin:Rhodopsin complexes (Alloway et al., 2000). We therefore think we can rule out that the reduced light sensitivity in triple and quadruple mutants can be caused by unspecific retinal dege neration; in fact, removal of Rh5 and/or Rh6 should restore photoreceptor integrity in a norpAP41 mutant background. Hence, the most likely explanation for the decrement of circadian light sensitivity obse rved in the triple and quadruple mutants is the lack of PLC-independent Rh5- and Rh6-mediated photoreception.
Light Input to the Molecular Clock
In Drosophila, light-induced degradation of the Timeless (Tim) protein mediated by Cry is thought to be central to molecular clock resetting (Peschel and Helfrich-Förster, 2011), although recent observations suggest the process is more complex (Tang et al., 2010). Nevertheless, observations with the original cryb mutant revealed that in some clock neurons, light-dependent Tim degradation occurs in the absence of functional Cry and even in norpAP41 cryb double mutants (Helfrich-Förster et al., 2001; Stanewsky et al., 1998). This strongly suggests that other Cry-independent molecular mechanisms must exist to reset molecular oscillations, and these may be mediated (at least in part) by the 2 Rhodopsins that were investigated here. Consistent with this idea, neurotoxin expression in the Rh5-expressing R8 cells and the H-B eyelet of norpAP41 cryb double mutants drastically interfered with the synchronization of Tim in subsets of the clock neurons that are not affected by the double mutant alone (Veleri et al., 2007). Moreover, Tim degradation is also mediated by a novel Cry-independent pathway involving the clock-controlled gene quasimodo. Quasimodo is a light-responsive membrane-attached ZP domain expressed in (largely Cry-negative) subsets of clock neurons, where it may be activated by yet unknown photoreceptive molecules (Chen et al., 2011).
CONCLUSIONS
We demonstrate here that the Drosophila Rhodopsins Rh5 and Rh6 contribute to light resetting of the Drosophila circadian clock independent of the norpA-encoded PLC-β enzyme that normally operates in the visual phototransduction cascade. This suggests that alternative opsin signaling mechanisms exist that may operate in photoreceptors, which participate in irradiance detection rather than image-forming vision. Except for Rh3, all other Drosophila Rhodopsins as well as the mammalian circadian photopigment melanopsin are able to function as thermoreceptors (Shen et al., 2011). Our findings suggest that this unexpected versatility of Rhodopsins may extend to the downstream signaling mechanisms, as already shown for other nonphotoreceptive functions of Rhodopsin (Chang and Ready, 2000).
Supplementary Material
Acknowledgments
The authors thank François Rouyer for the Rh52 and Rh61 mutants combined with cryb and Kofan Chen for technical support. They also thank Charlotte Helfrich-Förster, Alois Hofbauer, André Klarsfeld, Joel Levine, Nicolai Peschel, and members of their laboratory for comments on the article. This work was funded by the European Union through EUCLOCK, an Integrated Program (FP6).
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material for this article is available on the Journal of Biological Rhythms website at http://jbr.sagepub.com/supplemental.
References
- Alloway PG, Howard L, Dolph PJ. The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron. 2000;28:129–138. doi: 10.1016/s0896-6273(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Bachleitner W, Kempinger L, Wülbeck C, Rieger D, Helfrich-Förster C. Moonlight shifts the endogenous clock of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2007;104:3538–3543. doi: 10.1073/pnas.0606870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Chang HY, Ready DF. Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science. 2000;290:1978–1980. doi: 10.1126/science.290.5498.1978. [DOI] [PubMed] [Google Scholar]
- Chen KF, Peschel N, Zavodska R, Sehadova H, Stanewsky R. QUASIMODO, a novel GPI-anchored zona pellucida protein involved in light input to the Drosophila circadian clock. Curr Biol. 2011;21:719–729. doi: 10.1016/j.cub.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Cook B, Bar-Yaacov M, Cohen Ben-Ami H, Goldstein RE, Paroush Z, Selinger Z, Minke B. Phospholipase C and termination of G-protein-mediated signalling in vivo. Nat Cell Biol. 2000;2:296–301. doi: 10.1038/35010571. [DOI] [PubMed] [Google Scholar]
- Cook T, Pichaud F, Sonneville R, Papatsenko D, Desplan C. Distinction between color photo receptor cell fates is controlled by Prospero in Drosophila. Dev Cell. 2003;4:853–864. doi: 10.1016/s1534-5807(03)00156-4. [DOI] [PubMed] [Google Scholar]
- Davies WL, Hankins MW, Foster RG. Vertebrate ancient opsin and melanopsin: divergent irradiance detectors. Photochem Photobiol Sci. 2010;9:1444–1457. doi: 10.1039/c0pp00203h. [DOI] [PubMed] [Google Scholar]
- Dolezelova E, Dolezel D, Hall JC. Rhythm defects caused by newly engineered null mutations in Drosophila’s cryptochrome gene. Genetics. 2007;177:329–345. doi: 10.1534/genetics.107.076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P, Stanewsky R, Helfrich-Forster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- Fogle KJ, Parson KG, Dahm NA, Holmes TC. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science. 2011;331:1409–1413. doi: 10.1126/science.1199702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai S, Hamasaka Y, Ishida N. Circadian entrainment to red light in Drosophila: requirement of Rhodopsin 1 and Rhodopsin 6. Neuroreport. 2008;19:1441–1444. doi: 10.1097/WNR.0b013e32830e4961. [DOI] [PubMed] [Google Scholar]
- Hanai S, Ishida N. Entrainment of Drosophila circadian clock to green and yellow light by Rh1, Rh5, Rh6 and CRY. Neuroreport. 2009;20:755–758. doi: 10.1097/WNR.0b013e32832a7c4e. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Edwards T, Yasuyama K, Wisotzki B, Schneuwly S, Stanewsky R, Meinertzhagen IA, Hofbauer A. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J Neurosci. 2002;22:9255–9266. doi: 10.1523/JNEUROSCI.22-21-09255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249–261. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Riemensperger T, Coulom H, Iche M, Coupar J, Birman S. Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Curr Biol. 2010;20:209–214. doi: 10.1016/j.cub.2009.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer A, Buchner E. Does Drosophila have seven eyes? Naturwissenschaften. 1989;76:335–336. [Google Scholar]
- Kain P, Chakraborty TS, Sundaram S, Siddiqi O, Rodrigues V, Hasan G. Reduced odor responses from antennal neurons of G(q)alpha, phospholipase Cbeta, and rdgA mutants in Drosophila support a role for a phospholipid intermediate in insect olfactory transduction. J Neurosci. 2008;28:4745–4755. doi: 10.1523/JNEUROSCI.5306-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M. Families of phosphoinositide-specific phospholipase C: structure and function. Biochim Biophys Acta. 1998;1436:5–17. doi: 10.1016/s0005-2760(98)00125-8. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A, Malpel S, Michard-Vanhee C, Picot M, Chelot E, Rouyer F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Advanced analysis of a cryptochrome mutation’s effects on the robustness and phase of molecular cycles in isolated peripheral tissues of Drosophila. BMC Neurosci. 2002;3:5. doi: 10.1186/1471-2202-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG, editors. The Genome of Drosophila melanogaster. San Diego: Academic Press; 1992. [Google Scholar]
- Malpel S, Klarsfeld A, Rouyer F. Larval optic nerve and adult extra-retinal photoreceptors sequentially associate with clock neurons during Drosophila brain development. Development. 2002;129:1443–1453. doi: 10.1242/dev.129.6.1443. [DOI] [PubMed] [Google Scholar]
- Mealey-Ferrara ML, Montalvo AG, Hall JC. Effects of combining a cryptochrome mutation with other visual-system variants on entrainment of locomotor and adult-emergence rhythms in Drosophila. J Neurogenet. 2003;17:171–221. [PubMed] [Google Scholar]
- Meyertholen EP, Stein PJ, Williams MA, Ostroy SE. Studies of the Drosophila norpA phototransduction mutant, II: photoreceptor degeneration and rhodopsin maintenance. J Comp Physiol A. 1987;161:793–798. doi: 10.1007/BF00610221. [DOI] [PubMed] [Google Scholar]
- Ohata K, Nishyama H, Tsukahara Y. Action spectrum of the circadian clock photoreceptor in Drosophila melanogaster. In: Touitou Y, editor. Biological Clocks: Mechanisms and Applications. Amsterdam: Elsevier; 1998. pp. 167–170. [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Pearn MT, Randall LL, Shortridge RD, Burg MG, Pak WL. Molecular, biochemical, and electro physiological characterization of Drosophila norpA mutants. J Biol Chem. 1996;271:4937–4945. doi: 10.1074/jbc.271.9.4937. [DOI] [PubMed] [Google Scholar]
- Peschel N, Helfrich-Förster C. Setting the clock by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 2011;585:1435–1442. doi: 10.1016/j.febslet.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Rieger D, Stanewsky R, Helfrich-Forster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J Biol Rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar J, Raha D, Carlson JR. Requirement for a phospholipase C in odor response: overlap between olfaction and vision in Drosophila. Proc Natl Acad Sci U S A. 1995;92:2864–2868. doi: 10.1073/pnas.92.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Foster RG. Twilight times: light and the circadian system. Photochem Photobiol. 1997;66:549–561. doi: 10.1111/j.1751-1097.1997.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Salcedo E, Huber A, Henrich S, Chadwell LV, Chou WH, Paulsen R, Britt SG. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J Neurosci. 1999;19:10716–10726. doi: 10.1523/JNEUROSCI.19-24-10716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrelli F, Tauber E, Pegoraro M, Mazzotta G, Cisotto P, Landskron J, Stanewsky R, Piccin A, Rosato E, Zordan M, et al. A molecular basis for natural selection at the timeless locus in Drosophila melanogaster. Science. 2007;316:1898–1900. doi: 10.1126/science.1138426. [DOI] [PubMed] [Google Scholar]
- Sehadova H, Glaser FT, Gentile C, Simoni A, Giesecke A, Albert JT, Stanewsky R. Temperature entrainment of Drosophila’s circadian clock involves the gene nocte and signaling from peripheral sensory tissues to the brain. Neuron. 2009;64:251–266. doi: 10.1016/j.neuron.2009.08.026. [DOI] [PubMed] [Google Scholar]
- Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- Sprecher SG, Desplan C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature. 2008;454:533–537. doi: 10.1038/nature07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Tang CH, Hinteregger E, Shang Y, Rosbash M. Light-mediated TIM degradation within Drosophila pacemaker neurons (s-LNvs) is neither necessary nor sufficient for delay zone phase shifts. Neuron. 2010;66:378–385. doi: 10.1016/j.neuron.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veleri S, Brandes C, Helfrich-Forster C, Hall JC, Stanewsky R. A self-sustaining, light-entrainable circadian oscillator in the Drosophila brain. Curr Biol. 2003;13:1758–1767. doi: 10.1016/j.cub.2003.09.030. [DOI] [PubMed] [Google Scholar]
- Veleri S, Rieger D, Helfrich-Forster C, Stanewsky R. Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J Biol Rhythms. 2007;22:29–42. doi: 10.1177/0748730406295754. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Wolf R, Desplan C, Heisenberg M. Motion vision is independent of color in Drosophila. Proc Natl Acad Sci U S A. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA. Extraretinal photoreceptors at the compound eye’s posterior margin in Drosophila melanogaster. J Comp Neurol. 1999;412:193–202. doi: 10.1002/(sici)1096-9861(19990920)412:2<193::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Todo T, Wülbeck C, Stanewsky R, Helfrich-Förster C. Cryptochrome is present in the compound eyes and a subset of Drosophila’s clock neurons. J Comp Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.