Abstract
Age-related macular degeneration (AMD) is one of the most well-characterized late-onset, complex trait diseases. Remarkable advances in our understanding of the genetic and biological foundations of this disease were derived from a recent convergence of scientific and clinical data. Importantly, the more recent identification of AMD-associated variations in a number of complement pathway genes has provided strong support for earlier, paradigm-shifting studies that suggested that aberrant function of the complement system plays a key role in disease etiology. Collectively, this wealth of information has provided an impetus for the development of powerful tools to accurately diagnose disease risk and progression and complement-based therapeutics that will ultimately delay or prevent AMD. Indeed, we are poised to witness a new era of a personalized approach toward the assessment, management, and treatment of this debilitating, chronic disease.
Advanced age-related macular degeneration (AMD), which affects approximately 5% of persons older than age 75 years, is among the most debilitating of chronic human diseases. Given that age is the primary risk factor for AMD, the prevalence and severity of the condition are predicted to increase as human life expectancy increases. Fortunately for those who have AMD, remarkable advances in our understanding of the genetic and biological foundations of this disease have been made in the past 20 years. This knowledge came from an amazing confluence between a wealth of previously generated data relating to the etiology and biology of the disease on one hand, and the development of powerful new investigative tools on the other.
Early epidemiological studies established that the disease is heritable and clearly modulated by nongenetic environmental risk factors,1–3 whereas biochemical and histological studies demonstrated that the complement system—an important component of innate immunity—was involved in drusen biogenesis and the etiology of AMD.4,5 Simultaneously, more than a decade of genetic research directed our attention to multiple chromosomal loci that exhibited significant associations with AMD.6–10 More recently, near completion of the Human Genome Project, the development of the International HapMap Project library of single-nucleotide polymorphisms (SNPs), and the development of statistical tools for analyzing large quantities of data have provided powerful new tools to investigators seeking to identify AMD-associated genes.11,12 These high-throughput genotyping technologies allowed researchers to screen numerous unrelated individuals for common variations (eg, SNPs) that could be analyzed statistically for their probable relationship to AMD.
A convergence of the collective scientific and clinical observations and new technologies described above paved the way for a hallmark genetic discovery that changed the course of AMD research, and most certainly the future diagnosis, management, and treatment of this condition. Four articles published early in 2005 demonstrated an association between AMD and common variants (or polymorphisms) in the complement factor H gene (CFH [OMIM 134370]) on human chromosome 1q31, a gene that encodes the major regulator of the complement alternative pathway. Importantly, 1 of these 4 studies,13 which was based solely on the use of a candidate gene strategy, documented the existence of haplotypes of CFH that confer an increased risk of, or protection from, developing AMD. These important contributions ushered in a new era of research related to the genetics and biology of AMD and, likely, other chronic human diseases.13–18
The results of the CFH-AMD discovery have been confirmed repeatedly since their initial publication. Moreover, this observation led to accelerated research activities and the rapid identification of additional genes in the complement pathway that were associated with AMD risk. Variants in genes coding for complement factor B (CFB), complement component 2 (C2), and complement component 3 (C3) proteins have been discovered and shown to be associated with AMD risk and protection.19,20 Other studies documented the existence of a large, common deletion encompassing the complement factor H–related 1 (CFHR1) and complement factor H–related 3 (CFHR3) genes.19,21 In addition to significant associations of various complement genes with the AMD, it was also discovered that variants in a cluster of 2 genes on chromosome 10q26 (LOC387715/ARMS2 and PRS11/HTRA1), neither of which have any obvious relationship to the complement pathway, showed a strong and significant association with AMD risk.7,8 Studies indicate that the effects of the AMD-related genes identified to date on AMD risk are additive.7,20,22
This review provides a concise summary of our present knowledge relating to the role of genetic variation in determining AMD risk and protection, the potential biological mechanisms through which these variations modulate the development of AMD pathogenesis, an overview of the complement system, and insights relating to diagnostics and pharmacogenetic therapies that are most certain to become commonplace in the future management of this complex disease. Because of the clear genetic and biologic involvement of the complement system in AMD, a brief review of this system is provided. This background information should prove helpful for the expanded discussions relating to each of the major AMD-associated genes, their functional implications, and potential diagnostic and therapeutic implications that follow.
OVERVIEW OF THE COMPLEMENT SYSTEM
The CFH-AMD discovery provided additional robust support for the previously established hypothesis that aberrant regulation and/or activation of the complement pathway is associated with the development and progression of AMD. Beginning in the mid-1990s, important new observations were published. They were derived from immunohistochemical and biochemical studies of eyes from human donors with clinically documented histories of AMD and dense deposit disease (DDD, also termed membranoproliferative glomerulonephritis type II), a complement-associated renal disease in which drusen that are clinically indistinguishable from those associated with AMD develop at an early age. Interestingly, the drusen in both diseases are histologically and biochemically indistinguishable, containing a large proportion of complement pathway-associated proteins (Figure 1 and Figure 2).23,24 These observations formed the basis for the concept that drusen are a byproduct of chronic, local, inflammatory processes characterized, in part, by robust activation of the complement cascade at the retinal pigment epithelium–choroid interface, a major paradigm shift related to the cause of AMD. Moreover, they suggested that the pathobiology of DDD shares features with AMD and vice versa. Very recent studies have shown a significant association among the incidence of AMD, CFH genotype, and chronic renal disease,25,26 further supporting the concept of potential clinical and biological relationships between seemingly disparate diseases. Although these early studies strongly suggested that complement dysfunction or dysregulation was involved in the pathogenesis of AMD, traditional genetic studies conducted to this point had not yet yielded any significant clues regarding a potential role of complement system–related genes in the pathogenesis of this condition.
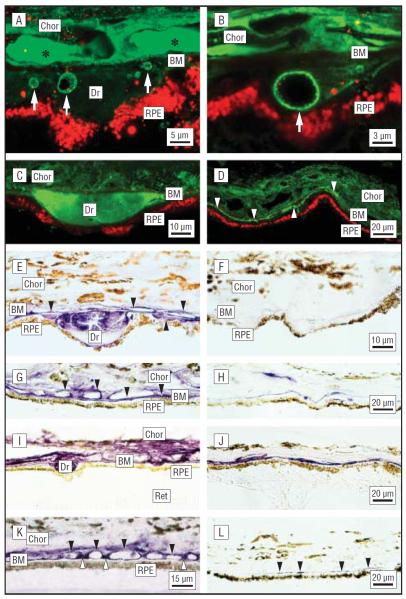
Figure 1.
Immunolocalization of complement factor H (CFH) and the membrane attack complex (MAC)/C5b-9 in the retinal pigment epithelium (RPE)/choroid (Chor) complex. A and B, Confocal immunofluorescence images from an 84-year-old man with atrophic age-related macular degeneration (AMD). Anti-CFH antibody labels substructural elements (arrows) in drusen (Dr) and the sub-RPE space (green; Cy2 channel). C and D, Localization of CFH in drusen and the sub-RPE space in an 83-year-old man with AMD (green). Drusen immunoreactivity (IR) is homogeneous; CFH IR is associated with the choriocapillaris and the sub-RPE space (arrowheads). E–L, The brown pigment in the RPE cytoplasm and Chor is melanin. E, Localization of CFH in drusen of a 79-year-old donor eye. Anti-CFH labeling (purple reaction product) is apparent in Dr, along the Bruch membrane (BM), and on the choroidal capillary walls (arrows). F, Control section of the same eye with no primary antibody; labeling is absent. G, Extensive labeling is present along the BM, the choroidal capillary walls, and intercapillary pillars (arrows) in a 78-year-old individual with AMD. H, Control section from the macula of a donor without AMD; much less labeling is apparent. Localization of C5b-9 in the RPE-Chor underlying the macula (I) and extramacula (J) in the same eye of an 81-year-old AMD donor. I, Intense anti-C5b-9 IR is associated with Dr, BM, and the choroidal capillary endothelium. J, Outside the macula, there is only sporadic labeling in the vicinity of the BM. Localization of C5b-9 in the macula from a donor with AMD (K) and from a second donor without AMD (L). K, Anti-C5b-9 labeling is associated primarily with the choroidal capillary walls (black arrowheads) and intercapillary pillars (white arrowheads). Labeling is much more intense in the AMD eye. Note the strong similarity to the anti-CFH labeling pattern in the macula from the same donor (G). Ret indicates retina. Reproduced with permission from Hageman et al.13
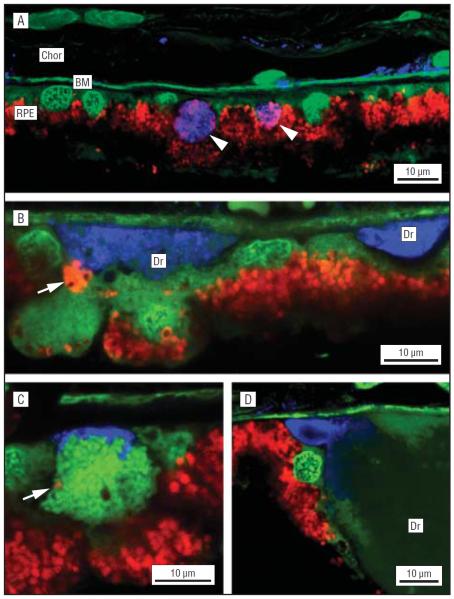
Figure 2.
Confocal microscopic localization of the membrane attack complex (C5b-9) in drusen (Dr) and in compromised retinal pigment epithelium (RPE) cells. A, Anti-C5b-9 labeling pattern at low magnification. Labeling in the choroid (Chor) (blue channel) is unremarkable except for staining in the Bruch membrane (BM) and around the choriocapillaris. Two RPE cells that may be the targets of complement attack are labeled (arrows). A nucleic acid-binding dye (YO-PRO; Invitrogen, Carlsbad, California) that also binds to the elastic layer of BM is used to visualize cell bodies on the green channel. No nucleic acid staining is evident in the anti-C5b-9–labeled RPE cells. B, Anti-C5b-9 labeling of Dr (blue channel). A condensed clump of lipofuscin granules (arrow) may have been expelled from the adjacent RPE cell. C, High magnification of a cell on the BM with anti-C5b-9 immunoreactivity on the basal surface. The only indication of its identity is the presence of a single red autofluorescent granule (arrow). Tightly packed vesicles in the cytoplasm are stained by the YO-PRO dye (green channel). D, C5b-9 immunoreactivity is present in the sub-RPE nodules that flank the Dr (blue channel). In this example, the nodule may have coalesced with the adjacent Dr. Reproduced with permission from Anderson et al.5
The complement system was initially described more than a century ago and, as suggested by its name, was initially thought to play a supplementary role in the immune system. It is now clear that this enzymatic cascade does more than just complement the body's ability to ward off disease. Importantly, it performs an essential role in the interplay between adaptive and innate immunity and contributes to overall physiological homeostasis by eliminating damaged, necrotic, and apoptotic cells. The complement system also maintains the solubility of circulating immune complexes, facilitating their elimination.27 This complement system is composed of several soluble and membrane-bound factors and is active in intravascular spaces, body fluids, and tissues (Figure 3). It is typically activated or triggered by 1 of 3 pathways—the classical, lectin, or alternative pathways—all of which converge at the point of C3 activation. Recently, a fourth complement-activation mechanism, referred to as the intrinsic pathway, has been discovered. In this pathway, serine proteases associated with the coagulation/fibrinolytic cascade activate the complement system directly through cleavage of C3 or C5, independently of the classical, alternate, and lectin pathways.28
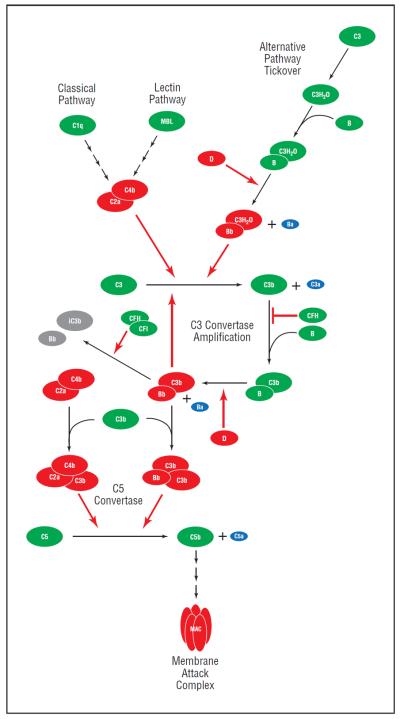
Figure 3.
The complement system is composed of proteins that are activated through a cascade of enzymatic cleavage. Precursor proteins are depicted in green, enzymes in red, byproducts in purple, and inactivated proteins in gray; red arrows indicate enzymatic reactions. Complement factor H (CFH) plays a key regulatory role by inhibiting the binding of complement component 3b (C3b) to complement factor B (CFB), thus preventing the formation of the alternative pathway's amplification loop C3 convertase (C3bBb) as well as acting as a cofactor for the complement factor I (CFI)–mediated degradation of the same convertase to inactive components iC3b and Bb. MAC indicates membrane attack complex; MBL, mannose-binding lectin.
In the classical pathway, the enzyme cascade is initiated by the interaction of pattern-recognition receptors on C1q with antibodies and molecules released by inflammatory processes such as serum amyloid protein and C-reactive protein. The lectin pathway is initiated by the binding of carbohydrates typically associated with microbes to lectin proteins such as mannose-binding lectin.29 C1q and mannose-binding lectin form complexes with serine proteases (C1r/C1s and MASP, respectively), which cleave C4 into functionally active components, C4a and C4b. C4b binds to C2, facilitating its cleavage to an active C2a component. The resulting C4bC2a complex functions as a C3 convertase, a protein complex that activates additional C3.27 The alternative pathway functions via a C3 convertase that is significantly different than that formed through the classical and lectin pathways. The alternative pathway C3 convertase is formed via a spontaneous hydrolysis of an internal C3 thioester. This spontaneous “tickover” forms C3(H20), a fluid-phase version of C3b, that binds to factor B, thus changing its confirmation and facilitating further cleavage by factor D into Ba and Bb fragments. C3(H20) binds with Bb, forming the initial alternative pathway convertase C3(H20)Bb.30 The spontaneous tickover of the alternative pathway allows the complement system to react immediately to pathogens or cellular damage by constitutively forming low levels of active C3 convertase. In addition to the spontaneous low-level activation of the alternative pathway (which may account for ≤80% of complement activation), various foreign substances can activate this pathway.27 Amplification is probably the most important function of the alternative pathway.17 Interestingly, components of cigarette smoke and some components of intraocular lenses have been demonstrated to activate the alternate complement pathway in vitro.31–37
Once a C3 convertase is formed through one of these pathways, C3 is cleaved into C3b and the anaphylatoxin C3a. Formation of C3b exposes an internal thioester, which binds to the surface of pathogens via patches of hydroxyl and amino groups. This binding-enhancement process, or opsonization, is critical for the elimination of pathogens and apoptotic cells by phagocytes.27 In addition to its opsonizing properties, when surface-bound C3b can bind to factor B and facilitate cleavage by factor D, forming the amplification loop C3 convertase of the alternative pathway C3bBb. This complex is stabilized by properdin.27,30
C3b also functions to create the classical/lectin pathway and alternative pathway C5 convertases by binding to form C4bC2aC3b and C3bC3bBb, respectively. These C5 convertases function similarly to cleave C5 to C5a, a potent anaphylatoxin, and C5b, which initiates the downstream formation of the membrane attack complex. The membrane attack complex, composed of C5b, C6, C7, C8, and 1 or more molecules of C9, creates a physical pore in the cell membrane of its targets (microbes, damaged cells, and when poorly regulated, healthy normal cells), ultimately leading to cell lysis and death.30 The ana-phylatoxins C3a and C5a (and C4a to a lesser extent) function to increase vascular permeability, initiate degranulation of mast cells and neutrophils, induce cytokine release from macrophages, and mediate leukocyte chemotaxis and extravasation.29
Alternative pathway regulation occurs via a number of structurally similar complement proteins, including CFH, decay accelerating factor, membrane cofactor protein, C4-binding protein, and complement receptor 1. It is noteworthy that CFH is the only fluid-phase regulator of the pathway; the other alternative complement pathway regulators are membrane-bound. Complement factor H, decay accelerating factor, and complement receptor 1 regulate the pathway by accelerating the decay of convertases, whereas CFH, membrane cofactor protein, and complement receptor 1 act as cofactors for factor I–mediated degradation of C3b to iC3b (inactive C3b). An inhibitor of the final common complement pathway, CD59, intercalates between C8 and C9 subunits, preventing formation of the membrane attack complex.27 Our discussion of the complement cascade illustrates the intricacy and complexity of this system.
Abnormalities in the structures and/or functions of complement pathway regulatory proteins can lead to an imbalance in normal homeostasis of the complement system, often resulting in “bystander” damage to healthy cells and tissues. This phenomenon accounts for substantial tissue damage in a variety of complement-mediated disease, including Alzheimer disease and atherosclerosis. Polymorphisms in genes that encode complement regulatory proteins are associated with numerous diseases, for example, atypical hemolytic uremic syndrome.17 Age-related macular degeneration and DDD can now be added to that growing list. Because CFH is the major fluid-phase regulator of the alternative pathway, any change in CFH function can have a profound effect on alternative pathway regulation. Thus, genetically determined protein dysfunction, a deficiency of CFH, the presence of an inhibitor of CFH, and an autoantibody directed against CFH can all lead to uncontrolled activation and/or regulation of the alternative complement pathway.17,38–40
AMD-ASSOCIATED COMPLEMENT PATHWAY GENES
CFH was the first major gene found to be linked to AMD, though we refer readers to earlier studies that showed minor associations of APOE and ABCA4 genes41–43 with AMD; these associations have been confirmed by most, but not all, other investigators. Most of the early studies—and in fact many of the more recent studies—focused on a single SNP in the coding region of CFH, Y402H, as the causal variant. However, it was clear from the beginning13 that multiple CFH variants (and their tagged haplotypes) rather than the Y402H variant in isolation, confer either an elevated or reduced risk of developing AMD. Three significant haplotypes—a major risk haplotype, tagged by the Y402H coding SNP and 2 protective haplotypes tagged by intronic variants and/or coding SNPs—were identified.13 Similar haplotype analyses were conducted subsequently in a Japanese population; Okamoto and colleagues44 confirmed the CFH association in a nonwhite population, identifying an additional risk haplotype. More recent studies further refined the analysis and association of the CFH locus, providing evidence for an additional risk haplotype45 and astrongly protective haplotype tagged by a complete deletion of 2 CFH-related genes, CFHR1 and CFHR3.19,21 Moreover, important associations between CFH genotype and environmental risk factors, such as smoking and obesity, disease progression, and the effect of genotype on response to treatment, have been determined. We refer readers to a host of excellent recent reviews pertaining to these associations.3,46–55
Associations between AMD and other complement genes have been identified since discovery of the CFH-AMD association. Single-nucleotide polymorphisms in the complement regulators CFB and C2 on chromosome 6p21 and C3 on chromosome 22q11, as well as a large deletion of the CFHR1 and CFHR3 genes,19,21 have been revealed. Although the influence of C2/CFB variation on AMD is less than that of the CFH and/or HTRA1 loci, combined analyses of the CFH and C2/CFB haplotypes showed that variation in the 2 loci can predict the clinical outcome in 74% of AMD cases, indicating that C2/CFB protective alleles can, to an extent, negate the effect of risk alleles from other loci.20,22 The precise role of CFB in AMD has not yet been established, although it has been shown that the protective CFB protein, which contains glutamine at position 32, has reduced hemolytic activity compared with the arginine-containing form.56 Based on these data, it has been postulated that CFB with reduced activity may provide a lower risk of chronic complement response, leading to AMD.20
Complement component 3 can be thought of as the pivotal protein on which the classical, lectin, and alternative complement cascade pathways merge. Owing to its critical role in the complement system, it was only logical to examine it as a candidate AMD gene. Yates and colleagues57 identified 2 nonsynonymous SNPs (rs2230199 and rs1047286) in C3 that was associated with late-stage AMD in 2 independent populations. Subsequent studies have validated the association of the rs2230199 SNP with AMD in several additional populations.58,59 The causal SNP has yet to be identified conclusively, though one common C3 polymorphism (rs2230199) that exhibits a strong association with AMD risk is known to encode proteins with functional differences. This Arg80Gly variant encodes the so-called C3S (slow) and C3F (fast) protein isoforms, the latter of which is associated with AMD risk. Although some in vitro studies have not been able to provide conclusive evidence of functional differences between the C3F and C3S alleles,60,61 other lines of evidence suggest that there are marked functional differences between proteins encoded by the C3F and C3S alleles. For example, C3F has lower activity than C3S in hemolytic assays.62 Moreover, the C3F allele is associated with a number of other diseases, including IgA nephropathy,63 systemic vasculitis,64 partial lipodystrophy,65 and DDD.66 The association with DDD and lipodystrophy is particularly relevant to this review, as individuals with these diseases also develop macular drusen. The patients with DDD also carry the same risk CFH genotype as individuals with AMD, once again suggesting that there are common pathways and causes among seemingly different diseases.
OTHER MAJOR NONCOMPLEMENT PATHWAY AMD-ASSOCIATED GENES
Evidence for a major AMD locus on chromosome 10q26 was initially provided from genomewide linkage analyses.7–10 In latter studies, the association was refined to variation within a chromosomal segment several kilobases in length and encompassing the 2 genes LOC387715/ARMS2 and PRS11/HTRA1.7,67–69 Genetic studies conducted thus far have made it impossible to assign causality to either of these 2 genes, and additional data will be required to determine which gene (and which specific variant) is associated with AMD risk. This aside, individuals homozygous for identified chromosome 10q26 risk variants and who are also homozygous for the CFH risk genotype almost invariably progress to late-stage AMD. One initial study suggested that the strongest association in the region was related to a coding polymorphism (Ala69Ser) within ARMS2, a gene that encodes a protein of undetermined function; whereas 2 subsequent groups suggested that a polymorphism in the promoter region of HTRA1 (rs11200638), a serine protease, was more significantly associated with risk.67,68 More recently, Fritsche and colleagues70 identified the presence of an insertion-deletion polymorphism in the 3′ region of ARMS2 that appears to mediate rapid turnover of messenger RNA in patients who carry a risk haplotype, data that support a functional role for ARMS2.
Other available data support a causal role for both HTRA1 and ARMS2 in disease pathogenesis. Both the HTRA1 and LOC387715/ARMS2 genes are transcribed in human and primate retina and the retinal pigment epthelium.71 One study provides preliminary data showing that the risk allele of HTRA1 is associated with elevated levels of HTRA1 messenger RNA and protein and that the HTRA1 antibody reacts with drusen.67 Yet another team examined the association of HTRA1 and ARMS2 variants in macaque monkeys with and without drusen,72 concluding that disease association was completely attributable to a promoter polymorphism (−558G>T) in the HTRA1 promoter region. HTRA1 is a serine protease known to participate directly or indirectly in the degradation of the extracellular matrix, to mediate cell death, and to localize with β amyloid deposits.69,73 Thus, it is intriguing to speculate that HTRA1 could participate in the development of late-stage AMD by the degradation of the Bruch membrane that most certainly precedes choroidal neovascularization membrane formation, the deposition of β amyloid peptide in drusen, and/or retinal pigment epithelium and photoreceptor cell death.5,24,74,75 However, only time and the generation of additional data will help to determine which of these 2 genes is associated with the development of AMD.
INTERACTION OF ENVIRONMENTAL AND GENETIC RISK FACTORS IN AMD
Age-related macular degeneration risk is also influenced by external, nongenetic risk factors. In addition to age, AMD risk factors that have consistently been identified in epidemiological studies include smoking, obesity, fat intake, and dietary antioxidant intake.76–83 More recent epidemiological studies carried out in light of genetic risk support the hypothesis that when external risk factors are superimposed on the genetic risk factors identified thus far, the genetic and environmental risk factors are not merely additive. In some cases, the resultant risk of AMD development conferred by a combination of environmental and hereditary risk factors is greater than that conferred by each risk factor individually.53,84–86
The Age-Related Eye Disease Study, as an example, demonstrated that a formulation of antioxidant multivitamins (ascorbic acid, dl-alpha tocopheryl acetate, beta carotene, and zinc) used in the study reduced the risk of developing advanced AMD by 25% and moderate vision loss by 19% at 5 years. A high dietary intake of these same vitamins also showed an association with a considerably reduced risk of AMD among elderly individuals in the Rotterdam Study. These studies were performed without the benefit of knowledge of genetic risk factors.87,88
The recent observations that one's genetic risk can be exacerbated by modifiable external risk factors raise the possibility that genetically susceptible individuals could be identified in preclinical stages of the disease when external risk factor modification might prevent or delay onset of clinical AMD. Although current studies such as the Age-Related Eye Disease Study 2 are not funded for genetic risk analysis, hopefully future large population-based studies will include genetic risk evaluation so that we can begin to grapple with this issue. One small study has demonstrated a reduced progression to late-stage AMD in individuals with a homozygous low-risk (TT) genotype at position 402 on the CFH gene compared with individuals with high-risk (CC) Y402H substitution when being treated with zinc.89 However, much more research in this area, including the development of more sensitive methods of detection of preclinical disease, will be necessary to determine if preventive strategies are having the desired effect many years before clinical disease develops.
A RATIONALE FOR CURRENT DIAGNOSTIC TESTING FOR AMD RISK AND PROTECTION
Age-related macular degeneration is posed to be one of the first major chronic human diseases that may benefit from the application of the new science of pharmacogenetics and personalized medicine. Taken together, 3 SNPs—CFH Y402H, ARMS2 A69S, and C3 R80G—account for approximately 76% of attributable risk of the development of AMD.59 Of these, CFH is the most strongly associated with disease, followed by ARMS2/HTRA1, smoking status, and finally C3 mutations. Despriet and colleagues90 calculated that when a population reference group (based on the average risk in a population) was compared with those with a high-risk genotype at these 3 loci, the risk of AMD was 14-fold higher than that of the general population, whereas individuals who carried only low-risk genotypes had a 20-fold decreased risk of disease. An even more recent multivariant analysis shows that the predictive value of genetic testing for CFH, ARMS2/HTRA1, C3, and CFB/C2 is 86% and diagnostic analyses can discriminate between in dividuals who will develop late-stage AMD and those who will not.91 Importantly, this same analysis shows that the risk of developing late-stage AMD by 80 years of age for individuals who do not carry a risk allele of any of these genes is less than 1%.
The ability to screen patients at a preclinical stage for risk of a genetic disease affords an opportunity to prevent or attenuate the disease later in life. Although pharmacogenetic therapy for AMD is still in its earliest stages of development, there does seem to be a role for AMD risk factor modification in light of genetic risk. As noted, genetic association can explain 75% of the attributable risk of AMD. It can be speculated that knowledge of an individual's genetic AMD risk should allow caregivers to better counsel potential AMD patients regarding risk factor modification before they have clinical disease. Genetic association should also provide patients and health care professionals with knowledge about which patients require more frequent eye screening for AMD. Once an individual has acquired early clinical AMD, there is still a defensible rationale for genetic screening. For example, the number and nature of AMD gene-associated alleles that patients possess may significantly influence the rate of progression of their disease. Current studies of AMD progression as it relates to genotype are under way and the field anxiously awaits their conclusions. Should such studies demonstrate an ability to stratify risk and rate of progression based on genetic risk profile, diagnostic testing will likely prove useful to the clinician in the management of patients.
Given increasingly limited health care resources and increasing interest in preventive medicine, diagnostic genetic testing will allow prudent application of resources directed toward risk factor modification and clinical disease screening. In the future, genetic risk determination will almost certainly influence the choice of therapy used.
PHARMACOGENOMIC THERAPY FOR AMD
There is early evidence that one's genetic profile may influence already existing therapies for AMD.89,92–97 The complexities of the complement system, superimposition of environmental/external factors, and recent discoveries of complement dysregulation in other chronic diseases raises important questions about potential pharmacologic and genetic manipulation of the complement system to treat and prevent AMD. Because modulation of the complement system is essentially immunomodulation, genetically directed (ie, pharmacogenetic) AMD therapies must take into account potential effects on other organ systems. In this final section, we discuss complement-based therapies currently under study as well as the potential for genetically directed complement-based therapies for AMD.
Because there is no cure for AMD, current treatment is palliative and has been mainly focused on delaying and reversing vision loss caused by late-stage, exudative (wet) AMD. Ophthalmologists are well acquainted with the treatment of exudative AMD, but a brief review of the chronology of AMD treatment will place the exciting potential for pharmacogenetic therapy in perspective. Treatment of exudative AMD between the years 1986 and 2000 was based on important findings made during the Macu lar Photocoagulation Study,98–101 which demonstrated that thermal laser photocoagulation delayed vision loss in patients with well-defined extrafoveal, juxtafoveal, and small subfoveal classic choroidal neovascular membranes. However, the fraction of patients with wet AMD for which this therapy applies is relatively small,102 and recurrence rates with subsequent vision loss are high.103,104
In 2000, the Food and Drug Administration (FDA) approved photodynamic therapy (PDT) with verteporfin as a therapy for exudative AMD. Visual results with verteporfin treatment were superior to those obtained with thermal laser,105,106 but 3% to 4% of patients experience severe vision loss, and even under the best circumstances, very few patients experienced an improvement in vision with PDT using verteporfin. Interestingly, 1 recent pharmacogenetic study shows visual acuity outcomes with PDT to be significantly worse in individuals with the CFH TT genotype compared with the TC or CC genotypes with classic neovascular lesions when treated with PDT. There was no significant difference in response to PDT for LOC387715 genotypes.96,107 In the current era of therapy for exudative AMD, antineovascular/antipermeability agents are administered by intraocular injection. In 2004, pegaptanib was the first of the vascular endothelial growth factor inhibitors to be approved by the FDA for exudative AMD.108
The FDA approved ranibizumab in 2006 for the treatment of exudative AMD. Ranibizumab not only stabilized vision in 94.6% of patients, but was the first therapy for exudative AMD to produce significant visual improvement, with a mean of 33.8% experiencing 3 or more lines of visual acuity increase compared with 5% in the control group.109 Bevacizumab, a monoclonal antibody derived from the same “parent” molecule from which ranibizumab was derived, is approved for use in colon cancer and has been used as an off-label therapy for wet AMD since 2005.110
The superimposition of AMD genetic risk information on the results of current therapies for AMD supports the concept that future treatment of AMD will be influenced positively by genetic risk information. One study of ranibizumab use in 156 patients reports a 37% higher risk of requiring additional injections for individuals with the homozygous high-risk CFH Y402H allele during a 9-month period.93 A study of bevacizumab use in 86 patients has shown that visual acuity outcomes are better for the CFH TT and TC genotypes compared with individuals with CC genotypes. Patients with TT and TC genotypes showed a mean increase in vision, with 53.7% of patients demonstrating improved visual acuity. Patients with the CC genotype showed a mean decrease in vision, with only 10.5% of patients demonstrating an increase in visual acuity.39,96
It is clear that the complement pathway, particularly the alternative pathway amplification loop, is a realistic target on which to base the development of new treatment modalities. Armed with a wealth of important new information relating AMD pathology to complement dys-function, numerous companies are currently developing genetically based and complement-targeted therapeutics with the goal of reducing complement-related AMD disease processes. Potential therapeutic target mechanisms include inhibition of C3 activation, inhibition of C3 convertase assembly, promotion of C3 convertase decay promotion of factor I-mediated C3B proteolysis, inhibition of C3 and/or C5 convertase activities, inhibition of membrane attack complex assembly by CD5-9, and reestablishment of normal alternative complement pathway control by augmentation with protective CFH protein (Figure 3).
With these therapeutic mechanisms in mind, consideration will have to be given to a number of issues that will influence the risk-benefit ratio of each potential therapeutic agent. Issues to consider include chronic vs acute administration, local ocular vs systemic delivery, intraocular vs extraocular delivery, the nature of the compound to be used (eg, small molecule vs large biologic), and potential systemic effects of chronic complement pathway modulation or inhibition. The issue related to the involvement of the systemic immune system is especially pertinent at this time given that recent studies have associated AMD with other chronic systemic diseases. If AMD is, indeed, the result of chronic, systemic complement dysregulation, then local ocular therapy may result in only a transient benefit. Alternatively, systemic therapy, possibly even genetic therapy, may be required to prevent, retard, or ameliorate AMD in the long run. Whereas preventive systemic therapy can be supported on both clinical and scientific grounds, potential risks and other adverse effects that might be associated with chronic inhibition of the complement system will need to be addressed.
Therapies to both treat exudative AMD and retard the progression of nonexudative AMD are under development (Figure 4).27 Phase 2 clinical trials are currently enrolling subjects to test compstatin/POT-4, a C3-peptide inhibitor, for intravitreal use in geographic atrophy. Compstatin/POT-4 effectively inhibits the central step of the complement cascade by preventing cleavage of C3 to its active fragments C3a and C3b. A trial using systemic administration of eculizumab, a humanized anti-C5 antibody used for the treatment of paroxysmal nocturnal hemoglobinuria, has been approved by the FDA to examine its use in geographic atrophy. Another complement component inhibitor, ARC1905, a pegylated, aptamer-based C5 inhibitor, inhibits the cleavage of C5 into C5a and C5b, thus blocking downstream complement activation. Intravitreal administration of ARC1905 is currently being examined in combination with ranibizumab in an open-label phase 1 safety study for future studies directed toward the treatment of wet AMD. Other complement pathway-modulating compounds currently being considered for and/or under preclinical development for possible use in AMD include TNX-234 (a humanized antibody directed against complement factor D), TA106 (a Fab fragment of an anti-CFB antibody), CR2-CFH hybrid proteins, JPE-1375 (a small molecule C5aR peptidomimetic), anti-properdin antibody (which should, in theory, destabilize the C3 convertase), C1-INH (a protein that inhibits activation of the classical pathway), and sCR1 (a soluble form of endogenous complement receptor 1, which promotes the degradation of active C3bBb). Yet another compound in pre-clinical development is a recombinant, protective form of CFH. The strategy here is based on the concept that augmentation of dysfunctional risk CFH protein with protective CFH protein should be effective in re-establishing homeostatic regulation of the complement alternative pathway, thereby protecting host cells from complement-induced damage.
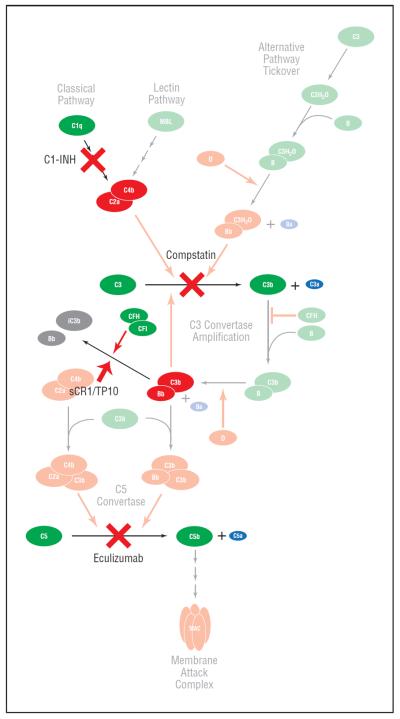
Figure 4.
A number of strategies for modulating the complement system are being considered for use in the treatment of various stages of age-related macular degeneration. These include, in general, approaches to (1) inhibit activation and the assembly of convertases, (2) promote the decay and proteolysis of macromolecular complexes, including the convertases, (3) block various effector molecules, such as C3a and C5a, and (4) reestablish control and homeostasis of the system, such as augmentation with protective complement factor H (CFH). This figure is a modified version of Figure 3; it depicts 4 potential targets within the complement pathway that are being considered for therapeutic intervention: C1-INH, which inhibits activation of the classical pathway; compstatin, which inhibits the activation of C3; sCR1, which promotes the degradation of the C3 convertase C3bBb; and eculizumab, which inhibits the system at the level of C5, thereby preventing formation of the membrane attack complex (MAC/C5b-9) and C5a. MAC indicates membrane attack complex; MBL, mannose-binding lectin.
COMMENT
In summary, decades of clinical, epidemiological, and biological studies demonstrated that AMD is heritable and strongly associated with complement dysfunction. The discovery in 2005 that genetic variation in the complement pathway gene CFH confers risk of or protection from developing AMD further enhanced our understanding of the disease, led to the rapid identification of yet other AMD-associated genes, and created a realistic potential that new diagnostics and complement-based therapeutic treatment modalities will be developed. Although there is much still to be learned about the complement system and its role in chronic diseases, including AMD, the pace of AMD-related research has accelerated substantially and is providing important new information almost daily. More importantly, the identification of roles for specific gene-directed pathways in the etiology of AMD, a chronic condition that develops over the span of many years, opens the door to the possibility that this and other chronic human diseases with similar genetic associations can be prevented or at least greatly ameliorated. In tandem with recent improvements in high-throughput and relatively inexpensive genetic technologies, we are poised to witness the development of a new era of a personalized approach toward the assessment, management, and treatment of this devastating disease.
Acknowledgments
Financial Disclosure: Dr Gehrs has been a consultant for Bausch & Lomb and Genentech and has received research support as a clinical principal investigator or investigator from Eli Lilly, Novartis, Allergan, Alcon, Genentech, and Pfizer. The University of Iowa Department of Ophthalmology and Visual Sciences has received educational program support from Allergan, Alcon, and Novartis; and the University of Iowa holds patents and patent applications, of which Dr Hageman is an inventor and for which he receives royalties. Dr Hageman has consulted for Genentech, Pfizer, and OccuLogix; has received grants from Pfizer and Optherion Inc; and has a financial interest (stock ownership) in and was the chief security officer of Optherion Inc.
Funding/Support: This study was supported by grants R24 EY017404 (Dr Hageman) and R01 EY13435 (Dr Allikmets) from the National Institutes of Health; unrestricted grants to the Department of Ophthalmology and Visual Sciences, University of Utah, the Department of Ophthalmology and Visual Sciences, University of Iowa, and the Department of Ophthalmology, and Columbia University from Research to Prevent Blindness Inc; the Macula Vision Research Foundation (Dr Allikmets); the Wallach Foundation (Dr Allikmets); the Elyachar Foundation (Dr Allik-mets); the Kaplen Foundation (Dr Allikmets); the Wigdeon Point Charitable Foundation (Dr Allikmets); the Ruth and Milton Steinbach Foundation (Dr Allikmets); and the Alcon Research Institute (Drs Allikmets and Hageman).
Footnotes
Additional Contributions: Drs Michael Dean, Seppo Meri, Anna Blom, John Atkinson, Lincoln Johnson, Dean Bok, Don Anderson, and Richard Smith provided helpful discussions and Drs Jamal Hoballah, Tim Johnson, Stacy Thompson, David Saggau, Charles Barnes, Jack Wells, Richard Rothman, Thomas Collins, Alan Reed, Antonio Sanchez, Michael Voigt, John Sharp, and Thomas Weingeist assisted in recruiting patients. Lisa Hancox, BS, Jill Hageman, RN, CCRN, Lori Davis, COT, Julie Donahue, BA, Sankar Baruah, BS, Mary Howard, Sheri McCormick, Norma Miller, BS, Rachel Wolf, BS, Shemin Zeng, MD, Gregory Bligard, Chelsie Kirschbaum, and the staffs of the Iowa Donor Network and Iowa Lions Eye Bank provided support and technical assistance. We are also extremely grateful to our study participants who have unselfishly donated their time to this research program.
REFERENCES
- 1.Klein R. Overview of progress in the epidemiology of age-related macular degeneration. Ophthalmic Epidemiol. 2007;14(4):184–187. doi: 10.1080/09286580701344381. [DOI] [PubMed] [Google Scholar]
- 2.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 3.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration: emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20(6):705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 6.Gorin MB. A clinician's view of the molecular genetics of age-related maculopathy. Arch Ophthalmol. 2007;125(1):21–29. doi: 10.1001/archopht.125.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14(21):3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 8.Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77(3):389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weeks DE, Conley YP, Tsai HJ, et al. Age-related maculopathy: a genomewide scan with continued evidence of susceptibility loci within the 1q31, 10q26, and 17q25 regions. Am J Hum Genet. 2004;75(2):174–189. doi: 10.1086/422476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher SA, Abecasis GR, Yashar BM, et al. Meta-analysis of genome scans of age-related macular degeneration. Hum Mol Genet. 2005;14(15):2257–2264. doi: 10.1093/hmg/ddi230. [DOI] [PubMed] [Google Scholar]
- 11.Shi J, Wang Y, Huang W. Development and application of genotyping technologies. Sci China C Life Sci. 2009;52(1):17–23. doi: 10.1007/s11427-009-0011-x. [DOI] [PubMed] [Google Scholar]
- 12.Guyer MS, Collins FS. The Human Genome Project and the future of medicine. Am J Dis Child. 1993;147(11):1145–1152. doi: 10.1001/archpedi.1993.02160350019003. [DOI] [PubMed] [Google Scholar]
- 13.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 15.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 17.Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 18.Józsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29(8):380–387. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Hageman GS, Hancox LS, Taiber AJ, et al. AMD Clinical Study Group Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: characterization, ethnic distribution and evolutionary implications. Ann Med. 2006;38(8):592–604. [PMC free article] [PubMed] [Google Scholar]
- 20.Gold B, Merriam JE, Zernant J, et al. AMD Genetics Clinical Study Group Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes AE, Orr N, Esfandiary H, Diaz-Torres M, Goodship T, Chakravarthy U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet. 2006;38(10):1173–1177. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]
- 22.Seddon JM, George S, Rosner B, Klein ML. CFH gene variant, Y402H, and smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum Hered. 2006;61(3):157–165. doi: 10.1159/000094141. [DOI] [PubMed] [Google Scholar]
- 23.Mullins RF, Aptsiauri N, Hageman GS. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye (Lond) 2001;15(pt 3):390–395. doi: 10.1038/eye.2001.142. [DOI] [PubMed] [Google Scholar]
- 24.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14(7):835–846. [PubMed] [Google Scholar]
- 25.Xing C, Sivakumaran TA, Wang JJ, et al. Complement factor H polymorphisms, renal phenotypes and age-related macular degeneration: the Blue Mountains Eye Study. Genes Immun. 2008;9(3):231–239. doi: 10.1038/gene.2008.10. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Knudtson MD, Lee KE, Klein BE. Serum cystatin C level, kidney disease markers, and incidence of age-related macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol. 2009;127(2):193–199. doi: 10.1001/archophthalmol.2008.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25(11):1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amara U, Rittirsch D, Flierl M, et al. Interaction between the coagulation and complement system. Adv Exp Med Biol. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171(3):715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markiewski MM, Deangelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med. 2008;12(6A):2245–2254. doi: 10.1111/j.1582-4934.2008.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mondino BJ, Nagata S, Glovsky MM. Activation of the alternative complement pathway by intraocular lenses. Invest Ophthalmol Vis Sci. 1985;26(6):905–908. [PubMed] [Google Scholar]
- 32.Mondino BJ, Rao H. Effect of intraocular lenses on complement levels in human serum. Acta Ophthalmol (Copenh) 1983;61(1):76–84. doi: 10.1111/j.1755-3768.1983.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 33.Gobel RJ, Janatova J, Googe JM, Apple DJ. Activation of complement in human serum by some synthetic polymers used for intraocular lenses. Biomaterials. 1987;8(4):285–288. doi: 10.1016/0142-9612(87)90116-5. [DOI] [PubMed] [Google Scholar]
- 34.Mondino BJ, Rajacich GM, Sumner H. Comparison of complement activation by silicone intraocular lenses and polymethylmethacrylate intraocular lenses with polypropylene loops. Arch Ophthalmol. 1987;105(7):989–990. doi: 10.1001/archopht.1987.01060070133042. [DOI] [PubMed] [Google Scholar]
- 35.Kochounian HH, Maxwell WA, Gupta A. Complement activation by surface modified poly(methyl methacrylate) intraocular lenses. J Cataract Refract Surg. 1991;17(2):139–142. doi: 10.1016/s0886-3350(13)80243-x. [DOI] [PubMed] [Google Scholar]
- 36.Kew RR, Ghebrehiwet B, Janoff A. Cigarette smoke can activate the alternative pathway of complement in vitro by modifying the third component of complement. J Clin Invest. 1985;75(3):1000–1007. doi: 10.1172/JCI111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kew RR, Ghebrehiwet B, Janoff A. Characterization of the third component of complement (C3) after activation by cigarette smoke. Clin Immunol Immunopathol. 1987;44(2):248–258. doi: 10.1016/0090-1229(87)90069-9. [DOI] [PubMed] [Google Scholar]
- 38.Walker PD. Dense deposit disease: new insights. Curr Opin Nephrol Hypertens. 2007;16(3):204–212. doi: 10.1097/MNH.0b013e3280bdc0f4. [DOI] [PubMed] [Google Scholar]
- 39.Fang CJ, Richards A, Liszewski MK, Kavanagh D, Atkinson JP. Advances in understanding of pathogenesis of aHUS and HELLP. Br J Haematol. 2008;143(3):336–348. doi: 10.1111/j.1365-2141.2008.07324.x. [DOI] [PubMed] [Google Scholar]
- 40.Kavanagh D, Richards A, Fremeaux-Bacchi V, et al. Screening for complement system abnormalities in patients with atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2007;2(3):591–596. doi: 10.2215/CJN.03270906. [DOI] [PubMed] [Google Scholar]
- 41.Allikmets R, Shroyer NF, Singh N, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277(5333):1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 42.Souied EH, Benlian P, Amouyel P, et al. The ε4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol. 1998;125(3):353–359. doi: 10.1016/s0002-9394(99)80146-9. [DOI] [PubMed] [Google Scholar]
- 43.Klaver CC, Kliffen M, van Duijn CM, et al. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998;63(1):200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto H, Umeda S, Obazawa M, et al. Complement factor H polymorphisms in Japanese population with age-related macular degeneration. Mol Vis. 2006;12:156–158. [PubMed] [Google Scholar]
- 45.Li M, Atmaca-Sonmez P, Othman M, et al. CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nat Genet. 2006;38(9):1049–1054. doi: 10.1038/ng1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Córdoba SR, de Jorge EG. Translational mini-review series on complement factor H: genetics and disease associations of human complement factor H. Clin Exp Immunol. 2008;151(1):1–13. doi: 10.1111/j.1365-2249.2007.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28(1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edwards AO. Genetics of age-related macular degeneration. Adv Exp Med Biol. 2008;613:211–219. doi: 10.1007/978-0-387-74904-4_24. [DOI] [PubMed] [Google Scholar]
- 49.Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age-related macular degeneration: a review of progress to date. Surv Ophthalmol. 2006;51(4):316–363. doi: 10.1016/j.survophthal.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Montezuma SR, Sobrin L, Seddon JM. Review of genetics in age related macular degeneration. Semin Ophthalmol. 2007;22(4):229–240. doi: 10.1080/08820530701745140. [DOI] [PubMed] [Google Scholar]
- 51.Patel N, Adewoyin T, Chong NV. Age-related macular degeneration: a perspective on genetic studies. Eye (Lond) 2008;22(6):768–776. doi: 10.1038/sj.eye.6702844. [DOI] [PubMed] [Google Scholar]
- 52.Scholl HP, Fleckenstein M, Charbel Issa P, Keilhauer C, Holz FG, Weber BH. An update on the genetics of age-related macular degeneration. Mol Vis. 2007;13:196–205. [PMC free article] [PubMed] [Google Scholar]
- 53.Seddon JM, Reynolds R, Maller J, Fagerness JA, Daly MJ, Rosner B. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables [published online ahead of print December 30, 2008] Invest Ophthalmol Vis Sci. 2009;50(5):2044–2053. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swaroop A, Branham KE, Chen W, Abecasis G. Genetic susceptibility to age-related macular degeneration: a paradigm for dissecting complex disease traits. Hum Mol Genet. 2007;16(Spec No. 2):R174–R182. doi: 10.1093/hmg/ddm212. [DOI] [PubMed] [Google Scholar]
- 55.Baird PN, Robman LD, Richardson AJ, et al. Gene-environment interaction in progression of AMD: the CFH gene, smoking and exposure to chronic infection. Hum Mol Genet. 2008;17(9):1299–1305. doi: 10.1093/hmg/ddn018. [DOI] [PubMed] [Google Scholar]
- 56.Lokki ML, Koskimies SA. Allelic differences in hemolytic activity and protein concentration of BF molecules are found in association with particular HLA haplotypes. Immunogenetics. 1991;34(4):242–246. doi: 10.1007/BF00215259. [DOI] [PubMed] [Google Scholar]
- 57.Yates JR, Sepp T, Matharu BK, et al. Genetic Factors in AMD Study Group Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357(6):553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 58.Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007;39(10):1200–1201. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- 59.Spencer KL, Olson LM, Anderson BM, et al. C3 R102G polymorphism increases risk of age-related macular degeneration. Hum Mol Genet. 2008;17(12):1821–1824. doi: 10.1093/hmg/ddn075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arvilommi H. Capacity of complement c3 phenotypes to bind on to mononuclear cells in man. Nature. 1974;251(5477):740–741. doi: 10.1038/251740a0. [DOI] [PubMed] [Google Scholar]
- 61.Bartók I, Walport MJ. Comparison of the binding of C3S and C3F to complement receptors types 1, 2, and 3. J Immunol. 1995;154(10):5367–5375. [PubMed] [Google Scholar]
- 62.Welch TR, Beischel L, Kleesattel A. Functional consequences of the genetic polymorphism of the third component of complement. J Pediatr. 1990;116(5):S92–S97. doi: 10.1016/s0022-3476(05)82709-x. [DOI] [PubMed] [Google Scholar]
- 63.Rambausek M, van den Wall Bake AW, Schumacher-Ach R, et al. Genetic polymorphism of C3 and Bf in IgA nephropathy. Nephrol Dial Transplant. 1987;2(4):208–211. [PubMed] [Google Scholar]
- 64.Finn JE, Zhang L, Agrawal S, Jayne DR, Oliveira DB, Mathieson PW. Molecular analysis of C3 allotypes in patients with systemic vasculitis. Nephrol Dial Transplant. 1994;9(11):1564–1567. [PubMed] [Google Scholar]
- 65.Finn JE, Mathieson PW. Molecular analysis of C3 allotypes in patients with nephritic factor. Clin Exp Immunol. 1993;91(3):410–414. doi: 10.1111/j.1365-2249.1993.tb05917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Appel GB, Cook HT, Hageman G, et al. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol. 2005;16(5):1392–1403. doi: 10.1681/ASN.2005010078. [DOI] [PubMed] [Google Scholar]
- 67.Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314(5801):992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 68.Dewan A, Liu M, Hartman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314(5801):989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 69.Grau S, Baldi A, Bussani R, et al. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc Natl Acad Sci U S A. 2005;102(17):6021–6026. doi: 10.1073/pnas.0501823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fritsche LG, Loenhardt T, Janssen A, et al. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet. 2008;40(7):892–896. doi: 10.1038/ng.170. [DOI] [PubMed] [Google Scholar]
- 71.Francis PJ, Appukuttan B, Simmons E, et al. Rhesus monkeys and humans share common susceptibility genes for age-related macular disease. Hum Mol Genet. 2008;17(17):2673–2680. doi: 10.1093/hmg/ddn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh KK, Krawczak M, Dawson WW, Schmidtke J. Association of HTRA1 and ARMS2 gene variation with drusen formation in rhesus macaques [published online November 5, 2008] Exp Eye Res. 2009;88(3):479–482. doi: 10.1016/j.exer.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 73.Grau S, Richards PJ, Kerr B, et al. The role of human HtrA1 in arthritic disease. J Biol Chem. 2006;281(10):6124–6129. doi: 10.1074/jbc.M500361200. [DOI] [PubMed] [Google Scholar]
- 74.Chong NH, Keonin J, Luthert PJ, et al. Decreased thickness and integrity of the macular elastic layer of Bruch's membrane correspond to the distribution of lesions associated with age-related macular degeneration. Am J Pathol. 2005;166(1):241–251. doi: 10.1016/S0002-9440(10)62248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73(6):887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 76.Smith W, Mitchell P, Leeder SR. Smoking and age-related maculopathy: The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114(12):1518–1523. doi: 10.1001/archopht.1996.01100140716016. [DOI] [PubMed] [Google Scholar]
- 77.Smith W, Mitchell P. Family history and age-related maculopathy: the Blue Mountains Eye Study. Aust N Z J Ophthalmol. 1998;26(3):203–206. doi: 10.1111/j.1442-9071.1998.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 78.Klein R, Klein BE, Moss SE. Relation of smoking to the incidence of age-related maculopathy: The Beaver Dam Eye Study. Am J Epidemiol. 1998;147(2):103–110. doi: 10.1093/oxfordjournals.aje.a009421. [DOI] [PubMed] [Google Scholar]
- 79.Klein R, Klein BE, Linton KL, DeMets DL. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking. Am J Epidemiol. 1993;137(2):190–200. doi: 10.1093/oxfordjournals.aje.a116659. [DOI] [PubMed] [Google Scholar]
- 80.François J. The inheritance of senile macule degeneration. Ophthalmologica. 1977;175(2):67–72. doi: 10.1159/000308637. [DOI] [PubMed] [Google Scholar]
- 81.Silvestri G, Johnston PB, Hughes AE. Is genetic predisposition an important risk factor in age-related macular degeneration? Eye (Lond) 1994;8(pt 5):564–568. doi: 10.1038/eye.1994.138. [DOI] [PubMed] [Google Scholar]
- 82.Silvestri G. Age-related macular degeneration: genetics and implications for detection and treatment. Mol Med Today. 1997;3(2):84–91. doi: 10.1016/S1357-4310(96)10057-5. [DOI] [PubMed] [Google Scholar]
- 83.Cho E, Hung S, Willett WC, et al. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr. 2001;73(2):209–218. doi: 10.1093/ajcn/73.2.209. [DOI] [PubMed] [Google Scholar]
- 84.Seddon JM, Francis PJ, George S, Schultz DW, Rosner B, Klein ML. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007;297(16):1793–1800. doi: 10.1001/jama.297.16.1793. [DOI] [PubMed] [Google Scholar]
- 85.Francis PJ, George S, Schultz DW, et al. The LOC387715 gene, smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum Hered. 2007;63(3–4):212–218. doi: 10.1159/000100046. [DOI] [PubMed] [Google Scholar]
- 86.Despriet DD, Klaver CC, Witteman JC, et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA. 2006;296(3):301–309. doi: 10.1001/jama.296.3.301. [DOI] [PubMed] [Google Scholar]
- 87.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report No. 9. Arch Ophthalmol. 2001;119(10):1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klein ML, Francis PJ, Rosner B, et al. CFH and LOC387715/ARMS2 genotypes and treatment with antioxidants and zinc for age-related macular degeneration. Ophthalmology. 2008;115(6):1019–1025. doi: 10.1016/j.ophtha.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 90.Despriet DD, Klaver CC, van Duijn CC, Janssens AC. Predictive value of multiple genetic testing for age-related macular degeneration. Arch Ophthalmol. 2007;125(9):1270–1271. doi: 10.1001/archopht.125.9.1270. [DOI] [PubMed] [Google Scholar]
- 91.Despriet DD, van Duijn CC, de Jong PTVM, Vingerling JR, Bergen AAB, Klaver CC. Genetic diagnosis of age-related macular degeneration: the role of molecular genetics in the identification of high risk eyes [ARVO abstract 1769/A498] Invest Ophthalmol Vis Sci. 2008 [Google Scholar]
- 92.Parmeggiani F, Gemmati D, Costagliola C, Sebastiani A, Incorvaia C. Predictive role of C677T MTHFR polymorphism in variable efficacy of photodynamic therapy for neovascular age-related macular degeneration. Pharmacogenomics. 2009;10(1):81–95. doi: 10.2217/14622416.10.1.81. [DOI] [PubMed] [Google Scholar]
- 93.Lee AY, Raya AK, Kymes SM, Shiels A, Brantley MA., Jr. Pharmacogenetics of complement factor H (Y402H) and treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2009;93(5):610–613. doi: 10.1136/bjo.2008.150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Francis PJ, Hamon SC, Ott J, Weleber RG, Klein ML. Polymorphisms in C2, CFB and C3 are associated with progression to advanced age-related macular degeneration associated with visual loss. J Med Genet. 2009;46(5):300–307. doi: 10.1136/jmg.2008.062737. [DOI] [PubMed] [Google Scholar]
- 95.Parmeggiani F, Costagliola C, Gemmati D, et al. Coagulation gene predictors of photodynamic therapy for occult choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(7):3100–3106. doi: 10.1167/iovs.07-1654. [DOI] [PubMed] [Google Scholar]
- 96.Brantley MA, Jr, Fang AM, King JM, Tewari A, Kymes SM, Shiels A. Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to intravitreal bevacizumab. Ophthalmology. 2007;114(12):2168–2173. doi: 10.1016/j.ophtha.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Parmeggiani F, Costagliola C, Gemmati D, et al. Predictive role of coagulation-balance gene polymorphisms in the efficacy of photodynamic therapy with verteporfin for classic choroidal neovascularization secondary to age-related macular degeneration. Pharmacogenet Genomics. 2007;17(12):1039–1046. doi: 10.1097/FPC.0b013e3282f12a4e. [DOI] [PubMed] [Google Scholar]
- 98.Macular Photocoagulation Study Group Argon laser photocoagulation for neovascular maculopathy: three-year results from randomized clinical trials. Arch Ophthalmol. 1986;104(5):694–701. [PubMed] [Google Scholar]
- 99.Macular Photocoagulation Study Group Krypton laser photocoagulation for neovascular lesions of age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1990;108(6):816–824. doi: 10.1001/archopht.1990.01070080058036. [DOI] [PubMed] [Google Scholar]
- 100.Macular Photocoagulation Study Group Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991;109(9):1220–1231. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- 101.Macular Photocoagulation Study Group Persistent and recurrent neovascularization after krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Arch Ophthalmol. 1990;108(6):825–831. doi: 10.1001/archopht.1990.01070080067037. [DOI] [PubMed] [Google Scholar]
- 102.Freund KB, Yannuzzi LA, Sorenson JA. Age-related macular degeneration and choroidal neovascularization. Am J Ophthalmol. 1993;115(6):786–791. doi: 10.1016/s0002-9394(14)73649-9. [DOI] [PubMed] [Google Scholar]
- 103.Han DP, Folk JC, Bratton AR. Visual loss after successful photocoagulation of choroidal neovascularization. Ophthalmology. 1988;95(10):1380–1384. doi: 10.1016/s0161-6420(88)33013-7. [DOI] [PubMed] [Google Scholar]
- 104.Sorenson JA, Yannuzzi LA, Shakin JL. Recurrent subretinal neovascularization. Ophthalmology. 1985;92(8):1059–1074. [PubMed] [Google Scholar]
- 105.Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Arch Ophthalmol. 1999;117(10):1329–1345. [PubMed] [Google Scholar]
- 106.Verteporfin In Photodynamic Therapy Study Group Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization–verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131(5):541–560. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 107.Brantley MA, Jr, Edelstein SL, King JM, et al. Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to photodynamic therapy. Eye (Lond) 2009;23(3):626–631. doi: 10.1038/eye.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 109.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 110.Ip MS, Scott IU, Brown GC, et al. American Academy of Ophthalmology Antivascular endothelial growth factor pharmacotherapy for age-related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115(10):1837–1846. doi: 10.1016/j.ophtha.2008.08.012. [DOI] [PubMed] [Google Scholar]