Abstract
The hedgehog (HH) family of growth factors is involved in many aspects of growth and development, from the establishment of left-right axes at gastrulation to the patterning and formation of multiple structures in essentially every tissue and the maintenance and regulation of stem cell populations in adults. Sonic hedgehog (Shh) in particular acts as a mitogen, regulating proliferation of target cells, a growth factor that triggers differentiation in target populations, and a morphogen causing cells to respond differently based on their positions along a spatial and temporal concentration gradient. Given its very broad range of effects in development, it is not surprising that many of the structures affected by a disruption in Shh signaling are also affected in Down syndrome (DS). However, recent studies have shown that trisomic cerebellar granule cell precursors have a deficit, compared to their euploid counterparts, in their response to the mitogenic effects of Shh. This deficit substantially contributes to the hypocellular cerebellum in mouse models that parallels the human DS phenotype, and can be corrected in early development by a single exposure to a small molecule agonist of the Shh pathway.
Here we consider how an attenuated Shh response might affect several aspects of development to produce multiple phenotypic outcomes observed in DS.
Therapeutic approaches in Down syndrome
Trisomy for human chromosome 21 (Hsa21) results in Down syndrome (DS) which is among the most complex genetic perturbations compatible with survival past term. While trisomy affects development of every tissue, reduced cognitive ability in DS is among the most limiting features, and DS is one of the leading genetic causes of intellectual disability. The development and characterization of mouse models of DS, especially Ts65Dn, demonstrates that orthologous gene dosage effects produce comparable outcomes for some phenotypes, including cognitive impairment (Fernandez et al., 2007; Hanson et al., 2007; Kleschevnikov et al., 2004; Reeves et al., 1995). As detailed elsewhere in this volume, several drugs with the potential to ameliorate cognitive deficits in DS are making their way to clinical trials.
Studies of mice have played an important role in understanding the brain regions that are especially affected in DS (Lott and Dierssen, 2010). Functional outcomes as well as anatomical and physiological studies demonstrate three regions among those with the largest effects: prefrontal cortex, a contributor to executive function; hippocampus, a crucial site for learning and memory; and cerebellum, which shows a dramatic reduction in size and cellularity. It is notable that initial studies of learning and memory deficits in trisomic mice, which showed affects on hippocampus, informed the development of the first cognitive tests focused on deficits associated with the hippocampus in DS (Pennington et al., 2003; Reeves et al., 1995). That effort has been carried forward, resulting recently in the Arizona Cognitive Test Battery for DS (ACTB) (Edgin et al., 2010). The ACTB is a sensitive set of tests focused on brain regions affected in DS. Clinical trials with the goal of ameliorating cognitive deficits in DS have begun; many proposed efforts with this goal will utilize the ACTB tests as part of their assessments (for updated information, see http://clinicaltrials.gov/).
Currently, approaches to therapy in DS may be thought of in three very broad areas. First, people with DS frequently exhibit early onset of geriatric diseases. The histopathology of Alzheimer Disease (AD) is present in all persons with DS along with the sequellae of the disease, including dementia in a substantial fraction of the DS population, and is certainly related at least in part to over-expression of the amyloid precursor protein gene, APP (Salehi et al., 2006). Age-related loss of afferents to the hippocampus from the locus coeruleus of neurons that use norepinephrine as a neurotransmitter, and degeneration of basal forebrain cholinergic neurons are also hallmarks of DS shared with AD (Salehi et al., 2006; Salehi et al., 2009).
A second general area for DS therapy involves correction of perturbed neuronal function in older children or adults. For example, restoration of an imbalance of inhibitory and excitatory inputs to the hippocampus forms the basis for major clinical trials going forward (Braudeau et al., 2011; Fernandez et al., 2007). This approach is based on the observation that down-regulation of the GABA-ergic inhibitory PV neurons in Ts65Dn mice restores the balance of inhibitory:excitatory inputs and normalizes performance in hippocampal-based tasks such as the Novel Object Recognition Task and the Morris Water Maze (see (Reeves and Garner, 2007; Rueda et al., 2008; Salehi et al., 2007). Several other efforts that have been carried out in trisomic mice and in some cases piloted in human studies look at a variety of hippocampal pathways (Lott and Dierssen, 2010).
A third potential area for therapy that is further downstream in the drug development pipeline addresses the initial basis of cognitive deficits, i.e., antenatal brain development (Haydar and Reeves, 2012). Anatomical and morphological changes in the developing trisomic brain are being studied in detail in animal models, while imaging techniques are increasingly providing information about development of the DS brain. One approach of this type has been shown to normalize early deficits in post-natal development of the cerebellum, which is markedly hypocellular in DS and mouse models (Roper et al., 2006b); this example involves Shh signaling and is considered in detail here.
Shh signaling
Canonical Shh pathway
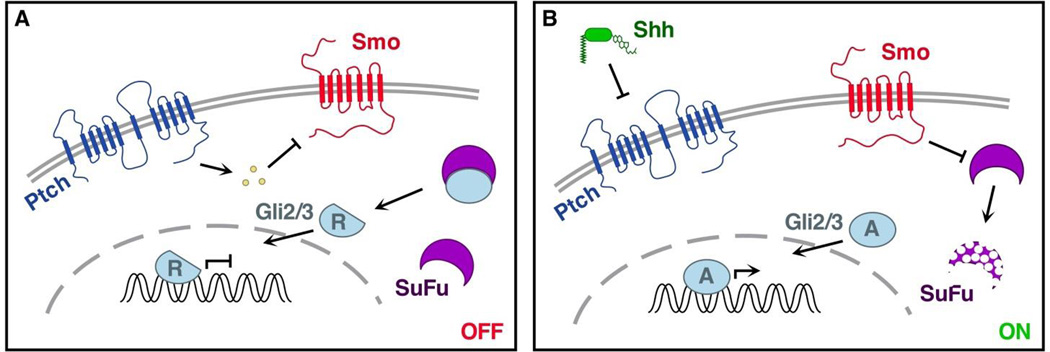
The Shh ligand is produced in cells distinct and often separated from those receiving the signal. The precursor protein is substantially modified by a cleavage that involves addition of a cholesterol moiety followed by palmitoylation (Mann and Beachy, 2004). Fully processed Shh (Shh-Np) is secreted from the producing cell and likely assembles into multimers (Zeng et al., 2001). Extracellular Shh-Np is sensed by the receiving cell via interactions with the 12-pass transmembrane protein, Patched (Ptch) (Marigo et al., 1996; Stone et al., 1996). In the pathway-off state (Fig. 1a), Ptch catalyzes the production of an unidentified repressor of Smoothened (Smo), a 7-pass transmembrane protein with possible G-protein coupled receptor activity (Ayers and Therond, 2010; Chen et al., 2002). When Smo is repressed (pathway-off), the transcription factors Gli2 and Gli3 are targeted to the proteasome for processing to produce their transcriptional repressor forms (Gli2R, Gli3R) (Asai et al., 2006; Wang et al., 2000).
Figure 1. The Shh Pathway.
A. In the pathway-off state Patched (Ptch) catalytically inhibits Smoothened (Smo) activity through an unidentified intermediate. Suppressor of Fused (SuFu) mediates cleavage of Gli2 and Gli3 into their repressor forms, lacking transactivation domains. The Gli2/3 repressors translocate to the nucleus where they repress transcription by binding target gene promoter sequences. B. Shh binding to Ptch inhibits its catalytic repression of Smo, resulting in activation of Smo and degradation of SuFu. In the absence of SuFu, Gli2/3 are phosphorylated to become the activator forms. Gli2/3 activators translocate to the nucleus where they promote transcription by recruiting other transcriptional activators to target gene promoters.
Another pathway element, Suppressor of Fused (SuFu), is found in both the cytoplasm and the nucleus and interacts with Gli1 and Gli2 proteins to further suppress pathway activity (Barnfield et al., 2005; Kogerman et al., 1999). SuFu/Gli complexes are exported from the nucleus and tethered in a SuFu dependent manner in the cytoplasm. Further, SuFu inhibits Gli mediated transcriptional activation by binding and inhibiting DNA-bound Gli1 or Gli2. The pathway is activated when Shh binds to Ptch, inhibiting the catalytic activity of the latter, thereby reversing the repression on Smo (Fig. 1b). This results in degradation of SuFu and Gli phosphorylation to produce activator Gli proteins that move to the nucleus and promote transcription (Chen et al., 2002; Humke et al., 2010; Yue et al., 2009; Zhang et al., 2004).
Non-canonical Shh signaling
Jenkins (Jenkins, 2009) broadly defines several mechanisms for pathway activation outside of the canonical derepression of Gli transcription factors following Shh binding to Ptch. For example, Ptch can interact directly with CyclinB1 to affect cell cycle progression (Barnes et al., 2001) and can initiate apoptosis in neuroepithelial cells until it is blocked by Shh binding (Thibert et al., 2003). Although Ptch is the primary receptor for Shh, several other membrane bound proteins compete for Shh and are capable of enhancing or inhibiting pathway activity. Cell Adhesion Molecule-Related/Down-Regulated by Oncogenes (CDO) and Brother of CDO (BOC) both bind Shh though Fn3 domains. Growth Arrest Specific 1 (Gas1) (Martinelli and Fan, 2007) and Hedgehog Interacting Protein (Hhip) (Bosanac et al., 2009) interact with Shh, but not through Fn3 domains. Of these, expression of CDO, BOC, or Gas1 increases Shh pathway activity, while Hhip negatively regulates the pathway (Beachy et al., 2010). SCUBE2, a secreted SCUBE protein family member, interacts with both Shh and Ptch and enhances Shh signaling (Tsai et al., 2009). The precise relationships between these receptor molecules and the Shh/Ptch interaction have yet to be described in detail.
The Gli transcription factors can also be regulated outside of the canonical Shh pathway. Borycki et al demonstrated that Wnt1 and Wnt4 can induce Gli2 expression and repress Gli3 expression in a quail segmental plate mesoderm explant culture system (Borycki et al., 2000). Others have suggested that Gli1 protein may be regulated independently of Shh through the MAPK pathway (Seto et al., 2009). This would raise some interesting possibilities for activating Gli regulated genes in the absence of Shh as well as for synergizing with Shh to super-activate the pathway.
While precise definitions of non-canonical pathways are lacking, results of multiple perturbations of the Shh pathway support the involvement of many of its components in multiple signaling paradigms. Indeed, it would be surprising if the enormous range of Shh effects as mitogen and morphogen in essentially every tissue could be reduced to one relatively simple pathway with three transcription factor effectors. Elaboration of these additional pathways for Shh signaling and their roles in specific processes will be a rich source for potential targets of therapeutic molecules that are fine-tuned to specific effects that are perturbed in disease states.
Phenotypes of Shh pathway mutants
Hedgehog signaling is a fundamental pathway involved in many aspects of prenatal development. Varied roles have been described from a number of studies in model organisms using constitutive and targeted gene “knockouts” in mice and chick/quail chimeras (Table 1). The first report of a constitutive Shh knockout demonstrated cephalic neural tube defects as early as day 8.5 of gestation (E8.5) that becomes more severe a day later, resulting in a markedly hypomorphic central nervous system (Chiang et al., 1996). Mutant embryos die around the time of birth and exhibit defects in development of heart, lung, kidney and foregut in addition to the forming CNS. Many of the same phenotypes have been observed in embryos lacking Smoothened (Smo), an intermediate member of the Shh pathway that functions to positively regulate pathway activation, and embryos lacking Dispatched A (mDispA), a factor that is essential for efficient Shh release from cells producing it. Embryos lacking Smo or mDispA have somewhat more severe phenotypes resembling Shh and Indian hedgehog (Ihh) double knockout. mDispA and Smo are required for Ihh as well as Shh signaling.
Table 1.
Phenotypes caused by alterations and interruptions in Shh signaling that may relate to deficits in DS.
| Perturbation | Affected System | Phenotypes | Age | Notes | Refs. |
|---|---|---|---|---|---|
| Shh−/− | Cardiac | Pharyngeal arch artery defects, ASD, VSD, Tetralogy of Fallot-like, Persistent truncus arteriosus (PTA) | E10.5 – E15.5 | (Hildreth et al., 2009; Smoak et al., 2005) | |
| Brain | Midline fusion of anterior lips of cephalic neural plate, incomplete separation of primitive optic vesicles† | E8.25 | (Abdelwahid et al., 2002) | ||
| Loss of ventral structures, Growth deficit in forebrain | E11.5 | (Chiang et al., 1996) | |||
| Branchial Arches | Reduced mandibular component† | E9.5 | (Abdelwahid et al., 2002) | ||
| Craniofacial Bones | Trace | E15.5 | (Chiang et al., 1996) | ||
| Nkx2.5Cre/+; Shhflox/− | Cardiac | Pharyngeal arch artery defects, PTA, AVSD | E10.5 | Shh ablated in Nkx2.5 expressing cells | (Goddeeris et al., 2007) |
| Mef2c-AHF-Cre; Smoflox/− | Cardiac | PTA, ASD, VSD, AVSD, rounded and short AV valves | E14.5 – E18.5 | Smo ablated in anterior heart field | (Goddeeris et al., 2008) |
| Wnt1-Cre; Smoflox/− | Cardiac | PTA | E10.5 | Smo ablated in neural crest | (Goddeeris et al., 2007) |
| SmoGli1-CreERT2 | Cardiac | ASD and AVSD | E13.5 | Floxed Smo allele under the control of inducible Gli1:Cre | (Hoffmann et al., 2009) |
| ShhNkx2.1-Cre | Cardiac | ASD | E13.5 | Shh ablated in Nkx2.1 expressing cells | (Hoffmann et al., 2009) |
| Shhc/Shhn, Pax2-Cre | Cerebellum | Absence of EGL, Disorganized PL, Fewer lobes | P5 | Shh ablated in Pax2 expressing precursors to Purkinje cells | (Lewis et al., 2004) |
| Shhc/Shhn, L7-Cre | Cerebellum | Absence of EGL, Disorganized PL, Fewer lobes | P5 | Shh ablated in precursors to Purkinje cells | (Lewis et al., 2004) |
| SuFu−/loxP, Hoxb.7-Cre | Cerebellum | Hypoplastic vermis, Lack of foliation, Disorganized cell layers | P21 | SuFu ablated in precursors to CGNPs | (Kim et al., 2011) |
| 5E1 Hybridoma | Neural Crest/Branchial Arches | Hypomorphic Branchial arches, Developmental delay | Stage 9–11 +24 hrs | Hybridoma cells injected lateral mesenchyme of developing chick | (Ahlgren and Bronner-Fraser, 1999) |
| Kif3afl/fl, hGFAP-Cre | Cerebellum | Atrophic cerebellum, Fused folia, Thicker PL | P25 | Kif3a ablated in GFAP expressing CGNPS, Bergman glia, and Radial glia | (Spassky et al., 2008) |
| Ptc−/− | Embryo | Failure of neural tube closure, Lethality | E10.5 | Constitutive Ptc knockout | (Goodrich et al., 1997) |
| Gli2flox/flox, En1-Cre | Cerebellum | Reduced foliation, Hypocellular EGL | P5/P8 | Gli2 is ablated by E9.0 in En1 expressing cells which give rise to the cerebellum | (Blaess et al., 2006) |
Similar phenotypes were seen in Smo −/− and mDispA −/− knock out mice
Similar results are seen after exposure to an inhibitor of Shh signaling, cyclopamine, the effective agent in Veratrum californicum that induces cyclopia in fetuses of pregnant sheep. In addition to cyclopia, malformations of the nose and skull, notably the premaxilla, are also present (Binns et al., 1963). From these studies it is clear that successful embryo development requires restriction of Shh activation to specific levels in both a temporal and spatial manner.
Shh response deficit as a “common denominator” of DS phenotypes
Trisomy and Shh in cerebellar development
The first direct demonstration of Shh response perturbation due to trisomy came from analysis of cerebellar development in the Ts65Dn “Down syndrome” mouse (Baxter et al., 2000). Mouse models play a critical role in the study of gene dosage mechanisms that produce the features of DS as reviewed in detail in this volume and elsewhere (Das and Reeves, 2011; Moore and Roper, 2007; O'Doherty et al., 2005). Ts65Dn mice, like people with trisomy 21, have a smaller cerebellum and show specific deficits of Purkinje cells and of the granule cell neurons that make up the internal granule layer (IGL) of the cerebellum. Further, the reduced density of GC in the IGL of Ts65Dn mice was shown to occur in people with DS, as well (Baxter et al., 2000).
The IGL is not present at birth in mice nor is it fully formed in newborn humans. Rather, granule cell precursors (GCPs) form the external germinal layer on the surface layer of the cerebellum. It forms over the first 3 weeks of life in mice (2–3 years in human beings). Purkinje cells produce Shh which stimulates GCPs to divide and migrate inward to form the IGL (Dahmane and Ruiz i Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999). The granule cell neuron deficit in Ts65Dn is already detectable from one week after birth (Roper et al., 2006b). On the day of birth, the number of GCPs in the external germinal layer is the same in Ts65Dn and euploid mice; however, the frequency of mitosis is significantly reduced in Ts65Dn. This reduced mitotic rate is a major contributor to the deficit in granule cell generation in trisomic mice (Roper et al., 2006b) and in DS (Guidi et al., 2011). Similarly, deleting a floxed Shh gene in late gestation by driving Cre expression with either the Pax2 or L7 promoters results in reduced cerebellar volume, hypocellularity and disorganization of GCPs in the EGL (Table 1) (Lewis et al., 2004).
When GCPs were isolated from trisomic and euploid cerebella and cultured in the presence of increasing amounts of Shh, two important things were observed (Roper et al., 2006b). First, trisomic GCP responded less to the mitogenic effects of Shh at every concentration (Fig. 2a). Second, the trisomic cells did exhibit a dosage response, suggesting that stimulation of Shh signaling in vivo might overcome some of the mitogenic deficit in trisomic cells that was observed in vitro. This was indeed the case. Trisomic mice that received a single dose of SAG on the day of birth had the same number of GCP and of mitotic GCP one week later, whereas vehicle-treated trisomic mice already showed a significant deficit of these cells (Fig. 2b).
Figure 2. SAG Corrects Ts65Dn Cerebellar Dysmorphology.
A. Ts65Dn GCPs display an attenuated response to Shh treatment over a range of concentrations. B. A single dose of SAG given on the day of birth restores GCP number at P6 to similar levels as Euploid littermates. (This figure derived from (Roper et al., 2006b).
The SHH hypothesis for DS
These results raise the question, is the attenuated response to Shh in trisomic mice restricted to GCP, or do all Shh-responsive cells in a trisomic individual show a reduced reaction to Shh stimulation? If the latter is the case, could stimulation of those developing populations at the appropriate stages of development represent a common approach to ameliorate diverse structural deficits in a wide range of cells and tissues that are affected to produce the DS phenotype? Based on the demonstration that trisomy results in a reduced response to the mitogenic effects of Shh in cerebellum, we consider here the possible effects of attenuated Shh response in three additional systems that are frequently or always affected during development in DS: craniofacial skeleton, heart and the enteric nervous system. Observations of parallel effects of Shh disruption and of trisomy suggest that this mechanism may contribute to multiple DS phenotypes. Effects in development of the face and enteric nervous system further suggest that Shh signaling effects may impinge on neural crest cells (NCC) which contribute to each of these structures.
Craniofacial development
The appearance of the DS face is very characteristic of this syndrome and is due substantially to hypoplasia of the midface skeleton. This is reflected in the Ts65Dn mouse and other models in an absolute correspondence between affected bones across the two species (Richtsmeier et al., 2000; Richtsmeier et al., 2002). In particular, the mid-face and mandible are significantly smaller and dysmorphic due to trisomy. These bones arise from an embryonic precursor, Meckel’s cartilage, which is itself a product of the neural crest cells (NCC) that contribute substantially to the first pharyngeal arch (PA1). To identify the earliest changes that lead to midface hypoplasia, we studied the formation of PA1 in Ts65Dn mice and their euploid littermates at embryonic day 9.5 (E9.5) in crosses with mice that express lacZ under control of the Wnt1 promoter, marking NCC (Roper et al., 2009).
Development of both trisomic and euploid embryos was highly variable at E9.5 with somite numbers ranging from 7 to 43, but no difference in developmental stage was observed between trisomic and euploid embryos (Roper et al., 2006a). When embryos at the 20–24 somite stage were considered, the trisomic PA1 was smaller, contained fewer neural crest-derived cells and these cells had a lower mitotic index than did their euploid counterparts. The number of migrating NCC (lacZ+ cells between the neural tube and PA1) was not significantly different at this stage, however, fewer migrating NCC were present in slightly less mature, 17–19 somite embryos (Roper et al., 2009). Earlier experiments in both chick and mice show that Shh from endoderm of the ventral foregut is required to maintain migrating NCC and to promote proliferation in PA1 (Ahlgren and Bronner-Fraser, 1999; Brito et al., 2006; Jeong et al., 2004). Note that if trisomic NCC, like GCP, respond less to Shh than their euploid counterparts, some of these migrating cells might differentiate since they would “see” less Shh signal at the same concentration.
We then dissected the neural tubes from trisomic or euploid embryos and cultured them ex vivo to determine whether the delamination of NCC from the tube is affected. Twenty-four hours after being placed in culture, Ts65Dn explants showed fewer cells migrating from the neural tube and those trisomic cells that did delaminate migrated for a shorter distance. Finally, we isolated cells from PA1 of euploid or trisomic embryos and cultured them to measure proliferation. Trisomic cells showed lower proliferation than did euploid. However, addition of Shh to the cultures increased cell division, bringing the rate in trisomic cells to that seen in euploid cultures (Roper et al., 2006a).
Thus, the earliest trisomy-related deficits leading to midface skeletal hypoplasia arise from reduced delamination and migration of NCC and from reduced proliferation of these cells in PA1, which provides the anlage for the cartilaginous model from which the mandible and mid-facial bones will form. The known effects of Shh as well as our observations are consistent with the hypothesis that an important contribution to this deficit is the reduced responsiveness to Shh in cranial neural crest from trisomic mice, with direct phenotypic consequences.
Trisomy and Shh in cardiovascular development
Nearly half of all children born with DS have a congenital heart defect (CHD) (Ferencz et al., 1989). Atrioventricular septal defects (AVSDs) are the most common followed by ventricular septal defects (VSDs) and atrial septal defects (ASDs). Several mouse models of DS show similar patterns of CHD, indicating conservation of the effects of trisomic genes during mammalian heart development (Liu et al., 2011; O'Doherty et al., 2005; Williams et al., 2008). Nearly half of Tc1 transchromosomic mice, which carry a freely segregating copy of Hsa21, present with heart defects. VSDs are most common in trisomic mice, while AVSD and patent truncus arteriousus (PTA) are also observed. About 15% of newborn Ts65Dn mice have cardiac defects, including ASD, VSD, PTA and various errors of branching of the pulmonary and outflow tracts. Mice that carry a duplication of the human chromosome 21 conserved synteny region on mouse chromosome 16 (Mmu16) show cardiovascular defects reminiscent of those seen in individuals with DS. They display ASD, VSD, and a tetralogy of Fallot-like phenotype (Li et al., 2007). Mice that are trisomic for all regions conserved with human chromosome 21 on mouse chromosomes 10, 16, and 17 have cardiovascular defects at a similar frequency (Yu et al., 2010).
Shh is secreted from cells in both the pulmonary endoderm, where it is required for proper atrial septation, and in the pharyngeal endoderm, where it is necessary for proper outflow tract septation (Goddeeris et al., 2008). Shh signaling marks cells within the second heart field (SHF) as progenitors of the atrial septum and outflow tract. Labeling of hedgehog-responsive cells early in heart development demonstrates that those cells migrate from the SHF and contribute to the primary atrial septum, dorsal mesenchymal protrusion (DMP), endocardial cushions and pulmonary trunk (Hoffmann et al., 2009). The atrial septum, DMP, and endocardial cushions all combine to form the mesenchymal complex of the atrioventricular septum (Snarr et al., 2007). The appearance of this complex is necessary to complete AV septation and to anchor AV valves.
NCC contribute to heart development by migrating into the outflow tract of the heart, contributing to septation and alignment. Smo is necessary for Shh pathway activation, and the loss of this gene in NCC resulted in errors in septation and alignment of the aorta and pulmonary trunk, as well as defects in pharyngeal arch arteries (Goddeeris et al., 2008). An Shh response deficit could thus contribute to heart defects through direct effects in SHF, or because of an impaired response of trisomic neural crest. As noted, several steps in NCC delamination, migration and proliferation require Shh signaling.
In support of this idea, several mouse models with impaired Shh signaling also display errors in septation (Table 1). A knockout of Shh−/− in which exon 2 and its flanking introns are removed displays AVSD and other structural defects (Hildreth et al., 2009; Smoak et al., 2005). Similarly, when Shh signaling is blocked by cyclopamine at HH stage 14 chick embryos, they exhibit PTA, VSD and pulmonary atresia secondary to reduced proliferation in the SHF (Dyer and Kirby, 2009). Similar outcomes occur when other components of the pathway are altered. Conditional knockouts of Smo and Shh result in AVSD and PTA in mouse embryos. Deletion of a floxed Shh allele in all cells expressing either Nkx2.5 or Gli1 results in AVSD (Goddeeris et al., 2007; Hoffmann et al., 2009). Thus Shh signaling mutants present AVSDs, VSDs, and ASDs, structural defects that are common in DS (Ferencz et al., 1989).
Septal defects were attributed primarily to errors in the endocardial cushions for many years, but evidence has emerged recently that points to a critical role for DMP as a contributing factor, especially to AVSD and secundum ASD (Goddeeris et al., 2008; Hoffmann et al., 2009). In this light, it is relevant that Shh signaling is not required for endocardial cushion contributions to septation, but is necessary for proper contributions to DMP from the second heart field. When Shh signaling is disrupted in DMP progenitors or the SHF o, the DMP is hypoplastic or does not form and an AVSD results (Goddeeris et al., 2008; Hoffmann et al., 2009). Hypoplastic DMP has also been described in human fetuses with DS and in mice trisomic for all of mouse chromosome 16 (Blom et al., 2003; Snarr et al., 2007; Webb et al., 1999). Thus there is an important role for Shh signaling in formation of the DMP, and for DMP involvement in AVSDs; DS is major risk factor for AVSD (Ferencz et al., 1989). Overall, there are substantial similarities between heart phenotypes caused by trisomy and those seen in Shh signaling mutants. These results do not prove causation but they are consistent with the effects expected from reduced response to Shh signaling in the developing heart.
Enteric Nervous System
The small and large intestines are innervated by vagal NCC that migrate along the primitive gut from the rostral toward the caudal end in response to Glial Derived Neurotrophic Factor (GDNF) (Young et al., 2001). In humans, these enteric neuron precursors (ENPs) colonize the gut beginning week 7 of gestation, with the primitive enteric ganglia reaching the rectum in week 12 (Kenny et al., 2010). Failure of the ENPs to reach the caudal end of the colon results in a condition known as aganglionic megacolon, or Hirschsprung’s disease (HSCR) (Kenny et al., 2010). Though still rare, the incidence of HSCR in conjunction with DS is significantly increased over the rate in the population at large (Arnold et al., 2009). Mutations in the mouse Ret gene, a receptor tyrosine kinase that is activated by GDNF, cause NCC colonizing the gut to migrate less efficiently and these mutants phenocopy HSCR (Asai et al., 2006). Human RET gene mutations contribute to susceptibility to the development of HSCR in people (Amiel et al., 2008; Angrist et al., 1995).
Shh is expressed by epithelial cells on the inner membrane of the gut and signals via BMP4 to inhibit differentiation of ENPs that are located in the central mesenchyme but are not close to the (outer) surface mesenchyme (Sukegawa et al., 2000). Inhibition of ENP differentiation could result in HSCR and, given the increased incidence in DS, it appears plausible that dosage sensitive genes located on Hsa21 may contribute to the aganglionic phenotype. Decreased responsiveness to Shh could result in the expansion of the pro-differentiation environment to a point deeper in the gut mesenchyme than normal. Early differentiation of these ENPs could then deplete the migratory pool of cells before the entire length of colon has been colonized.
Hsa21 genes and Shh signaling
None of the genes encoding canonical Shh signaling pathway components is encoded on Hsa21. However, up-regulation of Ptch (resulting in down-regulation of the SHH pathway) has been reported in Ts65Dn mice for a specific, small group of stem cells in the sub-ventricular zone (SVZ), the origin of granule cells in the dentate gyrus (Trazzi et al., 2011). In cultured neurospheres developed from the SVZ region, a C-terminal fragment of the APP protein, AICD, can contribute to the up-regulation of Ptch transcription (Trazzi et al., 2011). Since the APP gene is found on Hsa21 and thus is chronically upregulated in DS (and also in Ts65Dn mice), this provides a possible explanation for the attenuated mitogenic response to Shh by trisomic cells. At the phenotypic level, the number of cells in dentate gyrus is reduced by about 20% in Ts65Dn mice compared to euploid (Insausti et al., 1998; Lorenzi and Reeves, 2006). Drugs developed for Alzheimer disease that modulate APP cleavage to reduce C-terminal fragments might thus have an additional ameliorative benefit in DS.
Molecular pathway analysis has implicated several additional Hsa21 genes whose expression may impinge on Shh signaling directly or indirectly, especially on the regulation of Gli1, 2 and/or 3. To date, however, there is no direct demonstration of a dosage sensitive trisomic gene disrupting Shh signaling in the developing cerebellum, heart, or the cranial or vagal neural crest. Trisomic mouse models of DS provide a sensitized genetic background for dissection of these mechanisms.
Discussion
Trisomy for Hsa21 results in increased dosage for more than 300 genes and numerous studies of gene expression in DS and in animal models suggest that most of these will be up-regulated by ~50% whenever and wherever they are normally expressed. Viewed from this perspective, the challenge of finding “cures” based on modulation of individual gene function is daunting. The availability of segmental trisomies in animal models that recreate the dosage imbalances seen in DS and the demonstration that this produces features analogous to those in DS (Reeves et al., 1995) has led to a productive phenotype-based approach to the development of therapies (Reeves et al., 1995; Reeves and Garner, 2007; Salehi et al., 2007)
The phenotype-based approach suggests the possibility that multiple effects of trisomy in different tissues may result from perturbations in the same developmental pathways and regulatory processes, as we posit here for Shh. A deficit in response to the mitogenic effects of Shh has been demonstrated in trisomic cerebellar GCP. Trisomic NCC-derived cells in PA1 also appear to respond less to Shh than do their euploid counterparts. A similar response deficit in other trisomic cell types could affect development of the face, heart, enteric nervous system and perhaps other tissues affected in DS. The cerebellar GCP response deficit to Shh is amenable to amelioration through the application of a small molecule agonist of the Shh pathway (Chen et al., 2002; Roper et al., 2006b). Might a similar positive effect be possible in other cells and tissues that develop abnormally in DS if the Shh pathway could be stimulated to an appropriate degree at the appropriate time and place?
The Shh pathway is utilized in so many aspects of development that suggesting it as a therapeutic target seems highly improbable at first glance. Development is substantially disrupted in mice that are engineered to over- or under-express Shh. In some case the effects are concentration dependent, as when Shh acts as a morphogen to program cell response based on temporal and spatial gradients in anterior-posterior patterning of the limb (Harfe et al., 2004). Indeed, delivery of any molecule that stimulates or inhibits this pathway would likely need to be strictly limited in space and time to avoid deleterious side effects.
However, Shh should have no effect on cells that do not possess appropriate receptor and signal transduction pathways. We argue here that many if not all trisomic cells that are Shh-responsive might show the attenuated response seen in cerebellar GCP. To the degree that this is the case, off-target effects would be reduced and could possibly have a beneficial effect.
The basic tenets of this model are testable in cell and mouse model systems. While it is clear that there is a substantial amount to learn about Shh signaling in all situations where it occurs, models of DS can play an important part in understanding these pathways. If this single molecular mechanism does prove to be a “common denominator” of multiple trisomic phenotypes, there are attendant prospects that a single kind of pharmaceutical treatment might ameliorate multiple features of DS.
Acknowledgments
Critical support for this work was provided by the Down Syndrome Research and Treatment Foundation and Research Down Syndrome. This work was also supported by PHS awards 1R01 HD038384 from the National Institute of Child Health and Human Development and R01 HL083300 from the National Heart, Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- Shh
Sonic hedgehog
- DS
Down syndrome
- ACTB
Arizona Cognitive Test Battery
- AD
Alzheimer Disease
- GCP
granule cell precursor
- IGL
internal granule layer of the cerebellum
- EGL
external germinal layer of the cerebellum
- PA1
first pharyngeal arch
- NCC
neural crest cells
- ASD
atrial septal defect
- VSD
ventricular septal defect
- AVSD
atrioventricular septal defect
- PTA
patent truncus arteriosus
- SHF
second heart field
- DMP
dorsal mesenchymal protrusion
- ENP
enteric neuron precursors
- HSCR
Hirschsprung’s disease
- SVZ
subventricular zone
- SAG
Sonic agonist
Bibliography
- 1.Abdelwahid E, Pelliniemi LJ, Jokinen E. Cell death and differentiation in the development of the endocardial cushion of the embryonic heart. Microsc Res Tech. 2002;58:395–403. doi: 10.1002/jemt.10159. [DOI] [PubMed] [Google Scholar]
- 2.Ahlgren SC, Bronner-Fraser M. Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Current biology : CB. 1999;9:1304–1314. doi: 10.1016/s0960-9822(00)80052-4. [DOI] [PubMed] [Google Scholar]
- 3.Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, et al. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- 4.Angrist M, Bolk S, Thiel B, Puffenberger EG, Hofstra RM, Buys CH, Cass DT, Chakravarti A. Mutation analysis of the RET receptor tyrosine kinase in Hirschsprung disease. Hum Mol Genet. 1995;4:821–830. doi: 10.1093/hmg/4.5.821. [DOI] [PubMed] [Google Scholar]
- 5.Arnold S, Pelet A, Amiel J, Borrego S, Hofstra R, Tam P, Ceccherini I, Lyonnet S, Sherman S, Chakravarti A. Interaction between a chromosome 10 RET enhancer and chromosome 21 in the Down syndrome-Hirschsprung disease association. Hum Mutat. 2009;30:771–775. doi: 10.1002/humu.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asai N, Fukuda T, Wu Z, Enomoto A, Pachnis V, Takahashi M, Costantini F. Targeted mutation of serine 697 in the Ret tyrosine kinase causes migration defect of enteric neural crest cells. Development (Cambridge, England) 2006;133:4507–4516. doi: 10.1242/dev.02616. [DOI] [PubMed] [Google Scholar]
- 7.Ayers KL, Therond PP. Evaluating Smoothened as a G-protein-coupled receptor for Hedgehog signalling. Trends Cell Biol. 2010;20:287–298. doi: 10.1016/j.tcb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Barnes EA, Kong M, Ollendorff V, Donoghue DJ. Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J. 2001;20:2214–2223. doi: 10.1093/emboj/20.9.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnfield PC, Zhang X, Thanabalasingham V, Yoshida M, Hui CC. Negative regulation of Gli1 and Gli2 activator function by Suppressor of fused through multiple mechanisms. Differentiation. 2005;73:397–405. doi: 10.1111/j.1432-0436.2005.00042.x. [DOI] [PubMed] [Google Scholar]
- 10.Baxter LL, Moran TH, Richtsmeier JT, Troncoso J, Reeves RH. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Human molecular genetics. 2000;9:195–202. doi: 10.1093/hmg/9.2.195. [DOI] [PubMed] [Google Scholar]
- 11.Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010;24:2001–2012. doi: 10.1101/gad.1951710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binns W, James LF, Shupe JL, Everett G. A Congenital Cyclopian-Type Malformation in Lambs Induced by Maternal Ingestion of a Range Plant, Veratrum Californicum. Am J Vet Res. 1963;24:1164–1175. [PubMed] [Google Scholar]
- 13.Blaess S, Corrales JD, Joyner AL. Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development. 2006;133:1799–1809. doi: 10.1242/dev.02339. [DOI] [PubMed] [Google Scholar]
- 14.Blom NA, Ottenkamp J, Wenink AG, Gittenberger-de Groot AC. Deficiency of the vestibular spine in atrioventricular septal defects in human fetuses with down syndrome. Am J Cardiol. 2003;91:180–184. doi: 10.1016/s0002-9149(02)03106-5. [DOI] [PubMed] [Google Scholar]
- 15.Borycki A, Brown AM, Emerson CP., Jr Shh and Wnt signaling pathways converge to control Gli gene activation in avian somites. Development. 2000;127:2075–2087. doi: 10.1242/dev.127.10.2075. [DOI] [PubMed] [Google Scholar]
- 16.Bosanac I, Maun HR, Scales SJ, Wen X, Lingel A, Bazan JF, de Sauvage FJ, Hymowitz SG, Lazarus RA. The structure of SHH in complex with HHIP reveals a recognition role for the Shh pseudo active site in signaling. Nat Struct Mol Biol. 2009;16:691–697. doi: 10.1038/nsmb.1632. [DOI] [PubMed] [Google Scholar]
- 17.Braudeau J, Delatour B, Duchon A, Lopes-Pereira P, Dauphinot L, de Chaumont F, Olivo-Marin JC, Dodd RH, Herault Y, Potier MC. Specific targeting of the GABA-A receptor [alpha]5 subtype by a selective inverse agonist restores cognitive deficits in Down syndrome mice. J Psychopharmacol. 2011 doi: 10.1177/0269881111405366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brito JM, Teillet MA, Le Douarin NM. An early role for sonic hedgehog from foregut endoderm in jaw development: ensuring neural crest cell survival. Proc Natl Acad Sci U S A. 2006;103:11607–11612. doi: 10.1073/pnas.0604751103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 21.Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 22.Das I, Reeves RH. The use of mouse models to understand and improve cognitive deficits in Down syndrome. Dis Model Mech. 2011;4:596–606. doi: 10.1242/dmm.007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Dev Biol. 2009;336:137–144. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgin JO, Mason GM, Allman MJ, Capone GT, Deleon I, Maslen C, Reeves RH, Sherman SL, Nadel L. Development and validation of the Arizona Cognitive Test Battery for Down syndrome. J Neurodev Disord. 2010;2:149–164. doi: 10.1007/s11689-010-9054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferencz C, Neill CA, Boughman JA, Rubin JD, Brenner JI, Perry LW. Congenital cardiovascular malformations associated with chromosome abnormalities: an epidemiologic study. J Pediatr. 1989;114:79–86. doi: 10.1016/s0022-3476(89)80605-5. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007 doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- 27.Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, Klingensmith J. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development. 2008;135:1887–1895. doi: 10.1242/dev.016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goddeeris MM, Schwartz R, Klingensmith J, Meyers EN. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development. 2007;134:1593–1604. doi: 10.1242/dev.02824. [DOI] [PubMed] [Google Scholar]
- 29.Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science (New York, NY) 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 30.Guidi S, Ciani E, Bonasoni P, Santini D, Bartesaghi R. Widespread proliferation impairment and hypocellularity in the cerebellum of fetuses with down syndrome. Brain Pathol. 2011;21:361–373. doi: 10.1111/j.1750-3639.2010.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanson JE, Blank M, Valenzuela RA, Garner CC, Madison DV. The functional nature of synaptic circuitry is altered in area CA3 of the hippocampus in a mouse model of Down's syndrome. J Physiol. 2007;579:53–67. doi: 10.1113/jphysiol.2006.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Haydar T, Reeves RH. Direct and indirect effects of trisomy on early brain development. Trends in Neuroscience. 2012 doi: 10.1016/j.tins.2011.11.001. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hildreth V, Webb S, Chaudhry B, Peat JD, Phillips HM, Brown N, Anderson RH, Henderson DJ. Left cardiac isomerism in the Sonic hedgehog null mouse. J Anat. 2009;214:894–904. doi: 10.1111/j.1469-7580.2009.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP. sonic hedgehog is required in pulmonary endoderm for atrial septation. Development. 2009;136:1761–1770. doi: 10.1242/dev.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Insausti AM, Megias M, Crespo D, Cruz-Orive LM, Dierssen M, Vallina IF, Insausti R, Florez J. Hippocampal volume and neuronal number in Ts65Dn mice: a murine model of Down syndrome. Neurosci Lett. 1998;253:175–178. doi: 10.1016/s0304-3940(98)00641-7. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal. 2009;21:1023–1034. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 39.Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenny SE, Tam PK, Garcia-Barcelo M. Hirschsprung's disease. Semin Pediatr Surg. 2010;19:194–200. doi: 10.1053/j.sempedsurg.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Kim JJ, Gill PS, Rotin L, van Eede M, Henkelman RM, Hui C-c, Rosenblum ND. Suppressor of fused controls mid-hindbrain patterning and cerebellar morphogenesis via GLI3 repressor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1825–1836. doi: 10.1523/JNEUROSCI.2166-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleschevnikov AM, Belichenko PV, Villar AJ, Epstein CJ, Malenka RC, Mobley WC. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J Neurosci. 2004;24:8153–8160. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kogerman P, Grimm T, Kogerman L, Krause D, Unden AB, Sandstedt B, Toftgard R, Zaphiropoulos PG. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol. 1999;1:312–319. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- 44.Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Developmental biology. 2004;270:393–410. doi: 10.1016/j.ydbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Yu T, Morishima M, Pao A, LaDuca J, Conroy J, Nowak N, Matsui S, Shiraishi I, Yu YE. Duplication of the entire 22.9 Mb human chromosome 21 syntenic region on mouse chromosome 16 causes cardiovascular and gastrointestinal abnormalities. Hum Mol Genet. 2007;16:1359–1366. doi: 10.1093/hmg/ddm086. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Morishima M, Yu T, Matsui SI, Zhang L, Fu D, Pao A, Costa AC, Gardiner KJ, Cowell JK, et al. Genetic analysis of Down syndrome-associated heart defects in mice. Hum Genet. 2011 doi: 10.1007/s00439-011-0980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorenzi HA, Reeves RH. Hippocampal hypocellularity in the Ts65Dn mouse originates early in development. Brain Res. 2006;1104:153–159. doi: 10.1016/j.brainres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 48.Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down's syndrome. Lancet Neurol. 2010;9:623–633. doi: 10.1016/S1474-4422(10)70112-5. [DOI] [PubMed] [Google Scholar]
- 49.Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu Rev Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- 50.Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- 51.Martinelli DC, Fan CM. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 2007;21:1231–1243. doi: 10.1101/gad.1546307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore CS, Roper RJ. The power of comparative and developmental studies for mouse models of Down syndrome. Mamm Genome. 2007;18:431–443. doi: 10.1007/s00335-007-9030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Doherty A, Ruf S, Mulligan C, Hildreth V, Errington ML, Cooke S, Sesay A, Modino S, Vanes L, Hernandez D, et al. An aneuploid mouse strain carrying human chromosome 21 with down syndrome phenotypes. Science. 2005;309:2033–2037. doi: 10.1126/science.1114535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pennington BF, Moon J, Edgin J, Stedron J, Nadel L. The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Dev. 2003;74:75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- 55.Reeves R, Irving N, Moran T, Wohn A, Kitt C, Sisodia S, Schmidt C, Bronson R, Davisson M. A mouse model for Down Syndrome exhibits learning and behaviour deficits. Nature Genetics. 1995;11:177–183. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 56.Reeves RH, Garner CC. A year of unprecedented progress in Down syndrome basic research. Ment Retard Dev Disabil Res Rev. 2007;13:215–220. doi: 10.1002/mrdd.20165. [DOI] [PubMed] [Google Scholar]
- 57.Richtsmeier J, Baxter L, Reeves R. Parallels of craniofacial maldevelopment in Down Syndrome and Ts65Dn mice. Developmental Dynamics. 2000;217:137–145. doi: 10.1002/(SICI)1097-0177(200002)217:2<137::AID-DVDY1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 58.Richtsmeier JT, Zumwalt A, Carlson EJ, Epstein CJ, Reeves RH. Craniofacial phenotypes in segmentally trisomic mouse models for Down syndrome. Am J Med Genet. 2002;107:317–324. doi: 10.1002/ajmg.10175. [DOI] [PubMed] [Google Scholar]
- 59.Roper R, St. John H, Philip J, Lawler A, Reeves R. Perinatal loss of Ts65Dn mice, a model of Down syndrome. Genetics. 2006a;172:437–443. doi: 10.1534/genetics.105.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roper RJ, Baxter LL, Saran NG, Klinedinst DK, Beachy PA, Reeves RH. Defective cerebellar response to mitogenic Hedgehog signaling in Down [corrected] syndrome mice. Proceedings of the National Academy of Sciences of the United States of America. 2006b;103:1452–1456. doi: 10.1073/pnas.0510750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roper RJ, VanHorn JF, Cain CC, Reeves RH. A neural crest deficit in Down syndrome mice is associated with deficient mitotic response to Sonic hedgehog. Mech Dev. 2009;126:212–219. doi: 10.1016/j.mod.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rueda N, Florez J, Martinez-Cue C. Chronic pentylenetetrazole but not donepezil treatment rescues spatial cognition in Ts65Dn mice, a model for Down syndrome. Neurosci Lett. 2008;433:22–27. doi: 10.1016/j.neulet.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 63.Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, et al. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 64.Salehi A, Faizi M, Belichenko PV, Mobley WC. Using mouse models to explore genotype-phenotype relationship in Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13:207–214. doi: 10.1002/mrdd.20164. [DOI] [PubMed] [Google Scholar]
- 65.Salehi A, Faizi M, Colas D, Valletta J, Laguna J, Takimoto-Kimura R, Kleschevnikov A, Wagner SL, Aisen P, Shamloo M, et al. Restoration of norepinephrine-modulated contextual memory in a mouse model of Down syndrome. Sci Transl Med. 2009;1:7ra17. doi: 10.1126/scitranslmed.3000258. [DOI] [PubMed] [Google Scholar]
- 66.Seto M, Ohta M, Asaoka Y, Ikenoue T, Tada M, Miyabayashi K, Mohri D, Tanaka Y, Ijichi H, Tateishi K, et al. Regulation of the hedgehog signaling by the mitogen-activated protein kinase cascade in gastric cancer. Mol Carcinog. 2009;48:703–712. doi: 10.1002/mc.20516. [DOI] [PubMed] [Google Scholar]
- 67.Smoak IW, Byrd NA, Abu-Issa R, Goddeeris MM, Anderson R, Morris J, Yamamura K, Klingensmith J, Meyers EN. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Developmental Biology. 2005;283:357–372. doi: 10.1016/j.ydbio.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 68.Snarr BS, O'Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, Wessels A. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circulation Research. 2007;101:971–974. doi: 10.1161/CIRCRESAHA.107.162206. [DOI] [PubMed] [Google Scholar]
- 69.Spassky N, Han Y-G, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Developmental biology. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 71.Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development (Cambridge, England) 2000;127:1971–1980. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- 72.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–846. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 73.Trazzi S, Mitrugno VM, Valli E, Fuchs C, Rizzi S, Guidi S, Perini G, Bartesaghi R, Ciani E. APP-dependent up-regulation of Ptch1 underlies proliferation impairment of neural precursors in Down syndrome. Hum Mol Genet. 2011;20:1560–1573. doi: 10.1093/hmg/ddr033. [DOI] [PubMed] [Google Scholar]
- 74.Tsai MT, Cheng CJ, Lin YC, Chen CC, Wu AR, Wu MT, Hsu CC, Yang RB. Isolation and characterization of a secreted, cell-surface glycoprotein SCUBE2 from humans. Biochem J. 2009;422:119–128. doi: 10.1042/BJ20090341. [DOI] [PubMed] [Google Scholar]
- 75.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 76.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 77.Webb S, Anderson RH, Lamers WH, Brown NA. Mechanisms of deficient cardiac septation in the mouse with trisomy 16. Circ Res. 1999;84:897–905. doi: 10.1161/01.res.84.8.897. [DOI] [PubMed] [Google Scholar]
- 78.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 79.Williams AD, Mjaatvedt CH, Moore CS. Characterization of the cardiac phenotype in neonatal Ts65Dn mice. Dev Dyn. 2008;237:426–435. doi: 10.1002/dvdy.21416. [DOI] [PubMed] [Google Scholar]
- 80.Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Developmental biology. 2001;229:503–516. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
- 81.Yu T, Li Z, Jia Z, Clapcote SJ, Liu C, Li S, Asrar S, Pao A, Chen R, Fan N, et al. A mouse model of Down syndrome trisomic for all human chromosome 21 syntenic regions. Human molecular genetics. 2010;19:2780–2791. doi: 10.1093/hmg/ddq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yue S, Chen Y, Cheng SY. Hedgehog signaling promotes the degradation of tumor suppressor Sufu through the ubiquitin-proteasome pathway. Oncogene. 2009;28:492–499. doi: 10.1038/onc.2008.403. [DOI] [PubMed] [Google Scholar]
- 83.Zeng X, Goetz JA, Suber LM, Scott WJ, Jr, Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–720. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
- 84.Zhang C, Williams EH, Guo Y, Lum L, Beachy PA. Extensive phosphorylation of Smoothened in Hedgehog pathway activation. Proc Natl Acad Sci U S A. 2004;101:17900–17907. doi: 10.1073/pnas.0408093101. [DOI] [PMC free article] [PubMed] [Google Scholar]