Abstract
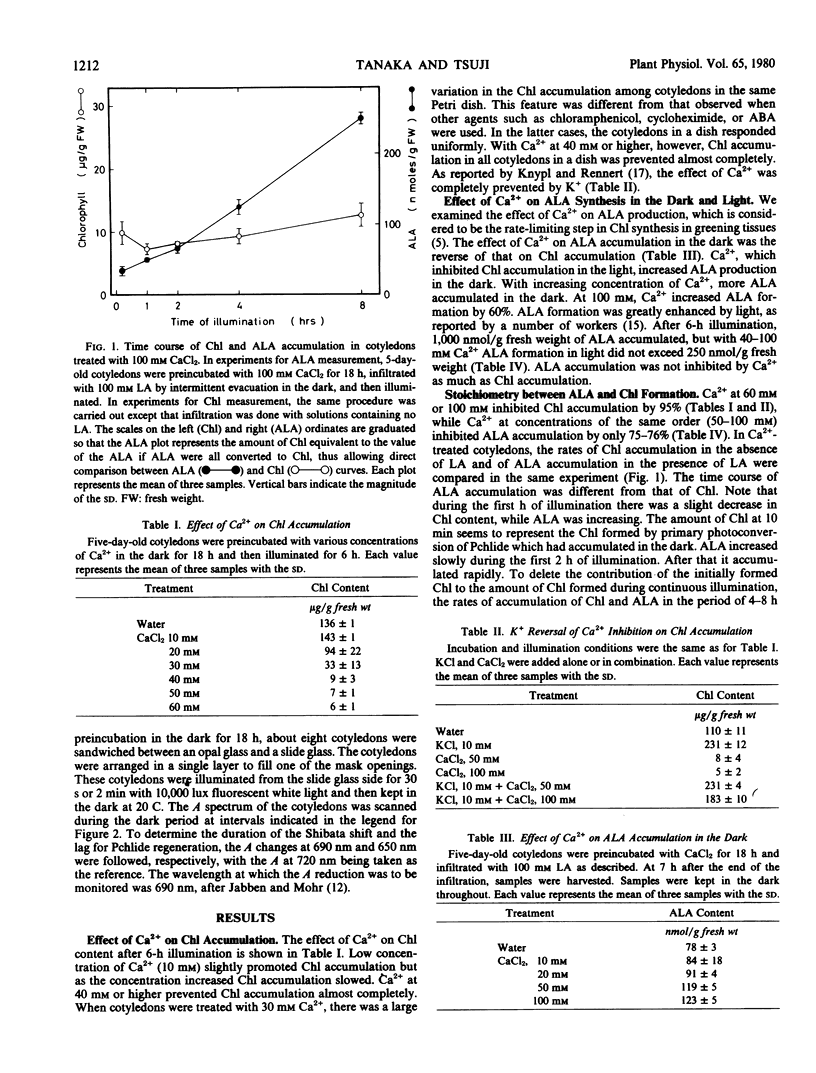
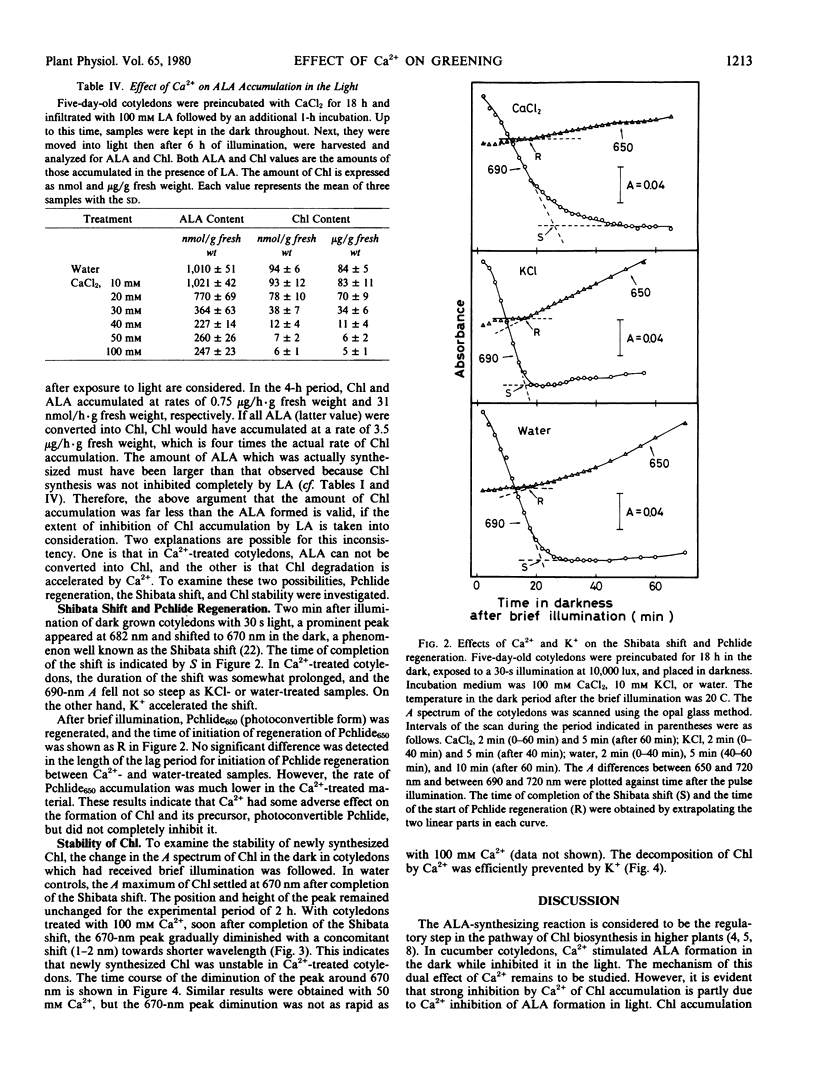
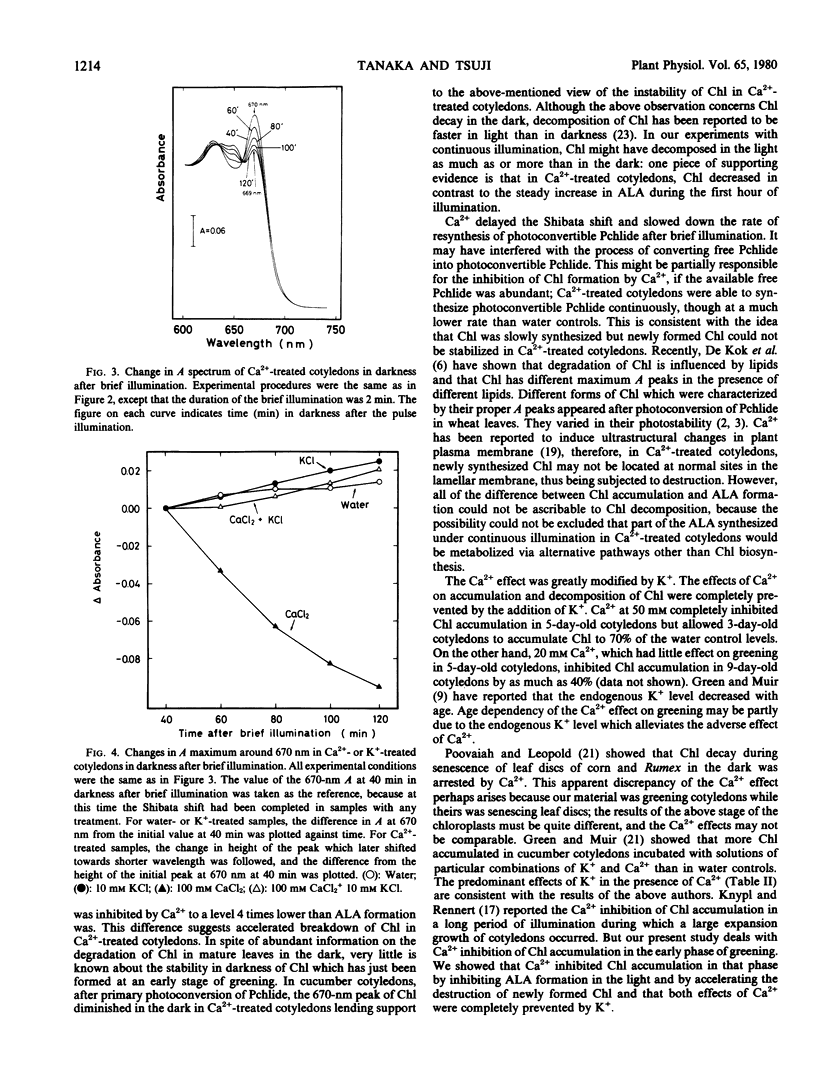
The effects of calcium on chlorophyll accumulation and its stability in the early phase of greening in cucumber cotyledons were investigated. Chlorophyll accumulation was hardly affected by dark preincubation of cotyledons with 10 millimolar calcium solution, but was inhibited almost completely when 50 or 100 millimolar solution was used. On the other hand, 50 millimolar calcium inhibited δ-aminolevulinic acid formation in the light by only 75%. Calcium had little effect on the lag for initiation of protochlorophyllide650 regeneration, but slowed down the rate of accumulation of protochlorophyllide650. In calcium-treated cotyledons, the chlorophyll formed by primary photoconversion was quickly decomposed in the dark. The present results show that calcium inhibited chlorophyll accumulation by inhibiting δ-aminolevulinic acid formation in the light and by stimulating the decomposition of newly formed chlorophyll, both effects being completely prevented by potassium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S., Beale S. I. Hemes, chlorophylls, and related compounds: biosynthesis and metabolic regulation. Adv Enzymol Relat Areas Mol Biol. 1978;46:33–203. doi: 10.1002/9780470122914.ch2. [DOI] [PubMed] [Google Scholar]
- Granick S., Gassman M. Rapid regeneration of protochlorophyllide(650). Plant Physiol. 1970 Feb;45(2):201–205. doi: 10.1104/pp.45.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel E., Klein S. Light dependent formation of -aminolevulinic acid in etiolated leaves of higher plants. Biochem Biophys Res Commun. 1972 Oct 17;49(2):364–370. doi: 10.1016/0006-291x(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Jabben M., Mohr H. Stimulation of the Shibata shift by phytochrome in the cotyledons of the mustard seedling Sinapis alba L. Photochem Photobiol. 1975 Jul-Aug;22(1-2):55–58. doi: 10.1111/j.1751-1097.1975.tb06721.x. [DOI] [PubMed] [Google Scholar]
- Klein S., Harel E., Ne'eman E., Katz E., Meller E. Accumulation of delta-Aminolevulinic Acid and Its Relation to Chlorophyll Synthesis and Development of Plastid Structure in Greening Leaves. Plant Physiol. 1975 Oct;56(4):486–496. doi: 10.1104/pp.56.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Katz E., Neeman E. Induction of delta-Aminolevulinic Acid Formation in Etiolated Maize Leaves Controlled by Two Light Systems. Plant Physiol. 1977 Sep;60(3):335–338. doi: 10.1104/pp.60.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Morré D. J., Bracker C. E. Ultrastructural alteration of plant plasma membranes induced by auxin and calcium ions. Plant Physiol. 1976 Oct;58(4):544–547. doi: 10.1104/pp.58.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Deferral of leaf senescence with calcium. Plant Physiol. 1973 Sep;52(3):236–239. doi: 10.1104/pp.52.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]



