Abstract
BACKGROUND
The role of patient age on the efficacy of mesenchymal stem cell (MSC) therapy in ischemic cardiomyopathy (ICM) is controversial.
OBJECTIVE
We sought to determine whether the therapeutic effect of culture-expanded MSCs persists even in older subjects.
METHODS
Patients with ICM who received MSCs via transendocardial stem cell injection (TESI) as part of the TAC-HFT (n = 19) and POSEIDON (N = 30) clinical trials were divided into 2 age groups: <60 versus ≥60 years. Functional capacity was measured by 6-minute walk distance (6MWD) and quality of life using the Minnesota Living with Heart Failure Questionnaire (MLHFQ) score, measured at baseline, 6 months, and 1-year post-TESI. Various cardiac imaging parameters, including absolute scar size, were compared at baseline and 1 year post-TESI.
RESULTS
Mean 6MWD was similar at baseline and increased at 1 year post-TESI in both groups: 48.5 ± 14.6 m (p = 0.001) for the younger and 35.9 ± 18.3 m (p = 0.038) for the older participants (p = NS between groups). The older group exhibited a significant reduction in MLHFQ score (−7.04 ± 3.54; p = 0.022), while the <60 age group had a borderline significant reduction (−11.22 ± 5.24; p = 0.058) from baseline (p = NS between groups). While there were significant reductions in absolute scar size from baseline to 1 year post-TESI, the effect did not differ by age.
CONCLUSION
MSC therapy via TESI in ICM patients improves 6MWD and MLHFQ score and reduces MI size. Importantly, age did not impair response.
Keywords: heart failure, infarct size, ischemic heart disease
INTRODUCTION
Based on preclinical studies and clinical trials, bone marrow-derived mesenchymal stem cells (MSCs) (1-3) have been shown to mitigate left ventricular (LV) remodeling associated with acute (2,4,5) myocardial infarction (MI) and chronic (1,6-8) ischemic cardiomyopathy (ICM). While the data are encouraging, evidence suggesting a deleterious effect of aging on autologous MSC transplantation has been highly controversial (9). Telomere length and shortening play crucial roles in the cellular molecular aging process (10,11) and there is a strong correlation between human MSC (hMSC) proliferative capacity and telomere length, in culture and with donor age (12). In addition to their diminished proliferative potential, aging hMSCs tend to have a compromised homing capability (13-16). Accordingly, these age-related impairments suggest that MSC therapy might produce a reduced effect when the cells are derived from older individuals.
While some proponents believe advanced stem cell donor age results in diminished function (17-21), other studies raise a clinically relevant issue as to whether recipient age is a crucial factor limiting response to cell therapy (22-24). This has led to the notion that MSC therapy outcome depends not only on stem cell age, and thus function, but also recipient age and comorbidities (9,22). Indeed, reduced responsiveness as a function of donor age would be a major limitation to the emerging development of cell therapy for heart disease, given the increasing incidence and morbidity of heart disease with age (25). Here, we tested the hypothesis that improvements in functional capacity, quality of life (QOL), and reverse remodeling by transendocardial injection of hMSCs in patients with ICM is actually preserved with recipient age. Our data here derive from the phase I/II randomized trials of TAC-HFT (Transendocardial Autologous Cells in Ischemic Heart Failure) (26) and POSEIDON (Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis) (27).
METHODS
Data from the TAC-HFT and POSEIDON were collected in a similar fashion in a central electronic data system. All ICM patients who received hMSCs from these trials were pooled together and dichotomized into 2 age groups, <60 years and ≥60 years. The associations between age and both clinical and imaging parameters were assessed. P values <0.05 were considered significant. Comprehensive statistical methods can be found in the Online Supplement.
RESULTS
A total of 49 patients who received hMSCs from both trials are included in this analysis. Thirty patients received hMSCs in the POSEIDON trial, of which 11 patients (36.7%) were <60 years old and 19 patients (63.3%) were ≥60 years old. In the TAC-HFT trial, a total of 19 patients received hMSCs, with 12 (63.2%) younger than 60 years of age and 7 patients (36.8%) in the ≥60 years age group. Average age at transplant was 51.95 (±7.33; 32.48 to 59.91 range) in the <60 years age group and 68.86 (±4.51; 62.84 to 79.01 range) in the older group. Mean time from MI to cell therapy was 6.26 ± 6.42 years for younger patients and 15.43 ± 9.23 years in the older group (p = 0.0002).
Baseline characteristics are shown and compared between age groups in Table 1. The majority of the cohort was male (89.8%). A borderline statistically significant difference was observed between age groups for the baseline 6MWD test (p = 0.0561). Scar size as percent of LV mass was significantly different between age groups at baseline (p = 0.0041). No other statistically significant differences were observed for demographic characteristics, MLHFQ, or other cardiac imaging parameters.
TABLE 1.
Baseline Characteristics
| Age Group | |||
|---|---|---|---|
| <60 years (n = 23) | ≥60 years (n = 26) | p value | |
| Age at Transplant, year | |||
| 51.95 (7.33) | 68.86 (4.51) | <0.0001 | |
| Time from MI to therapy, year | |||
| 6.26 (6.42) | 15.43 (9.23) | 0.0002 | |
| Sex | |||
| Male | 21 (42.9%) | 23 (46.9%) | |
| Female | 2 (4.1%) | 3 (6.1%) | 1.000 |
| Race | |||
| White | 12 (24.5%) | 19 (38.8%) | |
| European | 0 (0.0%) | 1 (2.0%) | 0.1547 |
| White North American | 3 (6.1%) | 1 (2.0%) | |
| Western European | 0 (0.0%) | 1 (2.0%) | |
| Black | 2 (4.1%) | 0 (0.0%) | |
| Indian/South Asian | 0 (0.0%) | 1 (2.0%) | |
| Filipino (Pilipino) | 1 (2.0%) | 0 (0.0%) | |
| Native American | 0 (0.0%) | 1 (2.0%) | |
| White Caribbean | 5 (10.2%) | 2 (4.1%) | |
| Ethnicity | |||
| Hispanic or Latino | 9 (18.4%) | 4 (8.2%) | |
| Not Hispanic or Latino | 14 (28.6%) | 20 (40.8%) | 0.0629 |
| Unknown | 0 (0.0%) | 2 (4.1%) | |
| 6MWD | |||
| 418.30 (71.57) | 372.12 (93.01) | 0.0561 | |
| MLHFQ Total Score | |||
| 42.33 (28.84) | 31.58 (27.81) | 0.2013 | |
| Scar size as absolute value | |||
| 26.93 (15.35) | 21.47 (13.29) | 0.2080 | |
| Scar size as % of LV mass | |||
| 22.09 (13.55) | 11.79 (6.06) | 0.0041 | |
| Ejection fraction | |||
| 31.96 (6.22) | 29.49 (12.65) | 0.3945 | |
| EDV | |||
| 289.36 (81.65) | 274.40 (86.66) | 0.5525 | |
| ESV | |||
| 199.50 (68.53) | 199.29 (86.15) | 0.9930 | |
| SI | |||
| 0.50 (0.07) | 0.47 (0.11) | 0.3077 | |
Values are mean (SD) or n (%).
6MWD = 6-minute walking distance; EDV = end-diastolic volume; EF = ejection fraction; ESV = end-systolic volume; LV = left ventricular; MI = myocardial infarction; MLHFQ = Minnesota Living with Heart Failure Questionnaire; SD = standard deviation; SI = sphericity index.
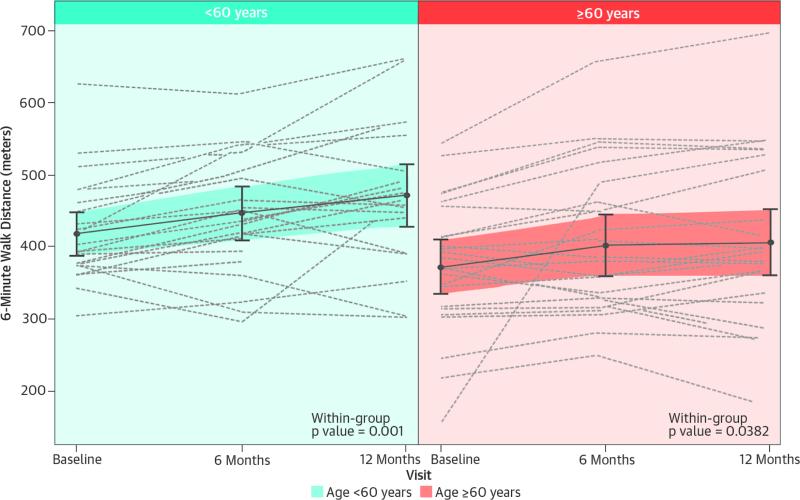
There was a statistical trend towards reduced functional capacity at baseline in the older age group, with 6MWD at baseline (418.30 ± 14.92 meters vs. 372.12 ± 18.24 meters, <60 years vs. ≥60 years, respectively; p = 0.056). A repeated measures model was used to estimate 6MWD at 6 months and 1 year post-TESI, which is shown in the Central Illustration. The 6MWD increased significantly over time in both the younger and older age groups (p = 0.001 and p = 0.038, respectively). A repeated measures model adjusting for baseline 6MWD showed no significant difference in 6MWD between age groups across time (p = 0.5621). Using the estimates from the model, we tested whether there were differences between age groups at each of the follow-up time points. The estimated difference at 6 months post-TESI was -1.01 (95% confidence interval [CI]: -35.51 to 33.49; p = 0.9538) and at 1 year 18.61 (95% CI: 15.95 to 53.17; p = 0.2882).
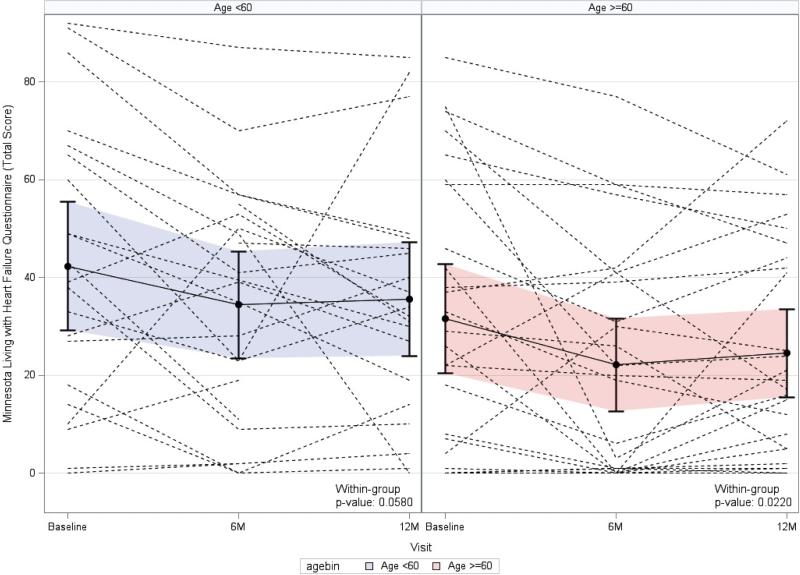
QOL measured by the MLHFQ was compared between age groups. A repeated measures model was used to estimate MLHFQ total score at 6 months and 1 year post-TESI, which is displayed in Figure 1. Patients <60 years of age showed an improvement in MLHFQ total score over time, although of only borderline significance (p = 0.0580), while those ≥60 years of age exhibited a more significant improvement in MLHFQ total score over time (p = 0.0220). A repeated measures model adjusting for baseline MLHFQ total score showed no significant difference in total score between age groups over time (p = 0.5859). Estimated differences between groups at 6 months and 1 year post-TESI were not significant (2.74; 95% CI: -5.74 to 11.23; p = 0.5237 and -0.72; 95% CI: -9.51 to 8.07; p = 0.8711, respectively). When 6MWD and MLHFQ were analyzed using the repeated measures model using age as a continuous covariate, neither outcome demonstrated a significant change with increase in age (p = 0.137 and p = 0.535, respectively).
FIGURE 1. Patient Quality of Life: Minnesota Living with Heart Failure Questionnaire.
In this graphic representation of estimated mean Minnesotta Living With Heart Failure Questionnaire, the total score values and individual patient values in each age group at each time point are depicted, using a repeated measures model. Both age groups’ total scores improved in a parallel fashion at 6 months post-TESI. Both groups plateaued by 1 year post-TESI with similar mean total scores in between-group comparison (p = 0.524 and p = 0.871 at 6 months and 1 year post-TESI, respectively; between-group comparison). Abbreviation as in the Central Illustration.
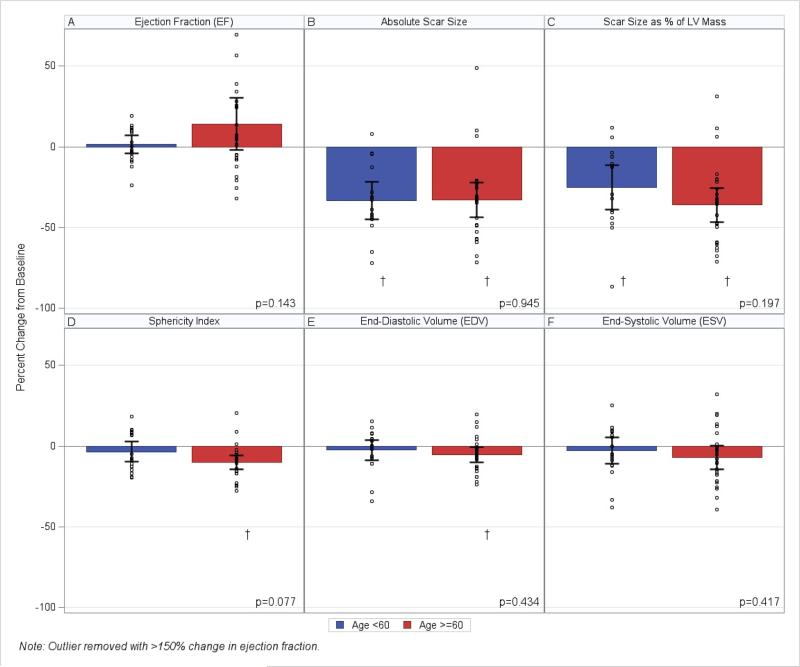
Next we examined the impact of age on reduction in MI scar size. Both age groups had a similar absolute scar size at baseline. MI size was 26.93 ± 15.35 g in the <60 years age group and 21.47 ± 13.29 g in the ≥60 years age group (p = 0.208). When testing within group changes from baseline to 1 year post-TESI, patients <60 and ≥60 years of age both had a significant decrease in absolute scar size (p < 0.0001 and p < 0.0001, respectively). Furthermore, this percent change over time was not significantly different between groups (-33.44 ± 5.41% in the younger versus -32.89 ± 5.25% in the older age group; p = 0.945) (Figure 2A and Figure 3). Scar size as a percentage of LV mass was significantly higher at baseline in the younger group (22.09 ± 13.55 g) when compared to older patients (11.79 ± 6.06 g; p = 0.004). However, at 1 year post-TESI, the percent change from baseline was significant within both age groups (p = 0.0013 in the younger age group and p < 0.0001 in the older age group). There was no significant difference in scar size as percent of LV mass when comparing percent change from baseline to 1 year post-TESI between age groups (p = 0.197) (Figure 2B).
FIGURE 2. Changes in Cardiac Structure and Function 1 Year Post-MSC Injection.
(A) Neither age group demonstrates significant improvement in EF. (B) Both younger and older patients show a significant decrease in absolute scar size, with no difference at 1 year post-TESI. (C) Scar size as a percentage of LV mass decreases in both age groups, and do not differ at 1 year. (D and E) Sphericity index and EDV significantly improve only in the older age group; however, there are no between-group changes 1 year post-TESI. (F) The older age group shows a trend in decreased ESV; neither ESV or EF were significantly different between groups at 1 year post-TESI. *< 0.05 within-group repeated measures ANOVA. EDV = end-diastolic volume; EF = ejection fraction; ESV = end-systolic volume; LV = left ventricular; MSC = mesenchymal stem cell; other abbreviation as in the Central Illustration.
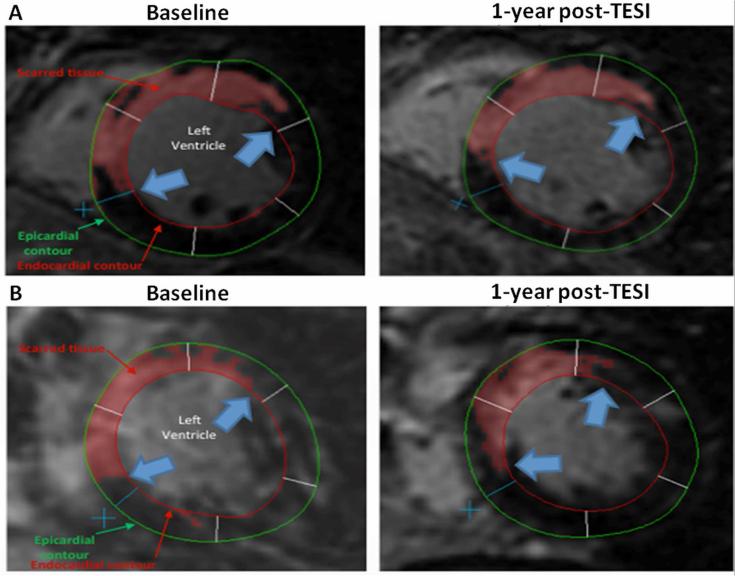
FIGURE 3. Impact of TESI on Scar Reduction.
(A) Short-axis views of the midventricular area of a younger patient's heart show delayed enhancement delineated at the anterior and septal walls. After MSC injection, scar size in <60-year-old patient decreased from 30.9 g at baseline to 21.2 g at 1 year with a -31.4% reduction. (B) Short-axis views of the midventricular area of a ≥60 year-old patient's heart, with delayed enhancement shown at the anterior and septal walls. Delayed tissue enhancement corresponds to scarred tissue and is depicted brighter than the nonscarred tissue (automatically detected and delineated with red using the full width at half maximum technique). Red, green, and white lines demarcating the endocardial, epicardial contours, and borders of the segments, respectively, were drawn manually. Extent of scar is represented by blue arrows. After MSC injection, scar size in the ≥60-year-old patient decreased from 36.2 g at baseline to 24.5 g at 1 year, with a similar -32.3% reduction in MI size. MI = myocardial infarction; other abbreviations as in Figures 1 and 3.
Cardiac computed tomography (CT) or magnetic resonance imaging (CMR) measured end-diastolic volume (EDV) and sphericity index (SI) were similar between age groups at baseline (p = 0.553 and p = 0.508, respectively). At 1 year post-TESI, within-group changes in EDV (p = 0.024) and SI (p < 0.0001) significantly decreased in the ≥60 years group, but no significant change was observed in the younger group. Neither EDV nor SI, as a percent change from baseline, differed between age groups at 1 year post-TESI (p = 0.434 and p = 0.077, respectively) (Figures 3C and D). Although mean ejection fraction (EF) and end-systolic volume (ESV) between groups were not different at baseline (p = 0.395 and p = 0.993, respectively) or at 1 year post-TESI (p = 0.143 and p = 0.417, respectively), there was a borderline significant decrease over time in ESV within the older age group (p = 0.054), that was not replicated in the younger group. There was no significant increase in EF in either age group. Linear regression analyses, using age as a continuous variable, did not indicate any significant association between cardiac structure or function and age (Table 2).
TABLE 2.
Association Between Cardiac Structure and Age Using a Linear Regression Model
| Cardiac Imaging Parameter | Regression Parameter Estimate | Standard Error | p Value |
|---|---|---|---|
| Scar size as absolute value | −0.25 | 0.38 | 0.5009 |
| Scar size as % of LV mass | −0.58 | 0.39 | 0.1501 |
| EF* | — | — | 0.5414 |
| (EDV | −0.25 | 0.18 | 0.1639 |
| (ESV | −0.28 | 0.26 | 0.2777 |
| SI | −0.19 | 0.18 | 0.2831 |
Due to the highly skewed distribution of ejection fraction, a non-parametric Spearman Rank Correlation Coefficient test was used to determine if a significant association was present. Correlation coefficient between age and EF = 0.0957.
Abbreviations as in Table 1.
DISCUSSION
The major new finding of this study shows that therapeutic responses to culture-expanded MSCs are not impaired in subjects of older age. This is particularly important to the emerging field of cell therapy for chronic heart failure due to ischemic cardiomyopathy, a disorder that increases dramatically in incidence with age (28). If cell therapy responses were impaired with age, this would impact patients at greatest risk. Our findings suggest that culture expansion of MSCs overcomes any limitation that endogenous cells might have and age of the host is not a limiting factor. These data support the developing this strategy for individuals of advanced age and, thus, they boast major public health implications.
Here, we analyzed efficacy outcomes, from the TAC-HFT (26) and POSEIDON (27) trials, to test whether older patients with chronic ICM receiving transendocardial mesenchymal stem cell therapy have impaired outcomes relative to young individuals. Notably, improvements in functional capacity were evident at 6 months following injection and persisted to 1 year after TESI to similar degrees in both age groups. To date, while several studies have examined whether donor cell age and function influence responses to cell therapy (17,19,20), no study has tested the hypothesis that recipient age diminishes the efficacy of MSC transplantation. Moreover, while previous studies examining aging and stem cell potency have tested bone marrow mononuclear cells (29-31) and peripheral blood progenitor cells (32), this relationship in culture-expanded mesenchymal stem cells has not previously been explored.
The 6-minute walk test has been widely used to assess functional capacity in patients with advanced heart failure (33), and is an independent predictor of all-cause mortality (34). We found a significant improvement in 6MWD in both age groups (Central Illustration), a result that parallels the overall results of TAC-HFT and POSEIDON. More importantly, the change from baseline at both 6 months and 1 year following TESI did not differ significantly between age groups. Such an improvement in functional capacity highlights the test's prognostic value (35) and strongly suggests that older cell recipients functionally recover just as well as younger patients.
Findings from the TAC-HFT and POSEIDON trials suggest that MSCs reduce infarct size over time. We found a similar significant decrease in absolute scar size irrespective of age groups at 1 year post-TESI (Figure 2 and Figure 3). These results corroborate findings from the POSEIDON trial where both autologous and allogeneic MSCs reduced infarct size over time. It is known that MSCs secrete anti-fibrotic matrix metalloproteases (36) via paracrine signaling. Although the reason remains mechanistically unclear, our study demonstrates that aging in stem cell recipients does not appear to influence the anti-fibrotic effects of MSC therapy. Interestingly, scar size as a percentage of LV mass in the younger age group was almost twice as large as the older age group at baseline (Table 1). This cannot be accounted for except possibly for the small sample size used in the study, which was further narrowed when focusing on the <60 years of age group. More importantly, the change from baseline to 1 year post-injection was similar between the two groups.
Quality of life in each age group was similar at baseline and comparisons of MLHFQ total score changes at both 6 months and 1 year from baseline did not differ significantly between groups (Figure 1). The questionnaire has been deemed a valid and effective instrument in measuring QOL in heart failure patients (37). Composed of 21 items that sum up to a total score, ranging from 0 (no effect) to 105 (strong effect of heart failure on daily life), the MLHFQ is a commonly used assessment tool in heart failure studies (38,39). Our analysis demonstrated a significant improvement in MLHFQ score in older patients and a trend, with borderline significance, towards an improved total score in patients <60 years of age.
Past studies (1,2) established the role of MSC therapy in LV reverse remodeling. We found that left ventricular chamber volumes between age groups were similar at baseline as well as 1 year following injection, albeit a significant improvement in EDV only in patients, aged ≥60 years. Correspondingly, sphericity index was reduced in the older age group, despite being the same between groups at baseline and 1 year post-TESI. While these findings may not completely corroborate those in POSEIDON and TAC-HFT, they do raise the important concept of recipient age not having an influence on MSC therapy efficacy. Improvements in EF have been inconsistent throughout clinical trials of stem cell therapy (40) and we did not find significant increases in either age group. Still, 1 year following injection, EF between older and younger age groups was not different. Notwithstanding these data, it is important to note that infarct size is a stronger predictor of future adverse cardiac events than EF (41).
STUDY LIMITATIONS
This study has several limitations that warrant mention. First, this work has a relatively small sample size, which may limit conclusions from certain measurements such as time to therapy. While a formal power or sample size calculation was not performed prospectively given that the patient population originates from a fixed cohort, we note that the sample size in each of the age groups would have 84% power to detect a difference in the distance walked in 6 minutes from 33 to -10 using a 2-sided alpha of 0.05, a relatively large difference. Second, because this analysis is a composite of 2 different clinical trials, data from 2 different imaging modalities are employed; multidetector CT scanning in POSEIDON and both cardiac CT and CMR in TAC-HFT. We corrected for this by calculating percent changes for cardiac imaging measurements. Finally, the issue of biological versus chronological age merits comment. Researchers generally feel that biological age predominates over chronological age and can be assessed with molecular assays such as telomere length (10-12). We did not incorporate this assessment, as telomere length assays were not part of either the POSEIDON or TAC-HFT study design. Importantly, whereas most telomere studies have correlated cellular and chronological age of donor cells, our study does not incorporate cellular characteristics of donor cells, rather the chronological age of recipients. Future studies are planned to measure telomere length in both donors and recipients.
CONCLUSIONS
In conclusion, our study suggests that recipient age does not reduce the effects of MSC therapy in patients with ischemic cardiomyopathy. Importantly, comparisons of 6-minute walk test and absolute scar size between age groups did not differ. Our findings document for the first time the relationship between advanced age and clinical outcomes in heart failure and show an important preservation in responses to cell therapy in a group of recipients of advanced age. These data support ongoing clinical trials on cell-based therapy and the need for future clinical investigation of MSC use in older age groups.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge: Aging patients respond similarly to younger patients after receiving human mesenchymal stem cell therapy for ischemic cardiomyopathy. Functional capacity, quality of life, and several cardiac function parameters may improve in these patients, even long after myocardial infraction, highlighting their ability to yield to mesenchymal stem cell anti-fibrotic and pro-myogenic effects.
Translational Outlook: Older patients may be eligible in future clinical stem cell therapy trials. Future randomized studies are required to further demonstrate equivalence in responsiveness between older and younger recipients.
CENTRAL ILLUSTRATION Patient Functional Capacity: 6-Minute Walking Distance.
This graphic representation shows estimated mean 6-minute walking distance values and individual patient values in each age group at each time point, using a repeated measures model. Both age groups increased similarly until 6 months post-TESI. Although the older age group plateaus by 1 year post-TESI, average distances between age groups do not differ significantly (p = 0.954 and p = 0.288 at 6 months and 1 year post-TESI, respectively; between-group comparison). Abbreviations: TESI = transendocardial stem cell injection.
Acknowledgments
Funding: This work was supported in part by NIH R01 Grants R01HL110737, R01HL084275, U54HL0810028 to JMH. JMH is also supported by NIH grants UM1HL113460, R01HL107110 and the Starr Foundation.
ABBREVIATIONS
- 6MWD
6-minute walk distance
- EDV
end-diastolic volume
- EF
ejection fraction
- ESV
end-systolic volume
- hMSC
human mesenchymal stem cell
- LV
left ventricular
- MI
myocardial infarction
- MLHFQ
Minnesota Living with Heart Failure Questionnaire
- SI
sphericity index
- TESI
transendocardial stem cell injection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Hare reports equity interest and board membership in Vestion, Inc. No other disclosures are reported.
REFERENCES
- 1.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–6. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (PROCHYMAL) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katritsis DG, Sotiropoulou PA, Karvouni E, et al. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65:321–9. doi: 10.1002/ccd.20406. [DOI] [PubMed] [Google Scholar]
- 4.Leistner DM, Fischer-Rasokat U, Honold J, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): Final 5-year results suggest long-term safety and efficacy. Clin Res Cardiol. 2011;100:925–34. doi: 10.1007/s00392-011-0327-y. [DOI] [PubMed] [Google Scholar]
- 5.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 6.de la Fuente LM, Stertzer SH, Argentieri J, et al. Transendocardial autologous bone marrow in chronic myocardial infarction using a helical needle catheter: 1-year follow-up in an open-label, nonrandomized, single-center pilot study (the TABMMI Study). Am Heart J. 2007;154:79.e71–7. doi: 10.1016/j.ahj.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 7.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 8.Hendrikx M, Hensen K, Clijsters C, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: Results from a randomized controlled clinical trial. Circulation. 2006;114:I101–7. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 9.Zhuo Y, Li SH, Chen MS, et al. Aging impairs the angiogenic response to ischemic injury and the activity of implanted cells: Combined consequences for cell therapy in older recipients. J Thorac Cardiovasc Surg. 2010;139:1286–94, 1294.e1281-2. doi: 10.1016/j.jtcvs.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 10.Wright WE, Shay JW. Historical claims and current interpretations of replicative aging. Nat Biotechnol;2002;20:682–8. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- 11.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–73. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 12.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–82. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 13.Mets T, Verdonk G. In vitro aging of human bone marrow derived stromal cells. Mech Ageing Dev. 1981;16:81–9. doi: 10.1016/0047-6374(81)90035-x. [DOI] [PubMed] [Google Scholar]
- 14.D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–22. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 15.Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–81. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 16.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–26. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Dimmeler S, Vasa-Nicotera M. Aging of progenitor cells: Limitation for regenerative capacity? J Am Coll Cardiol. 2003;42:2081–2. doi: 10.1016/j.jacc.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–20. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 19.Scheubel RJ, Zorn H, Silber RE, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–80. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;90:E89–93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 21.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–63. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 22.Kinkaid HY, Huang XP, Li RK, Weisel RD. What's new in cardiac cell therapy? Allogeneic bone marrow stromal cells as “universal donor cells”. J Card Surg. 2010;25:359–66. doi: 10.1111/j.1540-8191.2009.00984.x. [DOI] [PubMed] [Google Scholar]
- 23.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 24.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN trial. JAMA. 2012;307:1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clin Geriatr Med. 2009;25:563–77, vii. doi: 10.1016/j.cger.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA. 2012;308:2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Kook H, Chung I, et al. Telomere length changes in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24:411–5. doi: 10.1038/sj.bmt.1701923. [DOI] [PubMed] [Google Scholar]
- 30.Taraldsrud E, Grogaard HK, Solheim S, et al. Age and stress related phenotypical changes in bone marrow cd34+ cells. Scand J Clin Lab Invest. 2009;69:79–84. doi: 10.1080/00365510802419447. [DOI] [PubMed] [Google Scholar]
- 31.Sugihara S, Yamamoto Y, Matsuura T, et al. Age-related bm-mnc dysfunction hampers neovascularization. Mech Ageing Dev. 2007;128:511–16. doi: 10.1016/j.mad.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Lewis NL, Mullaney M, Mangan KF, Klumpp T, Rogatko A, Broccoli D. Measurable immune dysfunction and telomere attrition in long-term allogeneic transplant recipients. Bone Marrow Transplant. 2004;33:71–8. doi: 10.1038/sj.bmt.1704300. [DOI] [PubMed] [Google Scholar]
- 33.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–32. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 34.Ingle L, Cleland JG, Clark AL. The long-term prognostic significance of 6-minute walk test distance in patients with chronic heart failure. Biomed Res Int. 2014;2014:505969. doi: 10.1155/2014/505969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forman DE, Fleg JL, Kitzman DW, et al. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012;60:2653–61. doi: 10.1016/j.jacc.2012.08.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–40. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Middel B, Bouma J, de Jongste M, et al. Psychometric properties of the Minnesota Living With Heart Failure Questionnaire (MLHF-Q). Clin Rehabil. 2001;15:489–500. doi: 10.1191/026921501680425216. [DOI] [PubMed] [Google Scholar]
- 38.Perin EC, Silva GV, Henry TD, et al. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS-HF). Am Heart J. 2011;161:1078–87.e3. doi: 10.1016/j.ahj.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 39.Hong KU, Bolli R. Cardiac stem cell therapy for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16:324. doi: 10.1007/s11936-014-0324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367:113–21. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 41.Wu E, Ortiz JT, Tejedor P, et al. Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or endsystolic volume index: Prospective cohort study. Heart. 2008;94:730–6. doi: 10.1136/hrt.2007.122622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.