Abstract
Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor deriving from the thyroid parafollicular cell. Thyroidectomy continues to serve as the primary initial treatment for this cancer. Because standard cytotoxic chemotherapy has proven ineffective, reoperation and external beam radiation therapy had been the only tools to treat recurrences or distant disease. The discovery that aberrant activation of RET, a receptor tyrosine kinase, is a primary driver of MTC tumorigenesis led to clinical trials using RET-targeting tyrosine kinase inhibitors. The successes of those trials led to the approval of vandetanib and cabozantinib for treating patients with progressive or symptomatic MTC. The availability of these drugs, along with additional targeted therapies in development, requires a thoughtful reconsideration of the approach to treating patients with unresectable locally advanced and/or metastatic progressive MTC.
Keywords: adverse events, cabozantinib, tyrosine kinase inhibitor, vandetanib
Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor deriving from thyroid parafollicular cells (also called C cells). Approximately 2500 new cases are estimated for 2014 in the USA [1], which translates to an estimated 12,500 cases worldwide [2]. Despite this rarity, a wealth of mechanistic data and several constantly evolving treatment strategies exist. A major shift in prophylactic MTC treatment began with the discovery that germline mutations that activate the receptor tyrosine kinase (RET) cause hereditary MTC [3–5]. Current estimates suggest that approximately 25% of all MTCs are hereditary and, with the notable exception of a family member presenting with the disease, are diagnosed largely through germline DNA testing. Several excellent reviews discuss the management and treatment of hereditary MTC [6–8].
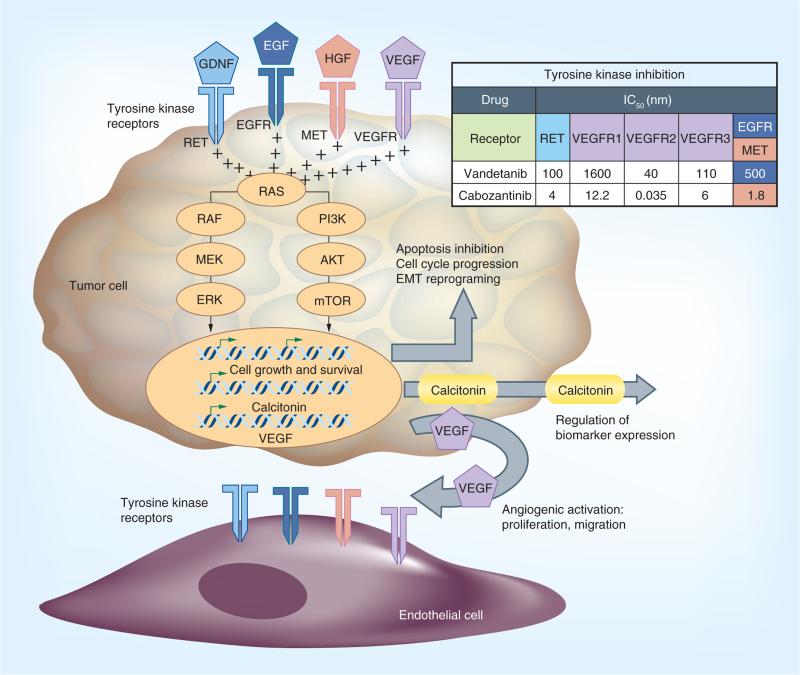
An emerging paradigm for the treatment of advanced MTC derives from our understanding of RET function and the role of angiogenesis in cancer. In the presence of its ligand and coreceptor, the dimerization of RET leads to its activation. Like other receptor tyrosine kinases, RET sends intra-cellular signals primarily through the MAPK cascade (Figure 1). Hereditary RET mutations induce ligand-independent receptor activation, imparting an unregulated proliferation signal. The observation that activating somatic RET mutations also exist in approximately 40% of sporadic MTCs provided a targeting strategy for stopping tumor cell growth: inhibition of activated RET [9–11]. There are currently two tyrosine kinase inhibitors (TKIs) – vandetanib and cabozantinib – approved by the US FDA for the treatment of MTC, with additional drugs in clinical development [12–14]. The availability of these new targeted therapies has led to another major change in MTC treatment. With the option of systemic therapy has come a new thought process for approaching surgery and metastatic disease and the need to manage the adverse events (AEs) associated with these new treatments. Recent censuses in the USA and Europe suggest that approximately 15% of MTC patients present with distant metastatic disease at their initial diagnosis. An additional 40% presenting with N1 disease are at an increased risk for recurrence [15–17]. The goals of this review are to present our evolving approach to the surveillance of advanced MTC and to provide guidance for the implementation of systemic treatment, a new MTC standard of care.
Figure 1. Signaling pathways implicated in medullary thyroid carcinoma tumorigenesis.
Although aberrantly activated RET is established as a primary driver in most medullary thyroid carcinoma, other tyrosine receptors, which are known targets of vandetanib and cabozantinib, have been detected. The antiangiogenic function of tyrosine kinase inhibitors coupled with their targeting of tumor receptors work to effectively disrupt the medullary thyroid carcinoma tumor microenvironment.
EGFR: EGF receptor; VEGFR: VEGF receptor.
Initial therapy
Although surgery is the cornerstone of treatment for MTC confined to the neck, once the disease spreads to the mediastinum or to distant sites the usefulness of surgical intervention is less apparent. The surgeon must weigh the benefits of locoregional control against the risks of the proposed procedure, a consideration that should be made in a multidisciplinary setting. Mediastinal disease that may be removed from a cervical incision is usually confined to nodes in level VII, the region inferior to the sternal notch above the innominate artery [18]. This location is usually considered part of a standard central neck dissection, and not designated as distant disease. Less clear in the current American Joint Committee on Cancer staging system [19] is whether the mediastinal region below level VII, often reached only by more invasive approaches, is considered locoregional or distant disease. Machens et al. suggests that this be considered distant metastasis since its presence portends an outcome equivalent to that of other distant disease [20]. We rarely perform an extensive operation in which sternotomy or thoracotomy is required to remove mediastinal nodal disease. Furthermore, patient outcomes have not been improved by such aggressive efforts. In the new era of targeted molecular therapy, progressive disease in this region would be considered a reason to initiate systemic therapy.
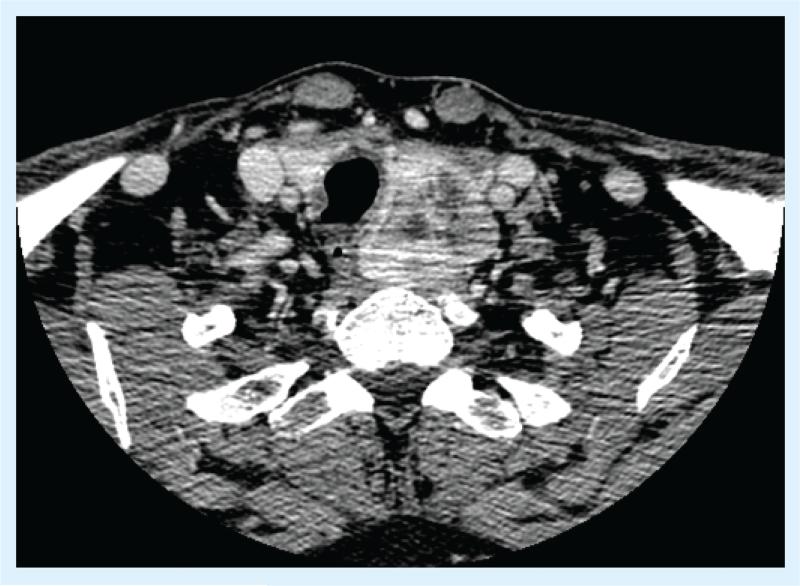
Cervical surgery in the setting of known distant metastatic disease is reasonable to consider when the local disease is in an anatomic position where future disease growth could be detrimental to key structures, such as the recurrent laryngeal nerve, trachea and esophagus (Figure 2 provides an example). Additionally, given that patients with widely metastatic MTC can live for years, palliative resection in the setting of bulky, symptomatic disease is often appropriate.
Figure 2. A 52–year-old man presenting with symptomatic L3 medullary thyroid carcinoma metastasis and a baseline calcitonin level of 4723 pg/ml.
Upon further workup, he was found to have several metastatic lesions in both lobes of his liver (largest, 2.3 cm) and signficant disease within his neck. After irradiation of his spine, he underwent a total thyroidectomy and bilateral level VI and left level III, IV neck dissection for bulky, symptomatic cervical disease. His distant disease has remained stable on no targeted therapy, and he has no evidence of recurrent cervical disease 4 years after initial diagnosis.
The decision to use external beam radiation to the cervical region after resection should now factor in the potential future use of targeted systemic therapies. Our current practice is to use radiation sparingly, only in aggressive disease in which further surgical intervention to eradicate potential recurrence is not feasible. To complicate matters further, some antiangiogenic therapies have been associated with upper airway fistula formation. Therefore, a careful consideration of the risk/benefit ratio of external beam radiation is required.
Evaluation of disease extent & progression in medullary thyroid carcinoma
Initial diagnosis & staging
All patients with suspected or confirmed MTC should undergo comprehensive neck ultrasonography at baseline. If cervical lymph node disease is present or if pre-operative calcitonin levels exceed 400 pg/ml, distant metastases are possible; thus, additional imaging with neck computed tomography, chest computed tomography, and three-phase contrast-enhanced computed tomography or contrast-enhanced MRI of the liver are indicated [6,21]. If skeletal metastases are suspected, axial skeleton MRI is the preferred imaging modality. The diagnostic and prognostic value of fluorodeoxyglucose positron emission tomography (18F-FDG PET) is still controversial [22].
The utility of biomarkers
Serum calcitonin is the primary biochemical marker used for detection, guiding initial staging evaluations, postoperative surveillance and prediction of progression in MTC patients. Carcinoembryonic antigen (CEA) is a less specific biomarker for MTC but can be useful in understanding extent of disease and prognosis. After initial surgery, it is necessary to allow 3–4 months to elapse before attributing any elevations in serum calcitonin or CEA to the presence of residual MTC. Patients with undetectable tumor markers should have annual measurements of serum calcitonin and CEA. The doubling times (dts) of calcitonin and CEA levels are predictive of both recurrence-free and overall survival [23–25]. Patients with calcitonin and CEA dts of less than 6 months have a worse prognosis and are more likely to experience disease progression than those with dts of over 24 months. Meijer et al. observed 5- and 10-year overall survival rates of 36 and 18%, respectively, in MTC patients with a calcitonin dt of less than 1 year and 95 and 95% in those with a calcitonin dt longer than 1 year. Similarly, if the CEA dt was less than 1 year, the 5- and 10-year overall survival rates were 43 and 21%, respectively, but if the CEA dt was greater than 1 year, the 5- and 10-year overall survival rates were both 100%. The American Thyroid Association has a web-based calculator to determine both values, which is most accurate when at least four values are available over a 2-year period [26].
Monitoring progression & the decision to treat
Because regional or distant metastatic disease at the time of initial thyroidectomy can vary from indolent to rapidly progressive, active surveillance for the presence of persistent or recurrent disease and the pace of disease change is needed after thyroidectomy. No optimal timing intervals for surveillance have been recommended by any published guidelines, but the authors typically recommend evaluating tumor markers every 3 months for the first 6 months after thyroidectomy with tumor-relevant radiologic imaging as needed. Thereafter, follow-up is performed every 4–6 months for the first 2 years to establish approximate serum calcitonin and CEA dts and to radiologically monitor for changes in metastatic disease. The majority of clinical trials investigating cancer treatments utilize the Response Evaluation Criteria In Solid Tumors (RECIST) rules and guidelines to standardize the measurement of solid tumors to determine whether disease improves (complete response or partial response), remains the same (stable disease) or worsens (progressive disease). The updated RECIST criteria were published in 2009 (version 1.1 [27]); relevant criteria are shown in Table 1 [28].
Table 1.
Summary of RECIST version 1.1 to determine measurability of disease and objective response.
| RECIST 1.1 | |
|---|---|
| Measurability and selection of target lesions at baseline | Measurable/target lesions: • Unidimensional measurement •Non-lymph nodes measured in the longest diameter only; size ≥20 mm with conventional techniques, ≥10 mm with spiral CT •Lymph nodes: short axis ≥15 mm •Lesions in a previously irradiated area can be considered measurable if progression is demonstrated and they meet size criteria •Maximum number of target lesions: two per organ, up to five total |
| Selection of non-target lesions | Non-target lesions: •Lymph nodes: 10-15 mm nodes •Non-lymph nodes: lesions <10 mm, bone lesions without soft tissue component, pleural and pericardial effusions, ascites or leptomeningeal disease |
| Objective response | •Target lesions (change in sum of diameter from baseline): CR: disappearance of all target lesions, confirmed at ≥4 weeks; PR: ≥30% decrease from baseline, confirmed at 4 weeks; PD: ≥20% increase over smallest sum observed (nadir) and overall 5 mm net increase or appearance of new lesions; SD: neither PR nor PD criteria met •Non-target lesions: CR: disappearance of all non-target lesions and normalization of tumor markers, confirmed at ≥4 weeks; PD*: unequivocal progression of non-target lesions or appearance of new lesions; non-PD: persistence of one or more non-target lesions or tumor markers above normal limits |
| Overall response | •Best response is recorded in measurable disease from treatment start to disease progression or recurrence •Non-PD in non-target lesions will reduce a best response of CR in target lesions to overall PR •Unequivocal new lesions are PD regardless of response in target and non-target lesions |
PD must be ‘unequivocal’ in non-target lesions (e.g., 75% increase in volume); PD can also be new ‘positive PET’ scan with confirmed anatomic progression. Stable positive PET is not PD if it corresponds to anatomic non-PD.
CR: Complete response; CT: Computed tomography; PD: Progressive disease; PR: Partial response; SD: Stable disease.
Systemic therapies
For the first time, patients with advanced or progressive MTC may benefit from systemic therapies that modulate and inhibit molecular pathways involved in cell activation, division, migration and survival (Figure 1). Prospective clinical trials have demonstrated that the antiangiogenic targeted inhibitors, such as vandetanib and cabozantinib, provide clinical benefit to these patients. While several drugs have been studied for MTC [8,29], only vandetanib and cabozantinib are currently approved by the FDA and should be used in the first-line setting [12–14]. Owing to the indolent nature of MTC, only a subpopulation of patients requires systemic therapy. Accepted indications for systemic therapy, listed in the left column of Table 2, include progressive disease (determined by RECIST), symptomatic disease that cannot be palliated with localized therapy or medication and disease that threatens organ function. Other indications that may be considered are listed in the right column of Table 2, but consultation with physicians with expertise in managing MTC is recommended if these indications but not those in the left column are present. Typically, once patients are started on a systemic therapy, they will continue treatment until it is no longer effective or they experience intolerable toxic effects that cannot be alleviated by dose reductions or other treatments. Except in the case of certain contraindications for vandetanib, there is currently no consensus on which drug should be used first. Importantly, response to cabozantinib treatment is observed following failed vandetanib treatment [30].
Table 2.
Indications for initiating systemic therapy in patients with medullary thyroid cancer.
| Start systemic therapy if any of the following are met: | Select cases meeting one of the following may be considered for treatment; clinical judgment indicated: |
|---|---|
| Evidence of clinically significant disease† progression defined as: •RECIST measurable disease, AND •Progressive disease (RECIST) within 12-14 months |
Calcitonin or CEA doubling time <6 months AND: •Structural evidence of clinically significant disease† but no serial imaging to document progression is available AND •Disease cannot be managed with local therapy/surgery |
| Symptomatic tumor burden:Tumor burden cannot be managed with local therapy (e.g., cryoablation, embolization, external beam radiation, surgical resection) | Severe, intractable MTC-related diarrhea or Cushing syndrome AND: •Symptomatic/other medical treatment is ineffective AND •Structural evidence of clinically significant disease† |
| Tumor involvement threatens organ function or vital structures and cannot be managed with local therapy |
Clinically significant disease is defined as disease measuring at least 1.5 cm or greater.
CEA: Carcinoembryonic antigen; MTC: Medullary thyroid carcinoma; RECIST: Response Evaluation Criteria In Solid Tumors.
Vandetanib
Vandetanib is a multi-TKI of RET (IC50 100 nM), VEGFR2 (VEGFR2; IC50 40 nM) and EGFR (EGFR; IC50 500 nM) (Figure 1). Like RET, VEGFR2 and EGFR are expressed on MTC cells, and VEGF is a well-established key mediator of signaling in tumor angio-genesis [31]. Vandetanib was approved by the FDA in 2011. The standard starting dose is 300 mg daily. Two Phase II clinical trials evaluated patients with hereditary MTC, one using a dosage of 100 mg and one with a dosage of 300 mg [32,33]. Of patients treated with vandetanib at 300 mg daily, 20% had a partial response and 53% had stable disease. Responses did not correlate with the presence of an underlying RET mutation. These findings led to a large, multicenter, randomized controlled Phase III clinical trial for patients with MTC measurable by RECIST (ZETA trial). This study included 331 patients who were randomized in a 2:1 ratio to vandetanib or placebo with crossover. Those treated with vandetanib had a significantly longer estimated progression-free survival (30.5 months) than those in the placebo group (19.3 months). The overall response rates were 45% in the vandetanib group and 13% in the placebo group [14]. All responses were partial; no complete responses were reported. An ongoing Phase IV trial is currently comparing 300 mg daily with 150 mg daily to understand the impact of dosing on tumor response (NCT01496313).
QTc prolongation was observed in 14% of the patients treated with vandetanib [14]. Thus, it is important to elicit a history of congenital QT prolongation, assess ECGs routinely in patients treated with vandetanib and to avoid medications that may prolong the QT interval. Hypocalcemia, hypokalemia, hypomagnesemia and hypothyroidism should be corrected before vandetanib is initiated. Participation in the risk evaluation and mitigation strategies program is mandatory in order to prescribe this drug.
Cabozantinib
Like vandetanib, cabozantinib is a multi-TKI of RET (IC50 4.5 nM) and VEGFR2 (IC50 0.035 nM) and c-MET as well (IC50 1.8 nM) (Figure 1). MET is expressed on tumor cells and endothelial cells and mediates hepatocyte growth factor signaling, leading to increased cell motility, proliferation and survival, and is upregulated in a wide range of malignancies, including MTC [34]. Cabozantinib was assessed against MTC in a Phase I clinical trial for patients with solid tumors. Of 35 patients with measurable and progressive MTC in this trial, 29% had partial responses [30]. Subsequently, a blind, multicenter, randomized, controlled Phase III trial (EXAM trial) enrolling 330 patients with MTC compared cabozantinib with a placebo. Patients were randomized to cabozantinib or placebo in a 2:1 distribution. All patients had had radiographic disease progression within 14 months before randomization. The primary endpoint was progression-free survival and the secondary end points were overall response rate and overall survival time. The median progression-free survival was significantly longer in the cabozantinib-treated patients (11.2 months) than in the placebo group (4.0 months), with partial response rates of 28% in cabozantinib-treated patients and 0% in placebo-treated patients. Responses were observed regardless of RET mutation status and correlations of radiologic response were made with changes in calcitonin and CEA levels from baseline [12]. As with vandetanib, no complete responses were reported. On the basis of these favorable trial results, cabozantinib was approved in 2012 at a starting dose of 140 mg daily; however, those experienced with the use of the drug rarely start at the recommended dose owing to its excessive toxicity (discussed in the next section).
The vandetanib (ZETA) and cabozantinib (EXAM) trials had some important differences. The EXAM trial required that enrollees demonstrate progression by RECIST within 14 months prior to study entry, whereas the ZETA trial included patients who had stable disease at baseline. Also, owing to its secondary objective of measuring overall survival, the EXAM trial did not allow crossover to active drug treatment if a patient on placebo demonstrated progression, whereas the ZETA trial did allow crossover.
Monitoring & management of AEs
The toxicities associated with TKIs require vigilant monitoring and management of AEs. Since targeted therapies can have life-threatening AEs, only physicians with the support and expertise required for managing these patients should prescribe them. Before initiating treatment with a TKI, a detailed history should be taken and a physical examination laboratory tests and EKG evaluation should be performed. Only patients with an adequate performance status (i.e., an Eastern Cooperative Oncology Group performance status of 2 or less) should be considered for systemic therapy. Otherwise, palliative treatments or hospice care should be considered. Our standard of care includes an AE assessment form [35] to document baseline symptoms and any new and ongoing AEs throughout the treatment period. Before starting treatment with vandetanib or cabozantinib, patients should sign an informed consent form and be instructed on the risks, common AEs and symptoms of serious AEs.
In general, most grade 1 AEs can be managed symptomatically and the dose continued, whereas most grade 3 AEs require treatment interruption and dose reduction. The management of grade 2 AEs is less straightforward and depends on the type of AE and whether the patient considers the AE to be tolerable (e.g., tolerable grade 2 fatigue does not require treatment interruption or dose reduction). Table 3 gives recommendations for the management of common AEs of vandetanib and cabozantinib. In general, grade 4 AEs require drug discontinuation [36,37].T
Table 3.
Dose interruption and modifications guidelines for common adverse events related to cabozantinib & vandetanib (defined by common terminology criteria for adverse events).
| Grade | Hypertension | Diarrhea† | Dermatologic AEs (acne, drug rash, HFSR) | Fatigue, weight loss | Proteinuria (confirmed by 24-h urinalysis) | QTcF prolongation | Liver transaminase elevation | Decreased potassium, magnesium, corrected calcium | Hematologic AEs (neutropenia, thrombocytopenia, anemia)‡ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | No dose change; manage hypertension | No dose change; manage diarrhea | No dose change; manage symptoms | No dose change | (<1 g/24 h); no dose change; monitor | (450-480 ms); monitor closely | No dose change; monitor labs | No dose change; correct abnormalities | No dose change |
| 2 | 1st occurrence: continue drug; manage hypertension | Tolerable: manage diarrhea | 1st occurrence: hold drug until grade ≤1; manage symptoms; resume at same dose level | Tolerable: manage symptoms | (1-3.4 g/24 h); hold drug for 1 week; manage proteinuria medically, then repeat 24-h urinalysis | (481-500 ms); monitor closely; correct electrolyte levels | Monitor labs closely; search for other causes; −1 dose reduction may be considered | Correct abnormalities | No dose change |
| 2nd or 3rd occurrence: hold drug; manage hypertension; resume drug at −1 dose level | Intolerable: 1st occurrence: hold drug, manage diarrhea, resume at same dose level 2nd occurrence: hold drug, manage diarrhea, resume at −1 dose level | 2nd occurrence: hold drug; manage symptoms; resume at −1 dose level | Intolerable: hold drug; resume at −1 dose level when grade ≤1 | If <1 g/24 h, resume drug at same dose level If >1 g/24 h, resume at −1 dose level | If caused by diarrhea: control diarrhea; consider a −1 dose reduction upon correction of abnormality | ||||
| 3 | Hold drug; manage hypertension; resume at −1 dose level | Hold drug, manage diarrhea, resume at −1 dose level | 1st or 2nd occurrence: hold drug; manage symptoms; resume at −1 dose level | Hold drug; manage symptoms; resume at −1 dose level when grade ≤1 | (≥3.5 g/24 h); hold drug for at least 1 week; manage proteinuria medically; repeat 24 h urinalysis | (≥500 ms); hold drug until QTcF normalizes; review electrolytes and medication list; resume drug at −1 dose level when QTc ≤450 ms | Hold drug; search for other causes; resume at −1 dose level when labs decrease to grade ≤2 | Hold drug; correct abnormalities | Dose reduction for neutropenia and thrombocytopenia |
| 3rd occurrence: consider discontinuation | If <1 g/24 h, resume at −1 dose level. If persistently >1 g/24 h, consider discontinuation | If caused by diarrhea: control diarrhea and resume at −1 dose level | |||||||
| If caused by non-compliance: consider discontinuation of drug | |||||||||
| 4 | Discontinue drug unless adequate control can be achieved: consider reduction of -2 dose levels under close supervision | Discontinue drug | Discontinue drug | Discontinue drug | Discontinue drug | Discontinue drug | Discontinue drug | Dose reduction for anemia, neutropenia and thrombocytopenia |
Grade ≥2 diarrhea may cause electrolyte abnormalities and therefore, laboratory evaluations should be performed.
Grade 3-4 hematologic AEs should trigger a search for causes not related to the drug.
AE: Adverse event; HFSR: Hand-foot skin reaction; QTcF: Correct QT interval by Fridericia formula.
Constitutional AEs
Fatigue, weight changes and loss of appetite are the most common constitutional symptoms encountered with the use of cabozantinib and vandetanib. Patients with fatigue should have laboratory testing performed to assess thyroid function and hemoglobin level. MTC patients should maintain a normal thyroid-stimulating hormone (TSH) level. Other causes of fatigue to consider are sleep apnea, depression and anxiety, insomnia and restless leg syndrome. Often, a correctable cause of fatigue is not found. In these cases, patients should be instructed to exercise and take short naps once a day. Patients with severe fatigue may have some mild benefit from psychostimulants, but these should be given only under expert supervision [38].
Interestingly, vandetanib has been shown to commonly cause weight gain [39] for reasons that are not yet clear, whereas cabozantinib causes weight loss in approximately 48% of patients [40]. Managing weight changes can be difficult and depends on the initial weight of the patient. Patients who have 10–20% weight loss from their baseline (grade 2), but who started out with a high BMI may need only nutritional supplements, without treatment interruption. However, patients with grade 2 weight loss and a low initial BMI may need treatment interruption or dose reduction in addition to nutritional supplements. Other causes of weight loss should be considered. With grade 3 weight loss, drug discontinuation must be considered. The initial weight gain described in patients on vandetanib is usually bothersome to the patient but does not require any intervention. However, other causes of weight gain, such as hypothyroidism and congestive heart failure (CHF), should always be kept in mind.
Loss of appetite is another difficult AE to manage, and the causes may be multifactorial. Loss of appetite occurs in 43% of cabozantinib-treated patients [40] and 21% of vandetanib-treated patients [41]. Both drugs may cause nausea, vomiting or dyspepsia; therefore, patients should be asked if they have experienced these symptoms and their cases managed appropriately. Dysgeusia (distortion of taste) has been described primarily in patients on cabozantinib. Stomatitis occurs in 51% of cabozantinib-treated MTC patients [40] and can be associated with dysgeusia. Stomatitis manifests as mouth pain, which can include a burning sensation when eating certain foods (spicy, acidic and salty), and may lead to ulcerations. Patients with stomatitis should avoid irritating foods and mouth products. Most cases are treated symptomatically with mouth rinses, such as sodium bicarbonate, and with topical or systemic analgesics [42]. For severe cases of stomatitis (grade ≥3, interfering with oral intake), treatment interruption or dose reduction may be indicated.
Gastrointestinal AEs
Vandetanib causes diarrhea in 57% of patients and cabozantinib causes diarrhea in 63% of patients [40,41]. Common Terminology Criteria for Adverse Events grading of diarrhea is based on the number of stools per day compared with the baseline stools per day. Since MTC patients may have baseline diarrhea, the average number of stools per day should be documented prior to commencing a TKI. Diarrhea at baseline should be controlled if possible before a patient starts either TKI, but treating diarrhea is especially important before starting vandetanib since the ensuing electrolyte abnormalities may lead to QT interval prolongation and arrhythmia. Diarrhea related to TKI may be managed with dietary modifications and antidiarrheal medications, such as loperamide and atropine sulfate/ diphenoxylate hydrochloride. If necessary, these two antidiarrheals may be alternated every 2 h to maximize the treatment. Severe cases of diarrhea can be managed by adding opium tincture, which is widely used for this indication despite the lack of literature to support this use. Budesonide, a nonabsorbable glucocorticoid, may benefit patients with inflammation of the bowel, but it has not been studied in the setting of TKIs. Octreo-tide, a somatostatin analog, may also be used in severe cases of diarrhea.
Liver transaminase increases are frequent, are usually mild (grade 1) and do not require dose interruptions or modifications. Grade 2 elevations should prompt a search for other causes, such as viral hepatitis, autoimmune hepatitis, alpha-1 antitrypsin deficiency and biliary tract diseases. A history of alcohol use should be elicited and patients should be counseled to avoid alcohol. The medication list should be reviewed and patients should be asked if they are taking any over-the-counter drugs or herbal supplements.
Cardiovascular AEs
Hypertension is the most common AE associated with vandetanib and cabozantinib, occurring in 33% of patients [40]. Blood pressure should be controlled prior to starting any antiangiogenic therapy, with a goal of less than 140/90 mmHg. Although there are no clear guidelines regarding the management of antiangiogenic TKI-induced hypertension, our practice is to administer an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker or beta blocker as first-line therapy. Calcium channel blockers are CYP3A4 inhibitors and therefore should be used with caution with cabozantinib, since concomitant use of CYP3A4 inhibitor drugs may increase the plasma concentration of cabozantinib [36]. If the patient is already on an antihypertensive and not at maximum dose, increasing the dose before initiating a new antihypertensive is the preferred strategy. In cases of severe hypertension, the TKI should be held until the blood pressure is controlled and then the TKI should be restarted at a lower dose (Table 3).
CHF due to the antiangiogenic activity of vandetanib and cabozantinib is less common but is serious. Patients with dyspnea, fatigue or orthopnea should be evaluated for CHF. Patients with risk factors for CHF (history of myocardial infarction, coronary artery disease etc.) should undergo echocardiography at baseline and again if symptoms of CHF develop. Prompt interruption of the TKI and treatment of CHF are necessary to avoid irreversible damage to cardiomyocytes [36].
Vandetanib carries a black box warning for QT interval prolongation, torsades de pointes and sudden cardiac death [41]. Thus, it should not be used in patients with a history of congenital long QT syndrome or in patients with a corrected QT interval of more than 450 ms. Vandetanib may be used with extreme caution in patients with a history of QT prolongation and those taking antiarrhythmic drugs or drugs known to cause QT prolongation. Serial monitoring of the electrocardiogram and electrolytes is necessary. Because MTC patients may have preexisting diarrhea or hypoparathyroidism and because the drug itself may cause diarrhea, aggressive diarrhea management and correction of electrolyte abnormalities is warranted to avoid QT interval prolongation. Patients with risk factors for prolonged QT should be considered for cabozantinib instead of vandetanib if possible.
Dermatologic AEs
Hand–foot skin reaction (HFSR) is a common AE of cabozantinib, reported in 50% of MTC patients taking this drug [40]. The symptoms of HFSR can include tingling, erythema, edema, hyperkeratosis, desquamation and ulceration of the palmar surfaces of the hands or plantar surfaces of the feet. Aggressive management is important because these symptoms can become debilitating. Patients should be instructed to keep their skin moisturized and to notify their physician of early signs of HFSR. Trauma, pressure points and extreme temperatures on the hands and feet should be avoided since these may worsen the condition. Table 3 describes the dose modifications for HFSR. Keratosis on the pressure points of the feet, in our experience, is best managed by referral to a podia-trist to shave the callused areas of the skin. Topical creams with salicylic acid or urea may also decrease keratosis.
Acneiform rash is associated with EGFR inhibitors, including vandetanib, and occurs in 35% of vandetanib-treated MTC patients [41]. It affects cosmetically sensitive areas, may be painful and may reduce quality of life. The severity of the rash as perceived by the patient is often the driving force for determining whether to interrupt the treatment. A topical corticosteroid and either doxycycline or minocycline may be used in patients with grade ≥2 rash [43]. The dose of vandetanib should be reduced in patients with a second occurrence of grade 2 or first occur-rence of grade 3 acneiform rash. All patients should be instructed to protect their skin from the sun, since sun exposure may predispose them to acneiform rash. Referral to a dermatologist with experience treating rashes induced by EGFR inhibitors is advised for grade ≥3 acneiform rash.
Drug rash is more common with vandetanib but can occur with cabozantinib. The rash is usually a maculopapular rash and may be pruritic. The rash may be managed with topical lotions or a short course of topical steroids and antihistamines. Patients with severe rashes should be questioned about fever, sore throat and ulcerations in the mucous membranes, which may indicate Stevens–Johnson syndrome. If this syndrome is suspected, the patient should discontinue the drug immediately and seek medical help.
Photosensitivity has been reported in up to 37% of patients on vandetanib [41,44]. Patients on vandetanib should be instructed to avoid or protect from sun exposure.
Hematologic AEs
Grade 1–2 thrombocytopenia is seen in 35% of cabozantinib-treated patients [40] but only 9% of vandetanib-treated MTC patients [41] and, in general, does not require a dose interruption or modification. Grade 3–4 thrombocytopenia has not been reported with either drug. Neutropenia has been reported in 35% of cabozantinib-treated MTC patients and in 10% of vandetanib-treated MTC patients [40,41]. Grade 3–4 neutropenia is rare, but if it occurs, should be managed with a dose reduction. Anemia is not a common AE in vandetanib- or cabozantinb-treated patients, likely because of the relative increase in erythrocyte levels with antiangiogenic drugs. Thus, the development of anemia while on an antiangiogenic drug should prompt an evaluation for secondary causes [36]. Cabozantinib carries a black box warning for hemorrhage, including hemoptysis and gastrointestinal hemorrhage in up to 3% of treated patients. Serious hemorrhagic events have also been reported with vandetanib. These drugs should be discontinued in patients with severe hemorrhagic events.
Hypothyroidism
Hypothyroidism or elevated TSH are common findings in vandetanib- and cabozantinib-treated patients. In the cabozantinib trials, 57% of patients had elevated TSH [40]. In the ZETA trial, thyroid hormone increases were necessary in 49% of vandetanib-treated patients but only 17% of placebo-treated patients [41]. TSH should be monitored in patients on either drug, but the guidelines for vandetanib require monitoring at baseline, 2–4 weeks and 8–12 weeks after starting treatment, and every 3 months thereafter. TSH should be maintained in the normal range in patients with MTC.
Rare but serious AEs
Rare but serious AEs require discontinuation of the offending drug. Both vandetanib and cabozantinib are associated with reversible posterior leukoencephalopathy syndrome, a syndrome of subcortical vasogenic edema that is seen on MRI of the brain. Patients present with seizures, headaches, visual disturbances, confusion or altered mental status. Hypertensive crisis may precede the neurologic symptoms.
In addition to Stevens–Johnson syndrome and CHF, other rare AEs associated with vandetanib are interstitial lung disease and ischemic cerebrovascular events. Cabozantinib is associated with thromboembolic events, osteonecrosis of the jaw and poor wound healing and carries a black box warning for perforations and fistulas. Gastrointestinal perforations occurred in 3% of cabozantinib-treated patients. A thorough history should include questions regarding any history of diverticulitis, chronic inflamma-tory intestinal disease and peptic ulcer disease, which could predispose patients to gastrointestinal perforations. Tracheoesophageal fistulas occurred in 1% of cabozantinib-treated patients. Invasion of the tumor into the trachea, esophagus or bronchi may increase the risk of tracheoesophageal fistulas and should be a consideration when starting patients on cabozantinib [45]. Patients should be warned of the risk of poor wound healing and advised to notify their physician of any elective, nonurgent surgery because cabozantinib treatment should be held prior to surgical intervention.
Monitoring for response to systemic therapy
Once a patient with MTC is on systemic chemotherapy, radiologic surveillance of areas of disease with cross-sectional imaging should be performed every 2–3 months to establish tumoral response to therapy. In our practice, we use RECIST guidelines to determine response to therapy (Table 1). Patients with a ≥30% decrease in the sum of target lesions are considered to have a partial response, while a ≥20% increase or new lesions represent progression of disease. Anything in between is stable disease.
Monitoring tumor markers alone is not sufficient and may actually be misleading because calcitonin and CEA levels occasionally rise or decline without relation to tumor response and their expression levels can be directly regulated by treatment (Figure 1). Bone scan and FDG-PET may be useful to monitor for new lesions, but should not be used to determine response to therapy.
Conclusion & future perspective
Standard therapy for primary MTC is total thyroidectomy with central lymph node dissection; unfortunately, a significant number of patients already have cervical lymph node metastases at the time of initial diagnosis, which increases the risk of residual disease and distant disease after surgical resection. MTC with distant metastases remains incurable.
However, the characterization of receptors and molecular pathways responsible for MTC development has led to the discovery of molecular targeted therapies for advanced MTC that reduce tumor size, stabilize disease and improve symptoms. In the last 3 years, two drugs – vandetanib and cabozantinib – have received regulatory approval for use in patients with progressive or symptomatic MTC that is not amenable to other treatments. Although these drugs offer long-awaited treatment options for patients with advanced MTC, neither has led to clinical remission of MTC. For patients with progressive disease who are unable to take one of these drugs (e.g., patients who are not candidates for treatment owing to comorbid conditions, failed or did not tolerate drug in the past, or who do not have access to these drugs), the standard recommendation is to refer the patient to a clinical trial. Further investigation is needed to understand which MTC patients would benefit most from targeted therapy, considering the significant toxicity profiles of these drugs, the need for chronic use of these drugs, the lack of survival benefit from the clinical data available to date [37]. Combination therapies may lead to greater effectiveness in targeting this disease. On the bright side, the past decade of basic science and clinical research has demonstrated great advancements in understanding the pathogenesis of MTC that may be instrumental in reaching the goal of a cure.
Executive summary.
Two tyrosine kinase inhibitors, vandetanib and cabozantinib, have been approved for the treatment of progressive, metastatic or unresectable medullary thyroid cancer (MTC).
The decision to treat with these inhibitors currently occurs independent of the diagnosis of hereditary or sporadic MTC.
Owing to the indolent nature of most MTC cases, only patients with clear evidence of progressive disease by imaging should be treated with these systemic therapies.
Serial measurements of calcitonin and carcinoembryonic antigen levels are useful to determine progression but should not be used as the sole criteria for treatment initiation.
Given their toxicity profiles and the need for chronic use, administration of these drugs should be managed by a physician experienced with their use.
Treatment of MTC, especially in the age of targeted therapy, requires a multidisciplinary approach.
Acknowledgements
The authors would like to thank Sarah Bronson, associate scientific editor from the department of scientific publications at MD Anderson Cancer Center for her editorial assistance.
ME Cabanillas has served on the Exelixis advisory board and has received research funding and consulting fees from Exelixis. MI Hu has received research funding from AstraZeneca Pharmaceuticals. Supported by the NIH/NCI under award number P30CA016672. C Jiminez, EG Grubbs and GJ Coter have nothing to disclose. The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant CA16672. Supported by the NIH/NCI under award number P30CA016672.
Footnotes
Financial & competing intrests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.The American Cancer Society Thyroid Cancer. 2014 www.cancer.org/acs/groups/cid/documents/webcontent/003144-pdf.pdf.
- 2.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. 2013 http://globocan.iarc.fr.
- 3•.Donis-Keller H, Dou S, Chi D, et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum. Mol. Genet. 1993;2(7):851–856. doi: 10.1093/hmg/2.7.851. [Taken together with [4,5], describes the initial association of RET activation as the cause of medullary thyroid carcinoma (MTC).] [DOI] [PubMed] [Google Scholar]
- 4•.Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367(6461):375–376. doi: 10.1038/367375a0. [Taken together with [3,5], describes the initial association of receptor tyrosine kinase (RET) activation as the cause of MTC.] [DOI] [PubMed] [Google Scholar]
- 5•.Mulligan LM, Kwok JB, Healey CS, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363(6428):458–460. doi: 10.1038/363458a0. [Taken together with [3,4], describes the initial association of RET activation as the cause of MTC.] [DOI] [PubMed] [Google Scholar]
- 6••.American Thyroid Association Guidelines Task Force. Kloos RT, Eng C, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19(6):565–612. doi: 10.1089/thy.2008.0403. [Key guidelines recognized for treatment of MTC.] [DOI] [PubMed] [Google Scholar]
- 7•.Waguespack SG, Rich TA, Perrier ND, Jimenez C, Cote GJ. Management of medullary thyroid carcinoma and MEN2 syndromes in childhood. Nature Rev. Endocrinol. 2011;7(10):596–607. doi: 10.1038/nrendo.2011.139. [Taken together with [8], provides an excellent resource for the treatment of hereditary MTC.] [DOI] [PubMed] [Google Scholar]
- 8•.Wells SA, Jr, Pacini F, Robinson BG, Santoro M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J. Clin. Endocrinol. Metab. 2013;98(8):3149–3164. doi: 10.1210/jc.2013-1204. [Taken together with [7], provides an excellent resource for the treatment of hereditary MTC.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bamford S, Dawson E, Forbes S, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br. J. Cancer. 2004;91(2):355–358. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eng C, Smith DP, Mulligan LM, et al. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum. Mol. Genet. 1994;3(2):237–241. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- 11.Wohllk N, Cote GJ, Bugalho MM, et al. Relevance of RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 1996;81(10):3740–3745. doi: 10.1210/jcem.81.10.8855832. [DOI] [PubMed] [Google Scholar]
- 12••.Elisei R, Schlumberger MJ, Muller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013;31(29):3639–3646. doi: 10.1200/JCO.2012.48.4659. [Taken together with [13,14], describes the key Phase III clinical trials that led to US FDA approval of vandetanib and cabozantinib..] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Thornton K, Kim G, Maher VE, et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. 2012;18(14):3722–3730. doi: 10.1158/1078-0432.CCR-12-0411. [Taken together with [12,14], describes the key Phase III clinical trials that led to US FDA approval of vandetanib and cabozantinib.] [DOI] [PubMed] [Google Scholar]
- 14••.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 2012;30(2):134–141. doi: 10.1200/JCO.2011.35.5040. [Taken together with [12,13], describes the key Phase III clinical trials that led to US FDA approval of vandetanib and cabozantinib..] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boostrom SY, Grant CS, Thompson GB, et al. Need for a revised staging consensus in medullary thyroid carcinoma. Arch. Surg. 2009;144(7):663–669. doi: 10.1001/archsurg.2009.122. [DOI] [PubMed] [Google Scholar]
- 16.Kandil E, Gilson MM, Alabbas HH, Tufaro AP, Dackiw A, Tufano RP. Survival implications of cervical lymphadenectomy in patients with medullary thyroid cancer. Ann. Surg. Oncol. 2011;18(4):1028–1034. doi: 10.1245/s10434-010-1363-y. [DOI] [PubMed] [Google Scholar]
- 17.Machens A, Dralle H. Prognostic impact of N staging in 715 medullary thyroid cancer patients: proposal for a revised staging system. Ann. Surg. 2013;257(2):323–329. doi: 10.1097/SLA.0b013e318268301d. [DOI] [PubMed] [Google Scholar]
- 18.American Thyroid Association Surgery Working Group, American Academy of Otolaryngology–Head and Neck Surgery et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19(11):1153–1158. doi: 10.1089/thy.2009.0159. [DOI] [PubMed] [Google Scholar]
- 19.Edge SB. American Joint Committee on Cancer. AJCC cancer staging manual. 7th Edition. Springer; NY, USA: 2010. [DOI] [PubMed] [Google Scholar]
- 20.Machens A, Holzhausen HJ, Dralle H. Contralateral cervical and mediastinal lymph node metastasis in medullary thyroid cancer: systemic disease? Surgery. 2006;139(1):28–32. doi: 10.1016/j.surg.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Machens A, Schneyer U, Holzhausen HJ, Dralle H. Prospects of remission in medullary thyroid carcinoma according to basal calcitonin level. J. Clin. Endocrinol. Metab. 2005;90(4):2029–2034. doi: 10.1210/jc.2004-1836. [DOI] [PubMed] [Google Scholar]
- 22.Ong SC, Schoder H, Patel SG, et al. Diagnostic accuracy of 18F-FDG PET in restaging patients with medullary thyroid carcinoma and elevated calcitonin levels. J. Nucl. Med. 2007;48(4):501–507. doi: 10.2967/jnumed.106.036681. [DOI] [PubMed] [Google Scholar]
- 23.Barbet J, Campion L, Kraeber-Bodere F, Chatal JF. GTE Study Group. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2005;90(11):6077–6084. doi: 10.1210/jc.2005-0044. [DOI] [PubMed] [Google Scholar]
- 24.Laure Giraudet A, Al Ghulzan A, Auperin A, et al. Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur. J. Endocrinol. 2008;158(2):239–246. doi: 10.1530/EJE-07-0667. [DOI] [PubMed] [Google Scholar]
- 25.Meijer JA, Le Cessie S, Van Den Hout WB, et al. Calcitonin and carcinoembryonic antigen doubling times as prognostic factors in medullary thyroid carcinoma: a structured meta-analysis. Clin. Endocrinol. 2010;72(4):534–542. doi: 10.1111/j.1365-2265.2009.03666.x. [DOI] [PubMed] [Google Scholar]
- 26.American Thyroid Association www.thyroid.org.
- 27.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Dadu R, Cabanillas ME. Optimizing therapy for radioactive iodine-refractory differentiated thyroid cancer: current state of the art and future directions. Minerva Endocrinol. 2012;37(4):335–356. [PMC free article] [PubMed] [Google Scholar]
- 29.Regalbuto C, Frasca F, Pellegriti G, et al. Update on thyroid cancer treatment. Future Oncol. 2012;8(10):1331–1348. doi: 10.2217/fon.12.123. [DOI] [PubMed] [Google Scholar]
- 30.Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J. Clin. Oncol. 2011;29(19):2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Antona C, Pallares J, Montero-Conde C, et al. Overexpression and activation of EGFR and VEGFR2 in medullary thyroid carcinomas is related to metastasis. Endocr. Relat. Cancer. 2010;17(1):7–16. doi: 10.1677/ERC-08-0304. [DOI] [PubMed] [Google Scholar]
- 32•.Robinson BG, Paz-Ares L, Krebs A, Vasselli J, Haddad R. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J. Clin. Endocrinol. Metab. 2010;95(6):2664–2671. doi: 10.1210/jc.2009-2461. [Demonstrates the role of vandetanib dose reduction in treatment of MTC.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells SA, Jr, Gosnell JE, Gagel RF, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J. Clin. Oncol. 2010;28(5):767–772. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papotti M, Olivero M, Volante M, et al. Expression of hepatocyte growth factor (HGF) and its receptor (MET) in medullary carcinoma of the thyroid. Endocr. Pathol. 2000;11(1):19–30. doi: 10.1385/ep:11:1:19. [DOI] [PubMed] [Google Scholar]
- 35••.Carhill AA, Cabanillas ME, Jimenez C, et al. The noninvestigational use of tyrosine kinase inhibitors in thyroid cancer: establishing a standard for patient safety and monitoring. J. Clin. Endocrinol. Metab. 2013;98(1):31–42. doi: 10.1210/jc.2012-2909. [Taken together with [35,36], establishes key practice concerns associated with treating MTC patients with tyrosine kinase inhibitors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Cabanillas ME, Hu MI, Durand JB, Busaidy NL. Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. J. Thyroid Res. 2011;2011:985780. doi: 10.4061/2011/985780. [Taken together with [34,36], establishes key practice concerns associated with treating MTC patients with tyrosine kinase inhibitors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Viola D, Cappagli V, Elisei R. Cabozantinib (XL184) for the treatment of locally advanced or metastatic progressive medullary thyroid cancer. Future Oncol. 2013;9(8):1083–1092. doi: 10.2217/fon.13.128. [Taken together with [34,35], establishes key practice concerns associated with treating MTC patients with tyrosine kinase inhibitors.] [DOI] [PubMed] [Google Scholar]
- 38.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J. Natl Cancer Inst. 2008;100(16):1155–1166. doi: 10.1093/jnci/djn250. [DOI] [PubMed] [Google Scholar]
- 39.Massicotte MH, Borget I, Broutin S, et al. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo-controlled study. J. Clin. Endocrinol. Metab. 2013;98(6):2401–2408. doi: 10.1210/jc.2013-1115. [DOI] [PubMed] [Google Scholar]
- 40.Cometriq (Cabozantinib) prescribing information . Exelixis, Inc.; CA, USA: www.cometriq.com/downloads/Cometriq_Full_Prescribing_Information.pdf. [Google Scholar]
- 41.Lp AP. Caprelsa (vandetanib) prescribing information. AstraZeneca Pharmaceuticals; LP, DE, USA: www.astrazeneca-us.com/pi/caprelsa.pdf. [Google Scholar]
- 42.Joensuu H, Trent JC, Reichardt P. Practical management of tyrosine kinase inhibitor-associated side effects in GIST. Cancer Treat. Rev. 2011;37(1):75–88. doi: 10.1016/j.ctrv.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Balagula Y, Lacouture ME, Cotliar JA. Dermatologic toxicities of targeted anticancer therapies. J. Support. Oncol. 2010;8(4):149–161. [PubMed] [Google Scholar]
- 44.Giacchero D, Ramacciotti C, Arnault JP, et al. A new spectrum of skin toxic effects associated with the multikinase inhibitor vandetanib. Arch. Dermatol. 2012;148(12):1418–1420. doi: 10.1001/2013.jamadermatol.192. [DOI] [PubMed] [Google Scholar]
- 45.Blevins DP, Dadu R, Hu M, et al. Aerodigestive fistula formation as a rare side effect of antiangiogenic tyrosine kinase inhibitor therapy for thyroid cancer. Thyroid. 2014;24(5):918–922. doi: 10.1089/thy.2012.0598. [DOI] [PMC free article] [PubMed] [Google Scholar]