Abstract
Neuronal activity is communicated to the cerebral vasculature so that adequate perfusion of brain tissue is maintained at all levels of neuronal metabolism. An increase in neuronal activity is accompanied by vasodilation and an increase in local cerebral blood flow. This process, known as neurovascular coupling (NVC) or functional hyperemia, is essential for cerebral homeostasis and survival. Neuronal activity is encoded in astrocytic Ca2+ signals that travel to astrocytic processes (‘endfeet’) encasing parenchymal arterioles within the brain. Astrocytic Ca2+ signals cause the release of vasoactive substances to cause relaxation, and in some circumstances contraction, of the smooth muscle cells (SMCs) of parenchymal arterioles to modulate local cerebral blood flow. Activation of potassium channels in the SMCs has been proposed to mediate NVC. Here, the current state of knowledge of NVC and potassium channels in parenchymal arterioles is reviewed.
Keywords: Arterial smooth muscle, Astrocytes, Neurovascular coupling, Parenchymal arterioles, Potassium channels
Maintenance of cerebral homeostasis requires dynamic regulation of oxygen and glucose supply so as to match nutrient delivery to metabolic demand.1 This is achieved through extensive and precise regulation of blood flow within the brain. In the normally functioning brain, increases in neuronal activity are accompanied by rapid, spatially localized increases in blood flow that serve to avoid the development of ischemic conditions in active regions of the brain.2–6 This coupling of increased neuronal activity to increased local cerebral blood flow (CBF), a process known as functional hyperemia or neurovascular coupling (NVC), occurs as a result of vasodilation at the level of the cerebral microcirculation and appears to depend on the generation of vasoactive substances.1 Abnormalities associated with cerebral microcirculatory function have been associated with a number of disorders, including Parkinson’s disease, stroke, and migraine.7 In addition, chronically insufficient CBF is an end result of most risk factors associated with neurodegenerative diseases such as Alzheimer’s disease and early onset dementia.8 Thus, elucidating the mechanisms by which neuronal activity regulates intracerebral arterioles is critical to the development of new targets and effective therapies for pathological conditions associated with cerebral microcirculatory dysfunction. Potassium (K+) channels play a central role in NVC and represent one such target for future therapeutics. The purpose of this review is to examine putative mechanisms of NVC and the role of K+ channels in this process.
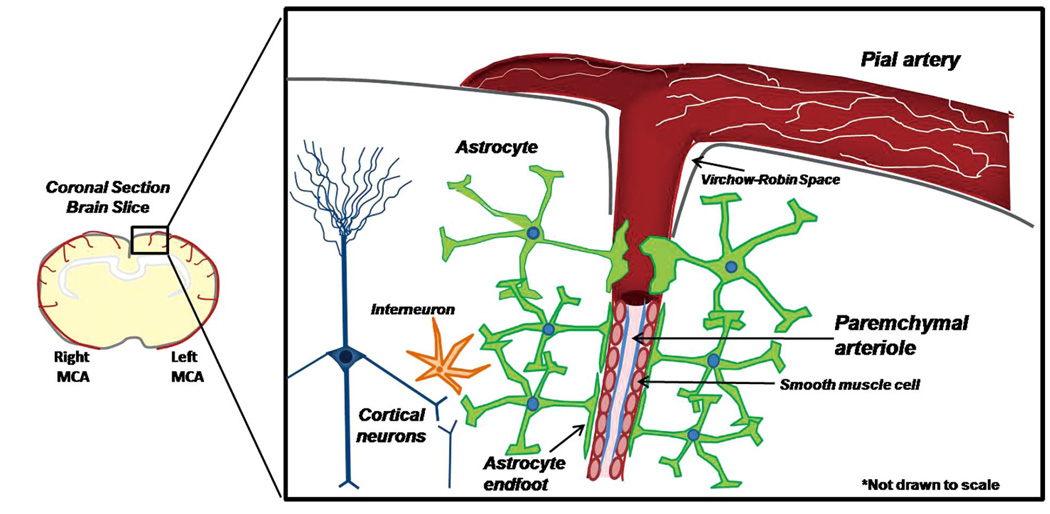
Blood flow to the brain is first conducted through a series of pial arteries on the surface of the brain, then through intracerebral arteries and arterioles within the brain tissue. Intracerebral (parenchymal) arterioles arise from branches of pial arteries that penetrate from the surface of the brain into the parenchyma (Figure 1).9 Parenchymal arterioles are structurally and functionally distinct from pial arteries. Within the cerebral cortex, these arterioles begin immediately distal to the Virchow-Robin space, are encased in astrocytic processes called ‘endfeet’, and terminate as an extensive capillary network. Parenchymal arterioles lack the extrinsic innervation of larger pial arteries, with primary regulation arising from astrocytes and neurons of central origin.2,10,11
Figure 1.
Pial arteries on the surface of the brain penetrate into the cortical parenchyma to give rise to parenchymal arterioles. Pial arteries are extrinsically innervated by sympathetic nerves (shown in white). As the arteriole penetrates into the brain parenchyma beyond the Virchow-Robin space, extrinsic innervation is lost and the arteriole becomes completely encased in astrocytic terminal processes called ‘endfeet’. Astrocytes integrate information from neurons and other cell types (ie, interneurons) and translate that information into dynamic astrocytic Ca2+ signals that propagate to the endfoot and regulate parenchymal arteriolar tone in order to regulate local cerebral blood flow according to the metabolic needs of the surrounding tissue.
Recent evidence suggests that astrocytes play a significant role in the regulation of local CBF.2,7,12–15 An individual astrocyte has numerous processes that surround multiple synapses, and thus forms a tripartite synapse with pre-and post-synaptic neurons.16 Astrocytes also possess additional processes that target the cerebral microcirculation. These astrocytic processes terminate as perivascular endfeet that encase smooth muscle cells (SMCs) of parenchymal arterioles and capillaries (Figure 1).2,12–15,17 This astrocytic architecture suggests that an individual astrocyte might be capable of integrating the activity of multiple neurons and translating this information into physiological signals, including those that regulate CBF by altering the vascular tone of parenchymal arterioles. Indeed, recent evidence points to a central role for astrocytes in functional hyperemia in the brain. Neurons themselves also release a number of important vasoactive substances, including nitric oxide, adenosine, and neurotransmitters; however, the potential role of these neuronal mediators in NVC is beyond the scope of the current review.7,18
Astrocytes act as vital regulators of neuronal function, serving to modulate extracellular potassium (K+) concentration ([K+]o) and extracellular volume, and remove neurotransmitters from synapses.19–21 Astrocytes possess numerous receptors that could be activated by neurotransmitters.22–24 Activation of metabotropic glutamate receptors (mGluRs) located on astrocytic projections that surround synapses of glutamatergic neurons results in an increase in cytosolic calcium concentration ([Ca2+]c) in the soma, which then propagates through astrocytic processes, ultimately resulting in a [Ca2+]c increase in the endfoot (Figure 2).25,26 These [Ca2+]c increases likely occur through activation of the phospholipase C/inositol trisphosphate (InsP3) cascade, and involve release of endoplasmic reticulum Ca2+ through InsP3 receptors and possibly ryanodine receptors.26,27 Zonta et al elegantly showed that the dilation of cortical arterioles is dependent on astrocytic [Ca2+]c increases induced by glutamate activation of mGluRs.14 Thus, the release of vasoactive substances from astrocytes in response to neuronal activation requires elevation of endfoot [Ca2+], although the mechanisms underlying the generation of these signals and the nature of these mediators are poorly understood.12–14
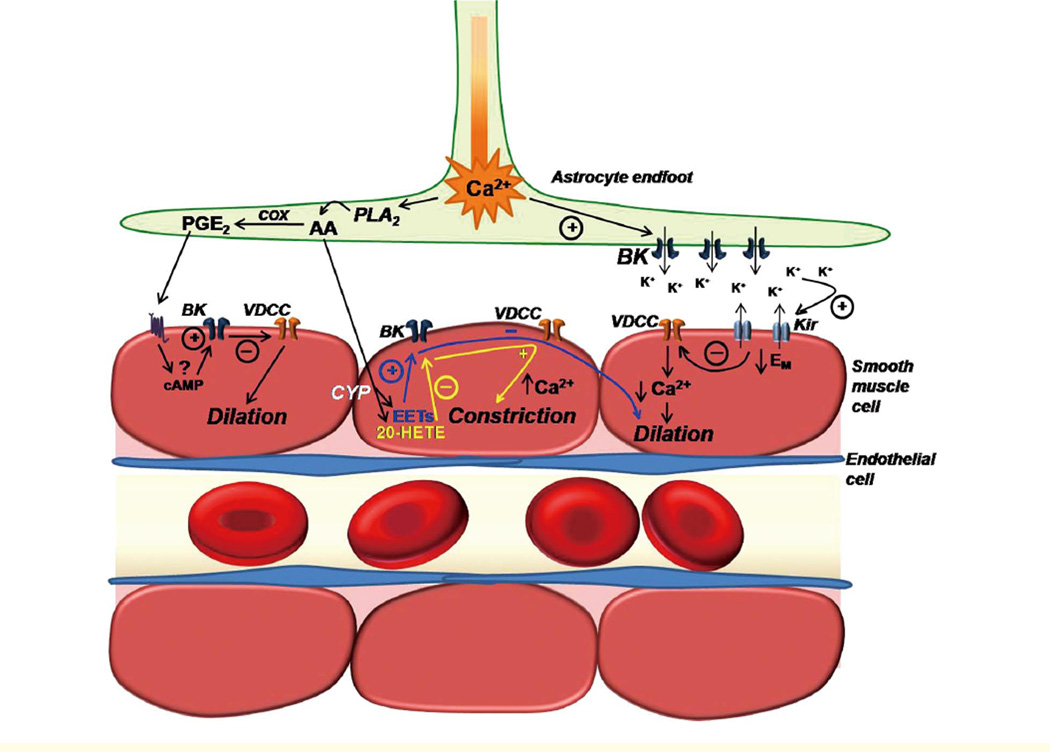
Figure 2.
Illustration depicting the involvement of parenchymal arteriolar smooth muscle cell (SMC) K+ channels in putative mechanisms of neurovascular coupling. Neuronal activity stimulates astrocytic metabotropic glutamate receptors (not shown) to produce a propagating rise in [Ca2+]i that terminates in perivascular endfeet. Increased astrocytic endfoot [Ca2+] activates BK channels to release K+ into the perivascular cleft. Moderate elevations in [K+]o in the perivascular cleft activate Kir channels in parenchymal arteriole SMCs, resulting in SMC membrane potential hyperpolarization, decreased Ca2+ entry through VDCCs, decreased [Ca2+]i, and vasodilation. Increased astrocytic endfoot [Ca2+] also activates the Ca2+ sensitive enzyme cytosolic phoshpolipase A2 (PLA2). PLA2 hydrolyzes membrane phospholipids to release the fatty acid arachidonic acid (AA). AA is then metabolized by cyclooxygenase (COX) and prostaglandin synthases to yield PGE2, or is believed to diffuse to the parenchymal arteriole SMC, where it is metabolized by cytochrome P-450 enzymes to generate EETs and/or 20-HETE. The mechanism of action of PGE2 on parenchymal arteriole SMCs is unknown, but based on studies done in pial arteries, it might involve cAMP-dependent activation of SMC BK channels, and subsequent SMC hyperpolarization and vasodilation. EETs formed in arteriolar SMCs by the metabolism of astrocytederived AA are proposed to activate SMC BK channels to elicit membrane hyperpolarization and vasodilation. Conversely, 20-HETE is believed to inhibit parenchymal arteriolar SMC BK channels resulting in membrane depolarization, activation of VDCCs, elevation of [Ca2+]i, and vasoconstriction.
Astrocytic Signals and NVC
Several signals based on arachidonic acid (AA) metabolism have been proposed to modulate vessel diameter in response to neuronal activity (Figure 2).12,14,28,29 Glutamate activation of astrocytic mGluRs leads to the Ca2+-dependent stimulation of phospholiase A2 (PLA2) and production of AA, which is metabolized to epoxyeicosatrienoic acid (EETs) by the action of cytochrome P450 (CYP) epoxygenases, with 11,12-EET and 14,15-EET being major vasoactive components.30–32 EETs, which are potent vasodilators, have recently been implicated in suppressing vasomotion and maintaining vasodilation of cortical arterioles following astrocytic AMPA receptor stimulation.30,33–35
Based on the effects of cyclooxygenase (COX) inhibitors in neonatal brain slices, Zonta et al suggested that elevation of astrocytic [Ca2+]c following glutamate activation of mGluRs leads to the generation of a COX product that activates receptors on vascular SMCs to generate vasodilation.14 Consistent with the speculation that this COX product is PGE2, Zonta and colleagues demonstrated that cultured astrocytes produce PGE2 in a pulsatile manner and PGE2 (20 µmol/L) dilates arterioles in brain slices.14,36 Importantly, the vasodilatory responses observed in these studies exhibited a significant delay (1–3 min) following neuronal activation by electrical field stimulation (EFS), activation of astrocytic mGluRs (t-ACPD [{±}-1-aminocyclopentane-trans-1,3-dicarboxylic acid]), patch seal-evoked stimulation, or by PGE2 application. This delayed response is a phenomenon that is not observed in vivo in adult animals. The slow response in brain slices might reflect the use of neonatal animals in which the neurons and vasculature are not fully developed. Alternatively, the time course of PGE2 action itself might be slow, which might suggest that this pathway does not mediate the rapid coupling of neuronal activity to vascular dilation but contributes instead to the regulation of blood flow on a longer time scale. Additionally, the observation that COX inhibition only partially suppresses vasodilation is consistent with the view that multiple mechanisms contribute to the neuronal regulation of arteriolar diameter.
In addition to inducing vasodilation, astrocyte-derived AA has been implicated in generating vasoconstriction. Mulligan and MacVicar have proposed that a rise in astrocytic endfoot [Ca2+] stimulates PLA2, which produces AA that then diffuses to the parenchymal arteriolar SMCs, where it is converted to 20-HETE by CYP ω-hydroxylase.12 The suggestion is that 20-HETE constricts arterioles by inhibiting SMC large-conductance calcium-sensitive K+ (BK) channels, an inference based on the inhibitory effects of the PLA2 inhibitor MAFP (100 µmol/L) and the 20-HETE synthesis-inhibitor HET0016 (100 µmol/L) on the observed constriction. However, these experiments examined non-preconstricted arterioles in brain slices, and the contribution of BK channels is expected to be negligible in the absence of tone.37 Accordingly, it is difficult to reconcile the observed constrictor effect of 20-HETE with a mechanism involving inhibition of vascular SMC BK channels. In addition, these observations must be interpreted with caution as the concentration of HET0016 used was very high (100 µmol/L), and likely to non-selectively inhibit the formation of other AA metabolites that might be involved in NVC. In human renal microsomes, the IC50 of HET0016 on CYP epoxygenase and COX activities is 2.8 µmol/L and 2.3 µmol/L, respectively.38 Also, 1 µmol/L HET0016 is sufficient to block 20-HETE production in intact cerebral arteries by 90%.39 Thus, the actual role of 20-HETE in NVC and its direct functional effects on parenchymal arterioles remains uncertain.
One astrocytic signal that clearly has the potential to generate both vasodilation and vasoconstriction is K+.40 The regulation of [K+]o by astrocytes has been extensively studied in the retina, where astrocytes are suggested to function as a K+ siphon, taking up K+ from regions of high concentration (perisynaptic regions) and releasing K+ to regions of lower extracellular concentration (perivascular regions).21 Consistent with this K+ buffering role, astrocytes display a large K+ conductance; a hyperpolarized Vm (approximately −80 mV) that is positive to the K+ equilibrium potential, EK (−102 mV); and exhibit polarized expression of K+ channels.41,42 Specifically, BK channels are abundantly expressed in perivascular endfeet.43 In brain slices, neuronal stimulation by EFS produces Ca2+-sensitive K+ (BK channel) currents in astrocytic endfeet that are activated by the rise in endfoot [Ca2+] and result in vasodilation.44 Activation of endfoot BK channels releases K+ into the perivascular space, producing a localized elevation in [K+]o in the spatially restricted microenvironment between the endfoot and the arteriole that is sensed by the arteriolar smooth muscle. By altering the [K+]o in the perivascular space through endfoot BK channel-mediated K+ release, the astrocyte can generate a signal that is capable of producing vasodilation or vasoconstriction. Strong evidence indicates that the vasodilatory response to K+ released from the endfoot is mediated by inward-rectifier K+ (Kir) channels in parenchymal arteriole smooth muscle. Barium, a selective inhibitor of Kir channels (at concentrations <100 µmol/L), blocks vasodilation elicited by neuronal stimulation with EFS in brain slices, but does not affect the increase in [Ca2+]c in astrocytic endfeet.44
K+ Channels in the Control of Parenchymal Arteriolar Tone
The membrane potential of arterial SMCs is a key determinant of vascular tone and is regulated by K+ channels. Opening of K+ channels in vascular SMC membranes allows K+ to flow out of the cell, resulting in membrane hyperpolarization, whereas inhibition of K+ channels results in membrane depolarization. K+ channel-mediated membrane hyperpolarization closes voltage-dependent Ca2+ channels (VDCCs), which decreases Ca2+ entry and leads to vasodilation.45,46 VDCCs are very sensitive to membrane potential, such that modest fluctuations in membrane potential can dramatically change Ca2+ entry.47 Consequently, minor changes in membrane potential can have a significant effect on arterial diameter.48 The membrane potential of SMCs in parenchymal arterioles pressurized to physiological levels (40 mmHg) is approximately −45 mV, and the arterioles are about 30% constricted.44 In cerebrospinal fluid, EK is about −102 mV. Membrane potential hyperpolarization to −60 mV causes a maximum dilation of cerebral arteries, and depolarization to approximately −30 mV causes maximal constriction.48 Therefore, even modest activation of SMC K+ channels can cause significant vasodilation.
The 4 different subtypes of K+ channels identified in arterial SMCs are inward rectifier K+ (Kir) channels, Ca2+-sensitive K+ (KCa) channels, voltage-dependent K+ (Kv) channels, and ATP-sensitive K+ (KATP) channels.49 The expression profile and functional contribution of each subtype can vary according to tissue bed and caliber of the arterial segment.50 There is functional evidence for all 4 K+ channel subtypes in SMCs of parenchymal arterioles, but their relative roles have not been as well characterized as in pial artery SMCs.51–54
Kir Channels
A number of arteries, including cerebral and coronary arteries, dilate to a modest elevation in extracellular K+. K+-induced dilations are caused by activation of strong inward rectifier K+ channels in the SMCs.49 In pial arteries, disruption of the gene for the strong inward rectifier K+ channel, Kir2.1, abolished K+-induced dilations.55,56 The property of inward rectification is likely conferred to Kir channels through the drawing out of positively charged polyamines plugging the inside of the pore by membrane potential hyperpolarization.57–59 The midpoint-of the-activation curve for Kir channels corresponds to EK, such that elevation of external K+ shifts the activation curve to more positive potentials.60 The only experimentally useful blocker of Kir channels is external barium, which blocks the pore in a voltage-dependent manner with an apparent half block constant of 10 µmol/L at −40 Mv.61 At concentrations below 100 µmol/L, barium ions do not cause significant block of smooth muscle KV, BK, or KATP channels.62 There are no selective activators of Kir channels, except external K+ and membrane potential hyperpolarization.
The activity of Kir channels in arteriolar myocytes is highly dependent on membrane potential and [K+]o. In parenchymal arteriolar SMCs, where the density of barium-sensitive Kir currents is greater than that observed in pial artery myocytes, elevation of [K+]o shifts the Kir activation curve to more positive potentials, and an increase in [K+]o from 3 mmol/L to 10 mmol/L increases the Kir current density 6.3-fold.44 The increase in Kir current density with elevated [K+]o is associated with suppression of SMC Ca2+ oscillations and vasodilation. Increasing [K+]o activates Kir, resulting in membrane potential hyperpolarization and vasodilation only up to a concentration of ~20 mmol/L – the [K+]o at which EK is similar to the SMC resting membrane potential. Further increases in [K+]o depolarize the arteriolar SMC membrane potential and cause vasoconstriction. Treatment with barium prevents the dilation of parenchymal arterioles to modest elevations in [K+]o, but does not affect the constriction to greater than 20 mmol/L [K+]o, indicating that Kir mediates K+-induced dilation but not K+-induced constriction in parenchymal arterioles. Exposure of parenchymal arterioles to barium alone does not affect vascular diameter, suggesting that SMC Kir channels are not tonically active and contribute minimally to tone in these vessels.
BK Channels
BK channels have been identified in virtually every type of smooth muscle. The characteristic smooth muscle BK channel complex is composed of an α pore-forming subunit and an auxillary β1 subunit.63–65 The β1 subunit acts to increase the apparent voltage- and Ca2+-sensitivity of the channel. Targeted disruption of the β1 subunit gene leads to hypertension and left ventricular hypertrophy.66 A number of recent animal studies have shown that the β1 subunit, and hence BK channel function, is downregulated in hypertension and diabetes.67–71 Two polymorphisms of the BK channel β1 subunit – KCNMB1-E65K and KCNMB1-V110L – are common in multiple ethnic and racial groups; an additional polymorphism (R140 W) is found in individuals of African descent. The first non-synonymous coding polymorphism was described by Fernandez-Fernandez et al, who identified the E65K polymorphism by direct sequence analysis of the 4 Kcnmb1 exons in a Spanish cohort.72 Heterologous expression studies in HEK293 cells established that the E65K polymorphism was a gain-of-function variant that increased BK-channel sensitivity to activation by Ca2+. Genetic association studies in the Spanish cohort demonstrated that carriers of E65K, which was present in 21% of the population examined, were less likely to have diastolic hypertension.72 In follow-up studies, the association of E65K with blood pressure appeared to be strongest among post-menopausal women.73
BK channels are selectively blocked by the scorpion toxins iberiotoxin and charybdotoxin, as well as the alkaloid paxilline.74–76 There are a number of synthetic openers of BK channels.77–79 However, the majority of these openers lack selectivity in intact tissues, and therefore their usefulness has been limited. An exception is NS11021, which has recently been shown to decrease smooth muscle excitability through activation of BK channels.80 BK channels are directly and indirectly activated by vasodilators that elevate cGMP and cAMP concentration.49,81,82 BK channels have also been shown to be modulated by AA metabolites. BK channel activity is inhibited by 20-HETE through protein kinase C (PKC) activation and increased by EETs.83–87
BK currents have been identified in parenchymal arteriolar SMCs; however, these currents have been suggested to be quite small in the physiologically relevant voltage range of −50 to −10 mV.88 In addition, whereas treatment of pressurized pial arteries with BK channel inhibitors causes pronounced vasoconstriction, parenchymal arteriolar tone does not appear to be substantially affected by BK channel inhibition, as BK channel block by subdural superfusion of paxilline (1 µmol/L) does not significantly affect resting cortical CBF in vivo.51 These observations suggest that, unlike pial arteries, where BK channels appear to play a major role in regulating tone, the functional significance of BK channel activity in parenchymal arterioles might not be substantial.37 Reduced BK channel-mediated opposition of myogenic tone could potentially explain the greater relative tone observed in parenchymal arterioles compared to pial arteries.89 The mechanism and physiological significance of diminished basal BK channel activity in pressurized parenchymal arterioles compared to pial arteries is unclear. However, BK channels in parenchymal arteriolar SMCs are involved in modulating vascular diameter in response to vasoactive chemical stimuli. For example, parenchymal arteriolar dilation to glutamate in newborn piglets is mediated by activation of SMC BK channels.90
Voltage-Dependent Potassium (KV) Channels
KV channels are present in all types of vascular smooth muscle, and serve to oppose depolarizing, contractile influences by virtue of their voltage-dependence.49 In vascular smooth muscle, the consensus is that these channels are composed of heteromers of KV1.2/1.5.91,92 KV channels can be inhibited by vasoconstrictors through PKC activation.93–95 Recent evidence indicates modest elevations of glucose can also inhibit KV channels through activation of PKC.53,96 The 4-aminopyridine (4-AP) is a potent inhibitor of these channels.62 Selective openers of these channels have not been identified. As in pial arteries, KV channels play a significant role in determining the level of myogenic tone in pressurized parenchymal arterioles. KV channel currents activated by membrane depolarization are observed in parenchymal arteriolar SMCs.53 The biophysical properties of these currents along with reverse transcription-polymerase chain reaction results indicate that KV channels in parenchymal arterioles are heterotetrameric channels composed of KV1.2 and KV1.5 subtypes. This is the same type of KV channel assembly present in pial artery SMCs. KV current in isolated parenchymal arteriolar SMCs is significantly reduced by application of 4-AP, which also constricts pressurized arterioles and arterioles in brain slices by roughly 40%. Hence, at physiological intravascular pressures KV channels actively oppose myogenic constriction in parenchymal arterioles. There is some evidence that KV channels also mediate changes in membrane potential and vascular tone in parenchymal arterioles in response to vasoactive agents, although this has not been well studied. However, elevated extracellular glucose concentration diminishes KV current in arteriolar SMCs and promotes vasoconstriction of parenchymal arterioles through activation of PKC.53
KATP Channels
KATP channels have been identified in a wide variety of smooth muscle types, and are likely an octomer of 4 sulfonylurea receptors (SUR2B) and 4 inward rectifier (Kir6.1) subunits.60,97 KATP channels lack voltage-dependence, and exhibit very weak inward rectification. They are also inhibited by intracellular ATP, and may open when ATP/ADP ratio changes during hypoxic or ischemic conditions.60 Under physiological conditions, SMC KATP channels are potently activated by vasodilators that activate cAMP-dependent protein kinase and are inhibited by vasoconstrictors that activate PKC.49,60 KATP channels in vascular smooth muscle are potently inhibited by sulfonylurea and hypoglycemic drugs, such as glibenclamide, and are selectively activated by a wide variety of synthetic compounds, such as pinacidil and cromakalim.60,98 There is no direct electrophysiological evidence for KATP channels in SMCs of parenchymal arterioles. However, preconstricted parenchymal arterioles dilate in response to levcromakalim, a KATP channel agonist.54 Additionally, dilation of parenchymal arterioles to mild hypercapnia is blocked by glibenclamide (5 µmol/L), a selective inhibitor of KATP channels.99 It has also been reported that KATP channel activation mediates parenchymal arteriolar membrane hyperpolarization in response to basic fibroblast growth factor.100 Glibenclamide-sensitive KATP currents measured in pial artery SMCs are activated by synthetic vasodilators such as pinacidil, as well as by endogenous vasodilators such as calcitonin gene-related peptide and VIP.101,102 While there is evidence that supports the presence of KATP channels in parenchymal arteriolar SMCs, the biophysical properties of KATP channels in these vessels are not known, and the functional role of KATP channels in regulating parenchymal arteriolar tone requires further characterization.
Parenchymal Arteriolar Smooth Muscle K+ Channels and NVC
Multiple parallel pathways are activated by the increase in [Ca2+]c in astrocytes in response to neuronal activation. This is evidenced by the fact that pharmacological inhibition or genetic manipulation of any of the putative mediators of NVC never fully blocks the vascular response. Also, a rise in astrocytic endfoot [Ca2+]c potentially activates a wide range of Ca2+-sensitive proteins. These Ca2+-sensitive proteins include BK channels, which release K+ ions as a vasoactive signal, as well as PLA2, which provides the substrate (AA) for the generation of eicosanoid and prostanoid vasoactive mediators. Because functional hyperemia in the brain is a prerequisite for survival, it is not surprising that redundant mechanisms have evolved to maintain it. However, redundant parallel mechanisms that ensure functional NVC might ultimately converge on parenchymal arteriolar SMC K+ channels to elicit their vascular response.
Vasodilation in Response to Neuronal Activity
Activation of parenchymal arteriolar smooth muscle K+ channels during NVC would cause vasodilation and thereby increase local CBF. Here, we consider evidence that putative astrocytic mediators of NVC – external K+, PGE2, and EETs – dilate parenchymal arterioles through activation of smooth muscle K+ channels.
External K+
As noted above, isolated SMCs from parenchymal arterioles have a relatively high density of strong inward rectifier K+ channels that are activated by external K+ and membrane hyperpolarization, consistent with the properties of Kir2 family members. Elevation of [K+]o from 3 mmol/L to 8 mmol/L hyperpolarizes parenchymal arteriolar membranes from −45 to −80 mV, and causes a rapid and profound dilation of isolated pressurized parenchymal arterioles as well as arterioles in brain slices.44 These effects are blocked by low concentrations of barium ions, which also block NVC responses to neuronal stimulation or Ca2+ uncaging in astrocytic endfeet in brain slices (by approximately 70%) and reduce hyperemic responses to whisker stimulation in vivo (by about 50%). Taken together, these results provide compelling evidence that parenchymal arterioles can dilate to external K+ through activation of Kir channels.
PGE2
Based on the effects of COX inhibitors, several groups have proposed that PGE2 released from astrocytic processes is responsible for NVC.14,103–105 PGE2 (20 µmol/L) has been shown to dilate arterioles in brain slices from neonatal rats.14 PGE2 might act through EP2 or EP4 prostanoid receptors in SMCs to elevate cAMP, which could lead to vasodilation by activating BK channels or KATP channels. This type of K+ channel-dependent mechanism of PGE2 dilation has been documented in renal arteries but has not been explored in parenchymal arterioles.106 PGE2 dilates pial arteries from cat and rat, and reportedly dilates human middle cerebral arteries through EP4 receptor activation.107,108 However, there is limited direct evidence of PGE2-mediated dilation in parenchymal arterioles. COX inhibition with indomethacin dilates pressurized parenchymal arterioles by 15%, but the mechanism of this effect is not known.109 We have observed that in pressurized mesenteric arteries, PGE2 only dilates the vessel in the presence of functional nitric oxide synthase, suggesting an endothelial effect rather than a SMC effect (unpublished observations). PGE2 has also been reported to elicit vasoconstriction in a variety of arterial segments, including cerebral arteries.110–112 The direct effect of PGE2 on parenchymal arteriolar tone and its mechanism of action requires further investigation.
EETs
The release of EETs has been proposed to be involved in NVC.113–116 Subdural superfusion of epoxygenase inhibitors reportedly reduces functional hyperemia in the rat somatosensory cortex by 28–69%.115 EETs hyperpolarize cerebral artery SM membrane potential and potently dilate pial arteries through direct activation of SM BK channels and through indirect activation of SM BK channels by increasing Ca2+ sparks.33,117–121 EET-mediated functional hyperemia in the brain very likely occurs through activation of K+ channels in parenchymal arteriolar SMCs. However, the direct effect of EETs on K+ channel activity, SMC membrane potential, and parenchymal arteriolar tone has not been investigated.
Vasoconstriction in Response to Neuronal Activity
Several groups have observed vasoconstriction of parenchymal arterioles in response to neuronal activation or Ca2+ uncaging in astrocytic endfeet.12,51,113 The function of vasoconstriction in NVC is unknown, and this phenomenon might be more pathological than physiological. Inhibition of parenchymal arteriolar smooth muscle K+ channels would depolarize the membrane and cause vasoconstriction, and there is evidence that the primary putative vasoconstrictor mediator of NVC, 20-HETE, acts through this mechanism.
As discussed above, inhibition of 20-HETE formation prevents vasoconstriction of parenchymal arterioles in response to neuronal stimulation in brain slices.12 The 20-HETE inhibition also prevents light-evoked vasoconstrictions in mammalian retina.113 This evidence suggests that 20-HETE constricts parenchymal arteriolar SMCs. Direct evidence in parenchymal arterioles is lacking, but 20-HETE potently constricts pial arteries.122–124 The 20-HETE constricts arteries by inhibiting SMC BK channels through PKC.84,85 The physiological significance of this mechanism is unclear, not only because of the questionable function of neuronal activity-induced vasoconstriction, but also because basal BK channel activity in parenchymal arteriole SMCs might be quite low. BK channel blockers have little effect on the diameter of arterioles in brain slices and on resting cortical CBF.51
External K+ can produce parenchymal arteriolar constriction in addition to dilation. While modest elevation of [K+]o dilates parenchymal arterioles through activation of Kir, elevation of [K+]o above 20 mmol/L evokes constriction through SMC membrane potential depolarization.51,125 In brain slices, raising [K+]o in the bath also converts evoked parenchymal arteriolar dilations with neuronal stimulation or endfoot Ca2+ uncaging into constrictions.51 In NVC, the astrocyte can tune the polarity of the arteriolar diameter response by altering the magnitude of K+ release from the endfoot.
PGE2 also has the potential to mediate neuronal activity-induced constriction. Human pial artery dilation in response to PGE2 occurs through EP4 receptors.108 In some types of cerebral arteries, PGE2 has been shown to cause constricton through EP1 or EP3 receptors.112 The specificity of the cerebral vascular response to PGE2 (dilation vs constriction) appears to be conferred by the prostanoid receptors stimulated. Which prostanoid receptor subtypes are expressed and their relative expression in SMCs of parenchymal arterioles is not known. Also, as stated above, the direct effect of PGE2 on parenchymal arterioles has not been studied.
In studies observing vasoconstriction with a rise in astrocytic endfoot Ca2+ or neuronal activation, a 20-HETE inhibitor, albeit at a high concentration, largely abolished the constrictor response. However, if there is a role for astrocyte-mediated vasoconstriction in normal physiology or in pathology, external K+, PGE2, and 20-HETE could potentially be mediators.
Indirect Role of Parenchymal Arteriolar SMC K+ Channels in NVC
Smooth muscle K+ channels also indirectly influence NVC by setting the resting level of tone in parenchymal arterioles prior to neuronal activation. In pial arterioles, the pre-existing level of tone in the vessel determines the magnitude and polarity (constriction vs vasodilation) of the vascular response to vasoactive substances.126,127 Similarly, the response of parenchymal arterioles to putative signals involved in NVC is dictated by the resting tone of the vessel.128 Varying the degree of preconstriction of parenchymal arterioles in brain slices with increasing concentrations of the thromboxane receptor agonist U-46619 alters the response of the arteriole to K+ such that the greater the initial level of preconstriction, the greater the magnitude of the vasodilator response to 10 mmol/L [K+]o. Stimulation of mGluR to elicit a rise in astrocytic [Ca2+]c evokes constriction in modestly preconstricted arterioles (70–100% of baseline) and dilation in arterioles with greater initial tone. The switch in polarity of vascular response appears to occur at a level of tone corresponding to approximately 50–70% of baseline diameter.
As key regulators of membrane potential, and therefore Ca2+ influx through VDCCs, SMC K+ channels are primary determinants of parenchymal arteriolar tone. Therefore, arteriolar SMC K+ channels likely play a role in determining the magnitude and polarity of the vascular response to neuronal activation by setting the initial level of tone of the arteriole.
Concluding Remarks
Precise regulation of CBF is essential for homeostasis in the brain. Smooth muscle K+ channels are critical regulators of vascular tone and blood flow in cerebral arteries and arterioles. Many mechanisms of chemical, mechanical, and humoral regulation of CBF involve modulation of SMC K+ channel activity. Parenchymal arteriolar SMC K+ channels are important in the process of NVC and functional hyperemia in the brain, and have potential as therapeutic targets in pathological conditions in which NVC is compromised, including Alzheimer’s disease, dementia, diabetes, and hypertension.
Acknowledgements
We thank Dr David Hill-Eubanks for comments on the manuscript. This work was supported by NIH grants DK053832, DK065947, HL44455, HL098243, HL077378, T32HL007944/6-10, and the Totman Trust for Medical Research.
References
- 1.Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol. 1890;11:85–158. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. author reply 344 – 345. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C. Regulation of the cerebral microcirculation during neural activity: Is nitric oxide the missing link? Trends Neurosci. 1993;16:206–214. doi: 10.1016/0166-2236(93)90156-g. [DOI] [PubMed] [Google Scholar]
- 4.Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science. 2003;301:1870–1874. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci USA. 2003;100:13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujikawa T, Tochikubo O, Kura N, Kiyokura T, Shimada J, Umemura S. Measurement of hemodynamics during postural changes using a new wearable cephalic laser blood flowmeter. Circ J. 2009;73:1950–1955. doi: 10.1253/circj.cj-09-0103. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 8.de la Torre JC. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 9.Edvinsson L, MacKenzie ET. General and comparative anatomy of the cerebral circulation. In: Edvinsson L, Krause DN, editors. Cerebral blood flow and metabolism. New York: Lippincott Williams and Wilkins; 2002. pp. 3–29. [Google Scholar]
- 10.Iadecola C. Neurogenic control of the cerebral microcirculation: Is dopamine minding the store? Nat Neurosci. 1998;1:263–265. doi: 10.1038/1074. [DOI] [PubMed] [Google Scholar]
- 11.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50:335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- 12.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 13.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- 14.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 15.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 17.Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: A confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- 18.Edvinsson L, Hamel E. Perivascular nerves in brain vessels. In: Edvinsson L, Krause DN, editors. Cerebral blood flow and metabolism. New York: Lippincott Williams and Wilkins; 2002. pp. 43–67. [Google Scholar]
- 19.Diamond JS. Deriving the glutamate clearance time course from transporter currents in CA1 hippocampal astrocytes: Transmitter uptake gets faster during development. J Neurosci. 2005;25:2906–2916. doi: 10.1523/JNEUROSCI.5125-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagelhus EA, Mathiisen TM, Ottersen OP. Aquaporin-4 in the central nervous system: Cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience. 2004;129:905–913. doi: 10.1016/j.neuroscience.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 21.Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience. 2004;129:1045–1056. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol. 2004;559:3–15. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy S, MacVicar BA. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci. 1995;15:5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: Long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 26.Parri HR, Crunelli V. The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience. 2003;120:979–992. doi: 10.1016/s0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- 27.Golovina VA, Blaustein MP. Unloading and refilling of two classes of spatially resolved endoplasmic reticulum Ca(2+) stores in astrocytes. Glia. 2000;31:15–28. doi: 10.1002/(sici)1098-1136(200007)31:1<15::aid-glia20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: Hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 29.Harder DR, Zhang C, Gebremedhin D. Astrocytes function in matching blood flow to metabolic activity. News Physiol Sci. 2002;17:27–31. doi: 10.1152/physiologyonline.2002.17.1.27. [DOI] [PubMed] [Google Scholar]
- 30.Harder DR, Roman RJ, Gebremedhin D, Birks EK, Lange AR. A common pathway for regulation of nutritive blood flow to the brain: Arterial muscle membrane potential and cytochrome P450 metabolites. Acta Physiol Scand. 1998;164:527–532. doi: 10.1111/j.1365-201x.1998.tb10702.x. [DOI] [PubMed] [Google Scholar]
- 31.Amruthesh SC, Boerschel MF, McKinney JS, Willoughby KA, Ellis EF. Metabolism of arachidonic acid to epoxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and prostaglandins in cultured rat hippocampal astrocytes. J Neurochem. 1993;61:150–159. doi: 10.1111/j.1471-4159.1993.tb03550.x. [DOI] [PubMed] [Google Scholar]
- 32.Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- 33.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992;263:H519–H525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- 34.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 35.Lovick TA, Brown LA, Key BJ. Neuronal activity-related coupling in cortical arterioles: Involvement of astrocyte-derived factors. Exp Physiol. 2005;90:131–140. doi: 10.1113/expphysiol.2004.028811. [DOI] [PubMed] [Google Scholar]
- 36.Zonta M, Sebelin A, Gobbo S, Fellin T, Pozzan T, Carmignoto G. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J Physiol. 2003;553:407–414. doi: 10.1113/jphysiol.2003.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 38.Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, et al. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature con tributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H2455–H2465. doi: 10.1152/ajpheart.00512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knot HJ, Zimmermann PA, Nelson MT. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol. 1996;492(Pt 2):419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newman EA. Regional specialization of retinal glial cell membrane. Nature. 1984;309:155–157. doi: 10.1038/309155a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gebremedhin D, Yamaura K, Zhang C, Bylund J, Koehler RC, Harder DR. Metabotropic glutamate receptor activation enhances the activities of two types of Ca2+-activated k+ channels in rat hippocampal astrocytes. J Neurosci. 2003;23:1678–1687. doi: 10.1523/JNEUROSCI.23-05-01678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price DL, Ludwig JW, Mi H, Schwarz TL, Ellisman MH. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res. 2002;956:183–193. doi: 10.1016/s0006-8993(02)03266-3. [DOI] [PubMed] [Google Scholar]
- 44.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 45.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi T, Ichikawa M, Iwata A, Nakata T, Lim YJ, Mishima M. Intracoronary nicorandil relieves multiple coronary vasospasm with hemodynamic collapse. Circ J. 2008;72:327–330. doi: 10.1253/circj.72.327. [DOI] [PubMed] [Google Scholar]
- 47.Rubart M, Patlak JB, Nelson MT. Ca2+ currents in cerebral artery smooth muscle cells of rat at physiological Ca2+ concentrations. J Gen Physiol. 1996;107:459–472. doi: 10.1085/jgp.107.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, et al. Heterogeneity in function of small artery smooth muscle BKCa: Involvement of the beta1-subunit. J Physiol. 2009;587:3025–3044. doi: 10.1113/jphysiol.2009.169920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, et al. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle Cell KCa channels. Circ Res. 2008;102:234–241. doi: 10.1161/CIRCRESAHA.107.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Straub SV, Girouard H, Doetsch PE, Hannah RM, Wilkerson MK, Nelson MT. Regulation of intracerebral arteriolar tone by K(v) channels: Effects of glucose and PKC. Am J Physiol Cell Physiol. 2009;297:C788–C796. doi: 10.1152/ajpcell.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinoshita H, Nakahata K, Dojo M, Kimoto Y, Hatano Y. Lidocaine impairs vasodilation mediated by adenosine triphosphate-sensitive K+ channels but not by inward rectifier K+ channels in rat cerebral microvessels. Anesth Analg. 2004;99:904–909. doi: 10.1213/01.ANE.0000133912.54318.0F. table of contents. [DOI] [PubMed] [Google Scholar]
- 55.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ Res. 2000;87:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- 56.Bradley KK, Jaggar JH, Bonev AD, Heppner TJ, Flynn ER, Nelson MT, et al. Kir2.1 encodes the inward rectifier potassium channel in rat arterial smooth muscle cells. J Physiol. 1999;515(Pt 3):639–651. doi: 10.1111/j.1469-7793.1999.639ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- 58.Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- 59.Lopatin AN, Makhina EN, Nichols CG. The mechanism of inward rectification of potassium channels: “Long-pore plugging” by cytoplasmic polyamines. J Gen Physiol. 1995;106:923–955. doi: 10.1085/jgp.106.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 61.Quayle JM, McCarron JG, Brayden JE, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol. 1993;265:C1363–C1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- 62.Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol. 1995;269:H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- 63.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 64.Knaus HG, Eberhart A, Glossmann H, Munujos P, Kaczorowski GJ, Garcia ML. Pharmacology and structure of high conductance calcium-activated potassium channels. Cell Signal. 1994;6:861–870. doi: 10.1016/0898-6568(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: Predominant alpha + beta subunit complexes. J Physiol. 1997;502(Pt 3):545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 67.McGahon MK, Dash DP, Arora A, Wall N, Dawicki J, Simpson DA, et al. Diabetes downregulates large-conductance Ca2+-activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100:703–711. doi: 10.1161/01.RES.0000260182.36481.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- 70.Chang T, Wu L, Wang R. Altered expression of BK channel beta1 subunit in vascular tissues from spontaneously hypertensive rats. Am J Hypertens. 2006;19:678–685. doi: 10.1016/j.amjhyper.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 71.Rusch NJ. BK channels in cardiovascular disease: A complex story of channel dysregulation. Am J Physiol Heart Circ Physiol. 2009;297:H1580–H1582. doi: 10.1152/ajpheart.00852.2009. [DOI] [PubMed] [Google Scholar]
- 72.Fernandez-Fernandez JM, Tomas M, Vazquez E, Orio P, Latorre R, Senti M, et al. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest. 2004;113:1032–1039. doi: 10.1172/JCI20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Senti M, Fernandez-Fernandez JM, Tomas M, Vazquez E, Elosua R, Marrugat J, et al. Protective effect of the KCNMB1 E65K genetic polymorphism against diastolic hypertension in aging women and its relevance to cardiovascular risk. Circ Res. 2005;97:1360–1365. doi: 10.1161/01.RES.0000196557.93717.95. [DOI] [PubMed] [Google Scholar]
- 74.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, et al. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion. Buthus tamulus. J Biol Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- 75.Gribkoff VK, Starrett JE, Jr, Dworetzky SI. The pharmacology and molecular biology of large-conductance calcium-activated (BK) potassium channels. Adv Pharmacol. 1997;37:319–348. doi: 10.1016/s1054-3589(08)60954-0. [DOI] [PubMed] [Google Scholar]
- 76.Li G, Cheung DW. Effects of paxilline on K+ channels in rat mesenteric arterial cells. Eur J Pharmacol. 1999;372:103–107. doi: 10.1016/s0014-2999(99)00188-0. [DOI] [PubMed] [Google Scholar]
- 77.Butera JA, Jenkins DJ, Lennox JR, Sheldon JH, Norton NW, Warga D, et al. Synthesis and bladder smooth muscle relaxing properties of substituted 3-amino-4-aryl-(and aralkyl-)cyclobut-3-ene-1,2-diones. Bioorg Med Chem Lett. 2005;15:2495–2501. doi: 10.1016/j.bmcl.2005.03.073. [DOI] [PubMed] [Google Scholar]
- 78.Hewawasam P, Gribkoff VK, Pendri Y, Dworetzky SI, Meanwell NA, Martinez E, et al. The synthesis and characterization of BMS-204352 (MaxiPost) and related 3-fluorooxindoles as openers of maxi-K potassium channels. Bioorg Med Chem Lett. 2002;12:1023–1026. doi: 10.1016/s0960-894x(02)00101-4. [DOI] [PubMed] [Google Scholar]
- 79.Siemer C, Bushfield M, Newgreen D, Grissmer S. Effects of NS1608 on MaxiK channels in smooth muscle cells from urinary bladder. J Membr Biol. 2000;173:57–66. doi: 10.1007/s002320001007. [DOI] [PubMed] [Google Scholar]
- 80.Layne JJ, Nausch B, Olesen SP, Nelson MT. BK channel activation by NS11021 decreases excitability and contractility of urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2010;298:R378–R384. doi: 10.1152/ajpregu.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993;265:C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- 82.Schubert R, Nelson MT. Protein kinases: Tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 83.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 84.Sun CW, Falck JR, Harder DR, Roman RJ. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension. 1999;33:414–418. doi: 10.1161/01.hyp.33.1.414. [DOI] [PubMed] [Google Scholar]
- 85.Obara K, Koide M, Nakayama K. 20-Hydroxyeicosatetraenoic acid potentiates stretch-induced contraction of canine basilar artery via PKC alpha-mediated inhibition of KCa channel. Br J Pharmacol. 2002;137:1362–1370. doi: 10.1038/sj.bjp.0704960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang W, Gauthier KM, Reddy LM, Sangras B, Sharma KK, Nithipatikom K, et al. Stable 5,6-epoxyeicosatrienoic acid analog relaxes coronary arteries through potassium channel activation. Hypertension. 2005;45:681–686. doi: 10.1161/01.HYP.0000153790.12735.f9. [DOI] [PubMed] [Google Scholar]
- 87.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, et al. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: Implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheong A, Quinn K, Dedman AM, Beech DJ. Activation thresholds of K(V), BK andCl(Ca) channels in smooth muscle cells in pial precapillary arterioles. J Vasc Res. 2002;39:122–130. doi: 10.1159/000057761. [DOI] [PubMed] [Google Scholar]
- 89.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: Effect of ischemia and reperfusion. Stroke. 2009;40:1451–1457. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanu A, Leffler CW. Carbon monoxide and Ca2+-activated K+ channels in cerebral arteriolar responses to glutamate and hypoxia in newborn pigs. Am J Physiol Heart Circ Physiol. 2007;293:H3193–H3200. doi: 10.1152/ajpheart.00274.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, et al. Voltage-gated K+ channels in rat small cerebral arteries: Molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen TT, Luykenaar KD, Walsh EJ, Walsh MP, Cole WC. Key role of Kv1 channels in vasoregulation. Circ Res. 2006;99:53–60. doi: 10.1161/01.RES.0000229654.45090.57. [DOI] [PubMed] [Google Scholar]
- 93.Rainbow RD, Norman RI, Everitt DE, Brignell JL, Davies NW, Standen NB. Endothelin-I and angiotensin II inhibit arterial voltage-gated K+ channels through different protein kinase C isoenzymes. Cardiovasc Res. 2009;83:493–500. doi: 10.1093/cvr/cvp143. [DOI] [PubMed] [Google Scholar]
- 94.Standen NB, Quayle JM. K+ channel modulation in arterial smooth muscle. Acta Physiol Scand. 1998;164:549–557. doi: 10.1046/j.1365-201X.1998.00433.x. [DOI] [PubMed] [Google Scholar]
- 95.Cole WC, Clement-Chomienne O, Aiello EA. Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochem Cell Biol. 1996;74:439–447. doi: 10.1139/o96-048. [DOI] [PubMed] [Google Scholar]
- 96.Rainbow RD, Hardy ME, Standen NB, Davies NW. Glucose reduces endothelin inhibition of voltage-gated potassium channels in rat arterial smooth muscle cells. J Physiol. 2006;575:833–844. doi: 10.1113/jphysiol.2006.114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seino S. ATP-sensitive potassium channels: A model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- 98.Watanabe N, Akasaka T, Fujimoto K, Kajita T, Shigeto F, Neishi Y, et al. Effect of nicorandil, a K+ATP-channel opener, on coronary capillary architecture and volume after early myocardial ischemiareperfusion: A 3-dimensional confocal laser microscopic study. Circ J. 2004;68:1210–1214. doi: 10.1253/circj.68.1210. [DOI] [PubMed] [Google Scholar]
- 99.Nakahata K, Kinoshita H, Hirano Y, Kimoto Y, Iranami H, Hatano Y. Mild hypercapnia induces vasodilation via adenosine triphosphate-sensitive K+ channels in parenchymal microvessels of the rat cerebral cortex. Anesthesiology. 2003;99:1333–1339. doi: 10.1097/00000542-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 100.Kajita Y, Takayasu M, Yoshida J, Dietrich HH, Dacey RG., Jr Vasodilatory effect of basic fibroblast growth factor in isolated rat cerebral arterioles: Mechanisms involving nitric oxide and membrane hyperpolarization. Neurol Med Chir (Tokyo) 2001;41:177–185. doi: 10.2176/nmc.41.177. discussion 185 – 176. [DOI] [PubMed] [Google Scholar]
- 101.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 102.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 103.Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 105.Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, Pertens E, Janssen LJ. 8-isoprostaglandin E(2) activates Ca(2+)-dependent K(+) current via cyclic AMP signaling pathway in murine renal artery. Eur J Pharmacol. 2005;520:22–28. doi: 10.1016/j.ejphar.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 107.Wahl M, Schilling L, Whalley ET. Cerebrovascular effects of prostanoids. In-situ studies in pial arteries of the cat. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:314–320. doi: 10.1007/BF00168516. [DOI] [PubMed] [Google Scholar]
- 108.Davis RJ, Murdoch CE, Ali M, Purbrick S, Ravid R, Baxter GS, et al. EP4 prostanoid receptor-mediated vasodilatation of human middle cerebral arteries. Br J Pharmacol. 2004;141:580–585. doi: 10.1038/sj.bjp.0705645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kimura M, Dietrich HH, Dacey RG., Jr Nitric oxide regulates cerebral arteriolar tone in rats. Stroke. 1994;25:2227–2233. doi: 10.1161/01.str.25.11.2227. discussion 2233 – 2224. [DOI] [PubMed] [Google Scholar]
- 110.van Rodijnen WF, Korstjens IJ, Legerstee N, Ter Wee PM, Tangelder GJ. Direct vasoconstrictor effect of prostaglandin E2 on renal interlobular arteries: Role of the EP3 receptor. Am J Physiol Renal Physiol. 2007;292:F1094–F1101. doi: 10.1152/ajprenal.00351.2005. [DOI] [PubMed] [Google Scholar]
- 111.Janssen LJ, Tazzeo T. Involvement of TP and EP3 receptors in vasoconstrictor responses to isoprostanes in pulmonary vasculature. J Pharmacol Exp Ther. 2002;301:1060–1066. doi: 10.1124/jpet.301.3.1060. [DOI] [PubMed] [Google Scholar]
- 112.Jadhav V, Jabre A, Lin SZ, Lee TJ. EP1- and EP3-receptors mediate prostaglandin E2-induced constriction of porcine large cerebral arteries. J Cereb Blood Flow Metab. 2004;24:1305–1316. doi: 10.1097/01.WCB.0000139446.61789.14. [DOI] [PubMed] [Google Scholar]
- 113.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: A mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Metea MR, Newman EA. Signalling within the neurovascular unit in the mammalian retina. Exp Physiol. 2007;92:635–640. doi: 10.1113/expphysiol.2006.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, et al. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283:H2029–H2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- 116.Liu X, Li C, Falck JR, Roman RJ, Harder DR, Koehler RC. Interaction of nitric oxide, 20-HETE, and EETs during functional hyperemia in whisker barrel cortex. Am J Physiol Heart Circ Physiol. 2008;295:H619–H631. doi: 10.1152/ajpheart.01211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leffler CW, Fedinec AL. Newborn piglet cerebral microvascular responses to epoxyeicosatrienoic acids. Am J Physiol. 1997;273:H333–H338. doi: 10.1152/ajpheart.1997.273.1.H333. [DOI] [PubMed] [Google Scholar]
- 118.Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol. 1990;259:H1171–H1177. doi: 10.1152/ajpheart.1990.259.4.H1171. [DOI] [PubMed] [Google Scholar]
- 119.Hercule HC, Salanova B, Essin K, Honeck H, Falck JR, Sausbier M, et al. The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BK alpha channel subunit in rodents. Exp Physiol. 2007;92:1067–1076. doi: 10.1113/expphysiol.2007.038166. [DOI] [PubMed] [Google Scholar]
- 120.Iliff JJ, Wang R, Zeldin DC, Alkayed NJ. Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels. Am J Physiol Heart Circ Physiol. 2009;296:H1352–H1363. doi: 10.1152/ajpheart.00950.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 122.Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke. 2003;34:1269–1275. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- 123.Yu M, Cambj-Sapunar L, Kehl F, Maier KG, Takeuchi K, Miyata N, et al. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol. 2004;486:297–306. doi: 10.1016/j.ejphar.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 124.Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, et al. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266:H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 125.Horiuchi T, Dietrich HH, Hongo K, Dacey RG., Jr Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke. 2002;33:2692–2699. doi: 10.1161/01.str.0000034791.52151.6b. [DOI] [PubMed] [Google Scholar]
- 126.Armstead WM, Mirro R, Busija DW, Leffler CW. Vascular responses to vasopressin are tone-dependent in the cerebral circulation of the newborn pig. Circ Res. 1989;64:136–144. doi: 10.1161/01.res.64.1.136. [DOI] [PubMed] [Google Scholar]
- 127.Rosenblum WI, Nelson GH. Tone regulates opposing endothelium-dependent and -independent forces: Resistance brain vessels in vivo. Am J Physiol. 1990;259:H243–H247. doi: 10.1152/ajpheart.1990.259.1.H243. [DOI] [PubMed] [Google Scholar]
- 128.Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294:H2855–H2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]