Abstract
Background
Longitudinal research is critical for understanding the biological mechanisms underlying the development of depression. Researchers recruit high-risk cohorts to understand how risk is transmitted from one generation to the next. Biological measurements have been incorporated into these longitudinal high-risk (LHR) studies in order to illuminate mechanistic pathways.
Methods
To frame our review, we first present heritability estimates along the gene-by-environment continuum as a foundation. We then offer a Biomarkers of Intergenerational Risk for Depression (BIRD) model to describe the multiple hits individuals at risk receive and to allow for greater focus on the interactive effects of markers. BIRD allows for the known multifinality of pathways towards depression and considers the context (i.e., environment) in which these mechanisms emerge. Next, we review the extant LHR cohort studies that have assessed central nervous system (electroencephalography (EEG), neuroimaging), endocrine (hypothalamic-pituitary-adrenal axis (HPA)/cortisol), autonomic (startle, heart rate), genetic, sleep, and birth characteristics.
Results
Results to date, in conjunction with the proposed model, point towards several pathways of discovery in understanding mechanisms, providing clear direction for future research examining potential endophenotypes.
Limitations
Our review is based on relatively narrow inclusion and exclusion criteria. As such, many interesting studies were excluded, but this weakness is offset by strengths such as the increased reliability of findings.
Conclusions
Blanket prevention programs are inefficient and plagued by low effect sizes due to low rates of actual conversion to disorder. The inclusion of biomarkers of risk may lead to enhanced program efficiency by targeting those with greatest risk.
Keywords: Longitudinal, High-risk, Depression, Development, Biology
1. Introduction
Studies of Longitudinal High-Risk (LHR) design identify offspring at increased risk for depression based on positive family history and follow offspring over time in order to capture first-onset of disorder. LHR designs are valuable in that they reduce the sample size needed to detect sufficient rates of illness because of increased incidence rates. LHR designs can aid in discovery of endophenotypes—that is, heritable phenotypes associated with illness risk that are measurable independent of illness state (Gottesman and Gould, 2003). LHR designs offer several benefits over studies of acutely depressed individuals. For example, studies of the acutely ill cannot discriminate between abnormalities representing the disease process (i.e., scars), active disease-state phenomena (i.e., state), byproducts of treatment (e.g., recovery, adaptation), or trait-related markers of risk that may exist prior to the onset of disorder. As such, case-control studies of ill patients compared to controls demarcate the end state of illness and are confounded by the scars of onset, chronic illness, compensatory mechanisms as well as acute state effects. Longitudinal follow-up of HR individuals is one way to address these confounds and to capture first-onset of disorder as offspring pass through windows of risk. The present review examines heritability estimates along the gene-by-environment continuum to establish a foundation for our proposed model of Biomarkers of Intergenerational Risk for Depression (BIRD). We integrate biological findings from LHR studies, provide theoretical and practical recommendations for existing cohorts and to-be-designed studies, and conclude with clinical implications.
Prospective psychiatric study of familial risk for psychopathology at the group level began in the 1980s following the introduction of structured diagnostic interviews with demonstrated reliability, the application of epidemiological methods, and the increased hope that genetic understanding would unravel the complex etiology of mental health disorders. As diagnostic methods for children and adolescents became available (e.g., Chambers et al., 1985) and as it became clear that most psychiatric disorders began in childhood and adolescence (Burke et al., 1990; Kessler et al., 2001; Kessler et al., 2005), several HR family studies including child and adolescent offspring were initiated. These early studies established the familial nature of many psychiatric disorders (Klein et al., 2001; Weissman et al., 1997) and led to the incorporation of longitudinal follow-up to capture the early signs, onset, and clinical course of disorder. As techniques became available for assessing putative physiological and neural biomarkers, LHR studies evolved from observational studies of clinical phenomena toward the explication of mechanisms, the search for neural endophenotypes, or what has been described as translational epidemiology (Weissman, 2012). This body of research has led to foundational knowledge of biological mechanisms in risk for depression. For example, whereas it was previously questioned whether or not children could experience depression, we now know that offspring of individuals with Major Depressive Disorder (MDD) are at increased risk for anxiety as children and MDD as they mature into adolescence and adulthood (Hammen et al., 2012; Klein et al., 2001; Kovacs et al., 1989; Weissman et al., 2006, 1997).
1.1. Heritability estimates along the continuum of gene-by-environment interactions
There are now a sufficient number of independent LHR studies using biological measurement of putative endophenotypes to examine the consistency of findings as they fall along the gene-by-environment continuum of heritablity. We present these estimates to offer a foundation in current knowledge about gene and environment contributions to mechanisms of risk. Fig. 1 illustrates heritability estimates for various putative biological mechanisms (corresponding references listed in Appendix A). Overall, heritability estimates for MDD are estimated to range between 31% and 42% (Sullivan et al., 2000; Wray and Gottesman, 2012). There is evidence of gender-specific heritability as well as evidence that heritability estimates may shift across developmental periods (Fanous et al., 2002). Heritability estimates for gene-by-environment mechanisms reviewed herein range from unmeasured (fMRI; both task-based and at rest) to 85% (alpha band power). These heritability estimates offer a context for the current review. This figure illustrates quite clearly that there are a number of sizable heritability risk factors for the intergenerational transmission of depression; however, there is limited information on the cumulative impact of these biological mechanisms. We believe that LHR studies can benefit from targeting these key markers and evaluating outcomes.
Fig. 1.
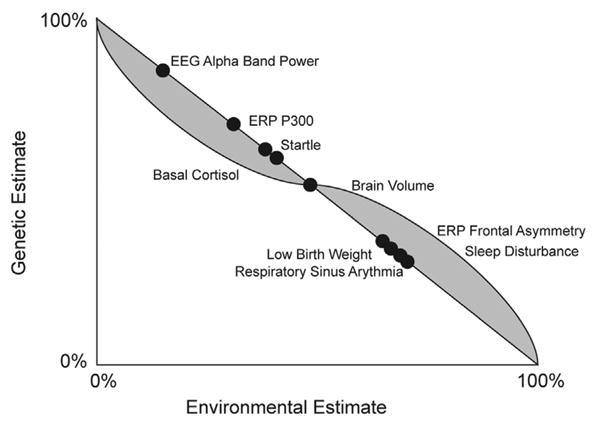
Documented heritability estimates along the gene dimension and the hypothesized environmental continuum. Grey shading illustrates hypothesized directionality of gene by environment interactions in that as markers have greater heritability, the influence of environment and gene by environment interactions may decrease in a non-linear fashion. As heritability decreases, environmental contributions and gene by environment interactions may increase in a non-linear fashion.
We propose Biomarkers of Intergenerational Risk for Depression (BIRD) to allow for the cumulative hit from multiple risk factors that individuals who are at high-risk based on family history may experience on the pathway to depression and resiliency. This model allows for the environmental context in which these mechanisms of risk emerge and evolve and also allows for the fact that some of these mechanisms may be correlated with one another or even derive from the same system dysfunction. We are particularly interested in environmental contributions as the environment is more amenable to manipulation, offering opportunities to study whether biological mechanisms can be altered through environmental intervention.
In light of this model, we review LHR studies of MDD that include physiological, neural, and genetic measures. Review at this stage can recommend new directions as well as highlight important findings of convergence and those in need of replication. It is time to examine the extent to which biological measures have been captured over developmental periods of risk, which will importantly advance the intergenerational transmission of depression. Furthermore our review can inform the selection of dependent and predictive variables of interest that can be pursued more extensively within existing cohorts and in the next generation of LHR studies. We examine the following systems: central nervous system including electroencephalography (EEG) and neuroimaging, endocrine including the hypothalamic-pituitary-adrenal axis (HPA), autonomic including heart rate and startle, genetics, sleep, and birth characteristics.
2. Methods
Inclusion criteria for our review included human studies that: (1) examine biological offspring of probands with MDD diagnosed via structured clinical interview; (2) include a biological measure in the protocol; (3) compare HR for depression to a Low-Risk (LR) control; and (4) follow the sample longitudinally across development. Literature searches were run in PubMed, Google Scholar, and Scopus and included search terms such as (risk AND depression AND mri/eeg/genetics/bio*) for published studies from January, 1990 through January, 2014. The initial search identified 385 studies. Studies were excluded for the following reasons: review paper or chapter without new results (n=46), no HR group or risk defined through self-report measures rather than diagnostic structured interviews of mental illness (n=102), no diagnostic measure of MDD (n=149), use of the family history method to interview offspring without directly interviewing the affected family member to establish risk group or questionnaire measures of symptoms only (n=7), and no comparison between HR and LR (n=8). We also excluded those without biological measures (n=14) and those that did not include longitudinal follow-up in the cohort reports to date (n=7).
3. Results
We identified six cohort studies. Table 1 details the Principal Investigator (PI) of each cohort study, overall sample size, recruitment focus, diagnostic interviews used, and length of longitudinal follow-up. Studies varied in whether or not they focused recruitment on adults who also had offspring or whether or not the offspring were the target of recruitment. These alternative strategies can be referred to as top-down and bottom-up respectively, with the former involving recruitment and focus on the characterization of adults with disorder and the subsequent study of offspring (e.g., Weissman), whereas the latter identifies child offspring from the outset based upon parental history of illness (e.g., Gotlib). Both strategies ultimately rely on adults to respond to recruitment efforts and to provide consent for multi-generational investigation but differ slightly in recruitment targets.
Table 1.
Cohort details.
| Primary investigator/university | Sample size | Recruitment | Diagnostic interviews | Longitudinal follow-up |
|---|---|---|---|---|
| Dawson University of Washington | 117 Mothers and their infant offspring | Community recruitment of mothers | Mothers=SCID Offspring=DISC-P | 5 years, 4 assessments: offspring age 24 months, 3.5 years, 4.5 years, and 6.5 years |
| Gotlib Stanford University | Overall sample of 200 offspring, varies by measure | Clinic and community recruitment focused on adolescent girls but accomplished by advertisements directed towards mothers | Mothers=SCID Daughters=K-SADS-PL | Every 18 months through offspring age 21–22 |
| Kovacs University of Pittsburgh | Up to 219 offspring depending on measure | Clinic and community recruitment of parents with depression | Parents=SCID Offspring=K-SADS-PL and K-SADS-YA | Annually through age 7 |
| Kutcher Dalhousie University | 83 offspring | Clinic and community recruitment of parents with offspring | Mothers=SCID Daughters=K-SADS-PL | 5 years, every 6 months |
| Garber Vanderbilt University | 240 mothers and their offspring, smaller cohort in EEG study (see Table 3) | Schools—letters sent to parents | Parents=SCID Adolescents = K-SADS-E | Annually for at least 2 years |
| Weissman Yale University & Columbia University | 182 offspring in 2nd generation, 137 in 3rd generation | Clinic and community recruitment of adults with offspring | Both parents and offspring: SADS Lifetime Version | 6 waves across 30 years: baseline (wave 1), 2 year (wave 2), 10 year (wave 3), 17 year (wave 4), 20 year (wave 5), 30 year (wave 6) |
Note: DISC=Diagnostic Interview Schedule for Children; KSADS=various versions of the Kiddie-Schedule for Affective Disorders and Schizophrenia; SADS=Schedule or Affective Disorders and Schizophrenia; SCID=Structured Clinical Interview.
Table 2 details which biological measures) each cohort study adopted and specifications of these measurements. The most commonly implemented biological measures include assessments of central nervous system function through EEG (5 cohorts) and autonomic measures such as heart period (3 cohorts). Additional implemented measures include MRI (2 cohorts), cortisol (2 cohorts), polysomnography measures of sleep (2 cohorts), genetics (1 cohort), startle (1 cohort), and birth-weight (1 cohort). Table 3 outlines ages and sample sizes of each investigation by manuscript and includes a summary of results.
Table 2.
Biological methods by Cohort.
| Primary investigator | MRI | EEG | Genetics | Sleep | Startle | Birth weight | HPA | Cardio |
|---|---|---|---|---|---|---|---|---|
| Dawson |
X Six scalp locations: F3, F4, P3, P4, T3, T4; International 10–20 system. EEG was recorded on a Grass Neurodata Acquisition System (Model 12). |
X Cortisol radio immunoassay kits from Pantex |
X The Porges-Bohrer moving polynomial algorithm estimates respiratory sinus arrhythmia (RSA), which is a measure of vagus nerve activity or vagal tone |
|||||
| Gotlib |
X 1.5-T imaging system (Signa; GE Medical Systems) |
X OXTR; Oxytocin receptor gene polymorphism (rs2254298) |
X Actiwatch 2 device; Philips Respironics |
X Cortisol via Salivettes |
X Biopac and ECG using modified lead II configuration scored with Mindware |
|||
| 5HTTPLR; serotonin-transporter-linked polymorphic region (SLC6A4) | ||||||||
| Kovacs |
X EEG was recorded at 12 pairs of sites–F3, F4, F7, F8, C3, C4, T7, T8, P3, P4, O1, and O2–using an electrode cap (Electro-Cap International) |
X RSA was computed using Fast Fourier transform analysis |
||||||
| Kutcher |
X C3 and C4 |
X Polysomnographic data (Melville Diagnostics Sandman System) |
||||||
| Garber |
X Fifteen scalp locations from the 10 to 20 system were used: F3, F4, F7, F8, T3, T4, T5, T6, P3, P4, C3, CZ, C4, Pz, and Fz. |
|||||||
| Weissman |
X 1.5 T scanner (Siemens Sonata) |
X 12 electrodes–F3, F4; F7,F8, C3,C4, T7,T8,P3,P4, P7, P8–using an electrode cap (Electro Cap International) |
In progress |
X |
X |
Note: MRI = magnetic resonance imaging; EEG = electroencephalography; HPA=Hypothalamic-pituitary-adrenal axis; T=Tesla; RSA=Respiratory sinus arrhythmia.
Table 3. Detailed results from each manuscript deriving from a HR cohort.
| Paper | Population Total N (mean age or age range); n within risk group (age within risk group where available) |
Findings | Sample | |
|---|---|---|---|---|
| CENTRAL NERVOUS SYSTEM | ||||
|
| ||||
| Magnetic Resonance Imaging (MRI): | ||||
| Chen, et al., (2010) | N=55 (9–15); 23 HR (12.76), 32 LR (12.90) | Total brain volume did not differ; however, ROI analyses using VBM found less gray matter density in bilateral posterior regions. | Gotlib | |
| Dubin, et al., (2012) | N=131; 66 HR (33.3), 65 LR (24.8) | SLF connectivity reductions correspond with depression, anxiety, and ADHD symptomology. | Weissman | |
| Gotlib, et al., (2010) | N=26 (10–14); 13 HR, 13 LR | HR daughters characterized by marked reductions in striatal activation during the anticipation and the receipt of reward. During gain anticipation, HR daughters exhibited higher right insula activation than did LR daughters. During punishment, HR greater activation in dACC than LR, who demonstrated greater activation in the caudate and putamen. | Gotlib | |
| Joormann, et al., (2012) | N=47; Females only (9–14); 20 HR, 27 LR | During the mood induction, HR girls exhibited greater activation than LR in amygdala and VLPFC. During mood regulation, LR exhibited greater activation than did HR in the DLPFC and dACC. | Gotlib | |
| Peterson, et al., (2009) | N=131; 66 HR (33.3), 65 LR (24.8) | The average reduction in cortical thickness in the lateral aspect of the right hemisphere of the HR group was a 28% reduction in average cortical thickness. Areas of statistically significant cortical thickening in the HR group were detected in the subgenual cortex, anterior and posterior cingulate gyrus, and medial orbitofrontal cortex of the right hemisphere. | Weissman | |
|
| ||||
| Electroencenphalography (EEG): | ||||
| Ashman, et al., (2008) | N=159 (6.5), 133 HR, 26 LR | Offspring of chronically depresses mothers demonstrated reduced frontal brain activation (EEG power). | Dawson | |
| Bruder et al., (2005) | N=87 (8–50); 18 two parents MDD, 40 one parent MDD, 29 LR | 2 MDD parent group showed greater medial alpha asymmetry, with less relative activity than 1 MDD parent group. Less relative frontal activity at lateral sites was associated with lifetime MDD in offspring with MDD but not with parents with MDD. | Weissman | |
| Bruder, et al. (2007) | N=49 (7–17); 19 HR parent and grandparent MDD, 14 HR 1 parent/grandparent MDD, 16 LR | HR grandchildren displayed a parietal alpha asymmetry similar to that seen in adolescents or adults with MDD. | Weissman | |
| Dawson, et al., (1997) | N=117 (13.7m); 23 postnatal MDD, 31 prenatal and postnatal MDD, 63 LR | HR infants were found to have reduced left frontal EEG activity. HR infants exhibited reduced left frontal EEG activity when compared to infants of mothers with subthreshold depression. | Dawson | |
| Dawson, et al., (1999a) | N=117 (13.7m); 54 HR, 63 LR | In infants with depressed mothers, reduced left frontal EEG correlated with lower levels of affection for mother but not infant temperament. Increased generalized frontal activation was related to higher levels of negative affect, hostility, tantrums, and aggression. | Dawson | |
| Dawson, et al., (1999b) | N=99; 59 HR (14.0), 40 LR (13.9) | HR infants were found to have reduced left, relative to right, activity during interactions with mothers and experimenters. | Dawson | |
| Dawson, et al., (2001) | N=159 (13–15m); 90 HR, 69 LR | HR infants demonstrated reduced left, relative to right, frontal brain activity in all conditions. | Dawson | |
| Dawson, et al., (2003) | N=124 (39.8 m); 65 HR, 59 LR | HR children whose mothers had chronic depression exhibited lower frontal and parietal brain activity as compared to HR mothers in remission and LR children. Frontal brain activation and contextual risk level were mediators between maternal depression and behavior problems. | Dawson | |
| Feng, et al., (2011) | N=73 (6–13); 43 HR, 30 LR | Results suggest that right frontal EEG asymmetry is consistent across situations and may be a marker of depression vulnerability. | Kovacs | |
| Forbes, et al., (2006) | N=57 (3–9); 41 HR, 16 LR | HR with greater relative left frontal activity had higher levels of internalizing and externalizing problems. | Kovacs | |
| Forbes, et al., (2008) | N=54 (4–7); 29 HR, 25 LR | Some evidence of maternal-child interaction influencing maternal depressive symptoms. Right frontal EEG symmetry in HR offspring during interaction tasks was associated with increased depressive symptoms in the mothers over time. | Kovacs | |
| Lopez-Duran, et al., (2012) | N=33 (6–10); 16 HR, 17 LR | HR children displayed greater relative right lateral frontal activation than LR during emotional film clips. Among HR, greater relative left lateral frontal activation moderated the association between stressful life events and internalizing symptoms. | Kovacs | |
| Perez-Edgar, et al., (2006) | N=74 (3–9); 44 HR, 30 LR | HR was significantly slower in reaction times on affective Posner task. HR group demonstrated larger P2 amplitudes to valid, relative to invalid, cues in frontal and central sites. LR group showed large mean amplitudes for valid trials, while HR showed large for the invalid trials, producing a large “reverse-validity” affect, most pronounced in frontal and parietal sites. | Kovacs | |
| Tomarken, et al. (2004) | N=38; 25 HR (13.1), 13 LR (13.0) | HR adolescents demonstrated a pattern of relative left frontal hypoactivity when compared to LR adolescents. | Garber | |
|
| ||||
| ENDOCRINE SYSTEM | ||||
|
| ||||
| HPA axis: | ||||
| Ashman, et al., (2002) | N=74 (7–8); 45 HR, 29 LR | Exposure to maternal depression in the first 2 years of life was the best predictor of elevations in baseline cortisol at age 7. | Dawson | |
| Dawson, et al., (2001) | N=159 (13–15m); 90 HR, 69 LR | HR infants demonstrated higher heart rates across all conditions. | Dawson | |
| Gotlib, et al., (2008) | N=67 (9–14); 25 HR (12.2), 42 LR (11.8) | Girls homozygous for s allele produced higher and more prolonged levels of cortisol in response to a lab stressor than girls with an l allele. | Gotlib | |
| Waugh, et al., (2012) | N=81 (9–14); 30 HR (11.9), 51 LR (11.8) | Among HR, decreased positive affect predicted increased cortisol reactivity, whereas increased negative affect was associated with poorer heart rate recovery and habituation. | Gotlib | |
|
| ||||
| AUTONOMIC NERVOUS SYSTEM | ||||
|
| ||||
| Startle: | ||||
| Grillon, et al., (2005) | N=108; 68 HR (34.9), 40 LR (35.8) | Generation 2 HR offspring increased startle magnitude to fear-potentiated startle test. Generation 3 HR offspring this pattern held in females only. | Weissman | |
|
| ||||
| Heart rate: | ||||
| Ashman, et al., (2008) | N=159 (6.5), 133 HR, 26 LR | Offspring of chronically depressed mothers demonstrated higher respiratory sinus arrhythmia reactivity. | Dawson | |
| Forbes, et al., (2006) | N=57 (3–9); 41 HR, 16 LR | Lower baseline RSA was linked with internalizing symptoms among HR but not LR participants. | Gotlib | |
| Gentzler, et al., (2009) | N=65 (5–13); 39 HR, 26 LR | No differences between HR and LR children; however, greater decreases in RSA in response to a sad film clip predicted more adaptive emotion regulation and lower depression levels. | Kovacs | |
| Gentzler, et al., (2012) | N=135 (6–13); 90 HR, 45 LR | LR children increase in resting RSA with age, but this does not occur in HR children, reflecting an atypical developmental trajectory. | Kovacs | |
| Santucci, et al., (2008) | N=54 (4–7); 29 HR, 25 LR | No differences between HR and LR children; however, lower vagal recovery and higher negative affectivity were associated with maladaptive emotion regulation in the face of frustration. | Gotlib | |
| Waugh, et al., (2012) | N=81 (9–14); 30 HR (11.9), 51 LR (11.8) | Among HR, decreased positive affect predicted increased cortisol reactivity, whereas increased negative affect was associated with poorer heart rate recovery and habituation. | Gotlib | |
|
| ||||
| GENES | ||||
|
| ||||
| Gotlib, et al., (2008) | N=55 (9–15); 23 HR (12.76), 32 LR (12.90) | Girls homozygous for s allele or serotonin transporter produced higher and more prolonged levels of cortisol in response to a lab stressor than girls with an l allele. | Gotlib | |
| Thompson, et al., (2011) | N=92 (12.1); 45 HR, 84 LR | HR youth heterozygous for the OXTR rs2254298 polymorphism (oxytocin gene) who also experienced early adversity had highest levels of depression and anxiety. | Gotlib | |
|
| ||||
| SLEEP | ||||
|
| ||||
| Chen, et al., (2012) | N=44 (14.1); 20 HR, 24 LR | HR girls reported lower sleep quality, with no significant difference in mood, actigraphy diary measured sleep, or sleep latency. | Gotlib | |
| Morehouse, et al., (2002) | N=81 (12–15); 41 HR, 40 LR | Temporal coherence was significantly lower among the HR girls than in controls. Additionally, 41% of those identified as having the most aberrant coherence values showed symptoms of depression or met diagnostic criteria. | Kutcher | |
|
| ||||
| LOW BIRTH WEIGHT | ||||
|
| ||||
| Nomura, et al., (2007) | N=224 (33); 162 HR, 82 LR | Offspring with low birth weight were at increased risk for MDD and other psychopathologies; this effect was stronger among the HR group. | Weissman | |
Notes: ROI = Region of Interest; VBM = Voxel-based morphometry; SLF = superior longitudinal fasciculus; MDD = Major Depressive Disorder; HR = High Risk; LR = Low Risk; RSA = Respiratory Sinus Arrhythmia, dACC = dorsal anterior cingulate cortex; VLPFC = ventro-lateral prefrontal cortex; DLPFC = dorso-lateral prefrontal cortex.
3.1. Central nervous system
3.1.1. Eeg
Table 3 illustrates remarkably consistent findings regarding greater relative right frontal EEG alpha asymmetries, greater parietotemporal asymmetries, and P300 abnormalities among LHR cohorts. In sum, five cohort studies have used various EEG measures in an attempt to capture brain-based putative endophenotypes of MDD. On the other hand, parietotemporal asymmetries are believed to reflect the arousal dimension of emotion (Heller et al., 1995), with decreased parietotemporal activity suggesting lower emotional arousal. Findings among HR individuals are consistent with documented abnormalities among acutely depressed individuals (e.g., Debener et al., 2000), suggesting they are potential trait vulnerability factors rather than state-related epiphenomena.
3.1.1.1. Frontal alpha asymmetries
One of the most well replicated findings across familial cohort studies is that HR offspring demonstrate asymmetries in alpha-band brain activity over frontal regions when compared to their LR peers. Greater left, relative to right, mid-frontal EEG activation (i.e., absence of alpha) is hypothesized to represent approach motivation, whereas greater right lateral frontal activation is hypothesized to represent withdrawal or blunted affect (Davidson, 1998). HR youth demonstrate greater right lateral frontal EEG activation, whereas LR youth demonstrate greater left lateral frontal EEG activation while watching emotional film clips (Lopez-Duran et al., 2012; Tomarken et al., 2004). Even among infants, demonstration of asymmetry reflecting emotion has been found (as displayed in Table 2). Specifically, links between left frontal hypoactivity with decreased positive affect and corresponding increases in negative affect have been observed among HR infants during mother-child interactions (Dawson et al., 1999a, 1999b). The reduced left frontal brain activity displayed by HR infants in this study was related to behavioral observations of less demonstrated affection during free play. In addition, increased frontal activation in both hemispheres was related to higher levels of negative affect, hostility, tantrums, and aggression longitudinally. Observed abnormalities were not specific to infant responses to depressed mothers but generalized to positive play interactions with non-depressed adults (in this case, a familiar lab experimenter).
The frontal alpha asymmetry research is not without inconsistency. Among HR children between the ages of three and nine who completed resting state EEG (see Table 3), results reveal contrasting patterns including correlations between greater relative left frontal activity and higher levels of internalizing and externalizing problems (Forbes et al., 2006). No EEG asymmetries were detected within Kovacs’ LHR study; however, relative right frontal EEG asymmetry both at baseline and in response to emotional (both happy and sad) film clips predicted elevated depression symptoms one year later (Feng et al., 2012), consistent with the hypothesis that asymmetry on the right compared to left is associated with the elaborated processing of emotion and traitlike tendencies towards withdrawal.
One benefit of following offspring longitudinally is observing the development of capacities that are implicated in risk for disorder. For example, at 14 months of age, HR infants exhibited reduced left frontal electrical brain activity (Dawson et al., 1997; Dawson et al., 1999a, 1999b). When assessed again at age three, HR offspring of mothers with chronic depression exhibited lower activations in frontal and parietal regions (higher alpha EEG power) compared to both LR offspring as well as HR offspring of remitted depressed mothers (Dawson et al., 2003). The combination of low frontal brain activation with exposure to contextual risks such as marital discord and maternal stress may mediate relations between maternal depression and offspring behavior problems. Findings were replicated when offspring were school-age; however, the association between maternal depression and higher EEG alpha power held for the frontal, but not parietal, region (Ashman et al., 2008). Collectively, these studies illustrate that differences in approach and withdrawal tendencies can be detected very early in development and may be predictive of a maladaptive trajectory in the development of emotion regulation that place a child at increased risk for MDD.
3.1.1.2. Parietotemporal asymmetries
Fewer investigations have examined asymmetries in parietotemporal regions, but findings are consistent across three generations within one cohort (Table 3). Individuals at the greatest level of risk (offspring of two depressed parents compared to one or no depressed parents) displayed parietotemporal asymmetries in comparison to their LR counterparts (Bruder et al., 2005). In the second generation of this same cohort, offspring of two depressed parents demonstrated greater central and parietal alpha asymmetry at medial sites (Bruder et al., 2005), again deriving from relatively less activity/greater alpha in the right hemisphere. Offspring in generation three with two depressed parents demonstrated greater alpha asymmetry deriving from relatively less right than left hemisphere activity in the parietal region during the resting state (Bruder et al., 2007). Thus, consistencies exist across generations within one cohort study suggesting that HR offspring demonstrate lower approach motivation or dampened responsiveness to positive stimuli.
3.1.1.3. P300 abnormalities
A smaller set of cohort studies have examined P300 abnormalities, which are hypothesized to measure cognitive processes such as the allocation of attention during stimulus evaluation (Kok, 2001). During a selective attention task, HR youth demonstrated slower reaction times and larger P300 and slow wave amplitudes in frontal regions than LR youth (Perez-Edgar et al., 2006). HR youth did not perform any worse than LR youth in neutral conditions; however, they recruited greater cognitive resources in executive regions while processing valent stimuli. These patterns suggest difficulty in the efficient mobilization of executive attention under conditions of negative affect, representing poorer emotion regulation. This is consistent with the hypothesis that processing emotional stimuli is cognitively taxing for at-risk individuals.
3.1.2. MRI
Two LHR cohort studies have used neuroimaging in an attempt to identify brain-based biomarkers for MDD (see Table 3 for details). These studies have identified differences between HR and LR groups in brain structure, function, and connectivity.
3.1.2.1. Volumetric MRI
Recent evidence among HR cohorts (Table 3) suggests that cortical thinning may be observed prior to illness onset. Cortical thinning representing a 30% reduction in the lateral surface of the right cerebral hemisphere was documented among HR offspring (Peterson et al., 2009). Among HR offspring with diagnoses of MDD or anxiety, thinning was also observed in the left hemisphere and thickening was observed in the medial portion of the right cingulate cortex. Exploration of the clinical phenotype associated with these patterns suggests that reductions in whole brain cortical thickness are associated with increasing symptoms of inattention and decreasing visual memory abilities for faces. To date, this is the only LHR cohort study to examine cortical thickness; however, specific brain regions have been examined in Gotlib's cohort (see Table 3). Volumetric reductions were identified in the left hippocampus and right parahippocampal gyrus among HR compared to LR girls (Chen et al., 2012). In addition to cortical thinning, smaller hippocampi may form a risk marker for depressive illness, a finding that until recently was thought to be a marker of present illness, scar, or burden (e.g., (Savitz and Drevets, 2009)). Structural endophenotypes are worthy of further exploration among HR cohorts as preliminary results are promising.
3.1.2.2. Structural connectivity
Structural connectivity has also been examined in one cohort (see Table 3) and suggests white matter reductions in the superior longitudinal fasciculus (SLF; (Dubin et al., 2012)). The SLF is significant in that it connects frontal and parietal regions and passes through the dorso-lateral prefrontal cortex (dlPFC). In this specific study, white matter hypoplasia in the right hemisphere was correlated with symptoms of mood, anxiety and attention difficulties. Moreover, cortical thickness and white matter volumes were highly intercorrelated, pointing towards an underlying common mechanism.
3.1.2.3. Task-based functional MRI
Task-based fMRI has been used in one LHR cohort (see Table 3) and focused on reward processing and the cognitive control of emotion. HR girls demonstrated marked reductions in striatal activation during anticipation and receipt of reward (Gotlib et al., 2010). During anticipation of reward, higher right insula activation was observed among HR compared to LR girls. Differential sensitivity to reward was also demonstrated by greater activations in the dorsal anterior cingulate cortex (dACC) among the HR group during loss, but not during reward, outcomes. Greater activations in the amygdala and ventro-lateral prefrontal cortex (vlPFC) have been documented among HR compared to LR girls in response to instructions to regulate emotions following a sad mood induction (Joormann et al., 2012). Differences in the vlPFC are significant as evidence suggests this region may serve an important top-down inhibitory link between the lateral PFC and the amygdala (Johnstone et al., 2007). Finally, LR girls demonstrated greater agility in their ability to use a positive autobiographical memory to induce positive mood as demonstrated by greater activation in the dlPFC and dACC. These findings suggest that further explorations of reward and cognitive control among offspring at risk for MDD are likely to be illustrative.
What is clear is that brain abnormalities can be observed in HR individuals before the onset of depression, providing evidence that these markers may represent endophenotypes. Structural and functional findings among LHR cohorts converge in regions involved in cognitive control and emotional salience consistent with the broader literature on MDD.
3.2. Endocrine
3.2.1. Hypothalamic-pituitary-adrenal (HPA) axis
It is well known that the stress contributes to the development of depression. The HPA axis is one of the primary biological stress response systems in humans. HPA axis dysfunction has long been implicated in depression (e.g., (Pariante and Lightman, 2008)). Cortisol—a hormone that can be measured in saliva or plasma during experimental manipulations— has been examined as a proxy for HPA axis dysfunction and inappropriate responses to stress in two LHR cohorts (Table 3). Indeed, evidence suggests that increased salivary cortisol can be detected prior to onset of disorder (Gotlib et al., 2008). In fact, high cortisol reactivity in reaction to a lab stressor predicted risk status among adolescent daughters of depressed mothers (Waugh et al., 2012). In an independent cohort, maternal depression during the child's first two years of life was the best predictor of elevations in baseline cortisol at age seven (Ashman et al., 2002). LHR cohort studies of cortisol are somewhat limited and inconsistent, partly because of small samples and variability in HPA measurement techniques.
3.3. Autonomic
Three cohort studies have measured heart rate or respiratory sinus arrhythmia (RSA) as putative mechanisms of risk (see Table 3). Heart period is inversely related to heart rate and is hypothesized to be a biological probe of emotion regulation as heart period tends to decrease during affective challenge and recover following challenge. RSA reflects variation in inter-beat intervals of the heart deriving from the frequency of breathing and is controlled by the vagus nerve and the parasympathetic branch of the autonomic nervous system. Higher RSA is believed to be more adaptive in that it reflects a greater capacity for self-regulation or physiological flexibility (e.g., (Porges, 1995)). Among HR, but not LR youth ages three to nine, lower baseline RSA was associated with symptoms of psychopathology (Forbes et al., 2006). Higher RSA reactivity has also been documented among HR offspring (Ashman et al., 2008). This study failed to find differences in baseline vagal tone; however, children of chronically depressed mothers demonstrated greater parasympathetic withdrawal in response to emotion-eliciting film clips. All children demonstrated RSA reductions and heart rate accelerations during neutral and negative emotion-eliciting film clips, indicating vagal withdrawal. In contrast, HR offspring of chronically depressed mothers exhibited a more pronounced reduction in RSA across all conditions, including the happy film condition (when increases in RSA would be expected).
Findings from three different cohorts (see Table 3) are consistent in documenting higher and less variable heart period during rest and recovery among HR youth; however, findings vary across development. For example, in the context of recovery from disappointment, HR youth continued to demonstrate an increased heart period compared to LR peers (Forbes et al., 2006), suggesting that HR youth have difficulties regulating emotions. However, it remains unclear whether these youth have difficulty recovering from distress as opposed to difficulty experiencing pleasure. Increased coupling between affective and cardiovascular responses to stress has also been documented among HR youth in addition to poor habituation to repeated exposure to negative affect (Waugh et al., 2012). Linear growth modeling was used to investigate developmental patterns and a significant risk by age interaction suggested that LR children demonstrated increasing resting RSA with age. Trajectories diverged during the pre-pubertal years (Gentzler et al., 2012). Lastly, HR infants as young as 13–15 months exhibited higher heart rates during the Strange Situation task, indicating that these infants were more aroused than their LR peers (Dawson et al., 2001).
LHR studies in this domain are consistent in documenting irregularities in the physiological processing of emotion; however, negative findings are worth noting. Two publications with RSA deriving from one cohort failed to detect differences between HR and LR groups. In the first, greater decreases in RSA in response to a sad film clip predicted more adaptive emotion regulation and lower levels of depression across both HR and LR groups (Gentzler et al., 2009). In the second, lower vagal recovery and higher negative affectivity were associated with maladaptive emotion regulation in the face of frustration but did not discriminate high from low risk (Santucci et al., 2008). Despite these contrasts, overall results in this domain are consistent with the broader depression literature.
3.3.1. Startle
One cohort to date (see Table 3) has examined fear-potentiated startle. Startle magnitude predicted risk status among second generation offspring, but among third generation offspring, this was only predictive among females (Grillon et al., 2005). Startle is a relatively inexpensive biological measure that has been underutilized to date.
3.4. Genes
Genetic results deriving from one LHR have been published (see Table 3), focusing on serotonin, a neurotransmitter posited to contribute to risk for depression, and oxytocin, a neuropeptide that functions to facilitate social affiliation and is also linked to mood. Beginning with the influential Caspi paper in 2003 (Caspi et al., 2003), much attention has been given to gene-by-environment interactions between stressful life events and reductions in transcription levels among individuals with a short (ss or sL) allele due to a polymorphism of the promoter region (5-HTTLPR) of the serotonin transporter gene (SLC6A4). The serotonin transporter impacts the production of a protein that moves serotonin from the synaptic gap. Current understanding of exactly how this may influence risk for depression is unknown, as replication has proven difficult (Risch et al., 2009). At best, evidence suggests that the 5-HTT polymorphism may play a moderating effect on the stress diathesis, particularly when experienced early in life (Hammen et al., 2010). Indeed, HR girls homozygous for the s allele produced more prolonged levels of cortisol than girls with an l allele (Gotlib et al., 2008).
Oxytocin has also been explored as a putative mechanism in MDD given its documented stress-attenuating effects (e.g., (Scantamburlo et al., 2007)). Phasic oxytocin has been shown to interact with responsiveness of the HPA axis in rats (Neumann, 2002), and low concentrations of oxytocin are associated with adverse early parenting environments (e.g., (Heim et al., 2008)). Indeed, HR girls heterozygous for the oxytocin rs2254298 (OXTR) polymorphism who were also exposed to early adversity demonstrated the highest levels of depression and anxiety (Thompson et al., 2011). Genetic research in LHR studies, although still in its infancy, is consistent with the broader depression literature in implicating 5HTTLPR and OXTR as putative mechanisms between stress and depression outcomes.
3.5. Sleep
Two LHR cohorts have included measures of sleep (see Table 3). Polysomnography (PSG) records biophysiological changes that occur during sleep including EEG measures of the brain, eye movements, muscle activity, and heart rhythm. Sleep disruptions have long been associated with depressive disorder (Benca et al., 1992; Breslau et al., 1996; Buysse et al., 2008) and offer an additional avenue for the study of endophenotypes given the influence genes have on sleep architecture (e.g, (Kimura and Winkelmann, 2007)). When sleep was measured via actigraphy and self-reported sleep quality, HR girls reported poorer sleep quality than LR girls; however, no differences in actigraphy were detected, suggesting that HR girls may perceive parallel levels of sleep as less qualitatively satisfying than their LR counterparts (Chen et al., 2012).
EEG was used to explore sleep rhythm within one cohort (Morehouse et al., 2002). Temporal coherence, as measured through EEG, can be interpreted to reflect a breakdown in the synchrony and organization of sleep rhythms. Indeed, temporal coherence was significantly lower among the HR girls when compared to control. What is more, coherence values correctly classified 70% of the HR girls. Forty-one percent of the girls with the most abnormal coherence values either showed symptoms of depression or met diagnostic criteria upon follow-up.
Published investigations with LHR cohorts in the domain of sleep are few, but preliminary evidence from two cohort studies suggests that sleep abnormalities may improve prediction of who is at greatest risk for future depression.
3.6. Birth
3.6.1. Low birth weight
Only one cohort (see Table 3) has been used to publish data on birth weight as it relates to risk for depression. Results suggest an interactive relation between risk status and birth weight in predicting offspring depression (Nomura et al., 2007). Eighty-one percent of HR youth who also had low birth weight, defined as weight less than 2.5 kg, developed MDD. Across both HR and LR groups, low birth weight was associated with increased risk of MDD, anxiety disorders, suicidal ideation, and impaired functioning. Several theories exist that attempt to explain how low birth weight is related to increased risk for depression, including decreased prenatal nutrition, maternal nutrition, and brain development, and increased maternal stress and drug use; however, this area has been understudied within LHR design and overall this mechanism is not well understood.
4. Discussion
Results from our review suggest several consistencies across cohorts and ways in which findings previously only observed among acutely ill individuals may be identifiable prior to onset of disorder. Existing findings provide support for BIRD in suggesting that biological measurements may tap system dysfunction that can be measured through various forms of biological assessment. Several opportunities exist to capitalize on existing findings as they fall along the gene-by-environment heritability continuum to expand the applicability and usefulness of LHR studies. We suggest the following approaches, pointing to relevant examples that have not yet reached general usage. We note that the nature of retrospective review limits the bandwidth of what can be covered and conclude with clinical implications.
4.1. Recommendations
4.1.1. Combine modalities
Combining data across modalities may contribute to the demarcation of “biosignatures” of disorder in LHR studies. Indeed, within the Weissman cohort, anatomical MRI and resting EEG were combined to reveal correlations in regions where alpha asymmetry was greatest, in the medial and parietal lobes. Interestingly, cortical thinning did not mediate the relation between risk and alpha asymmetry (Bruder et al., 2012). Less cortical activity, reflected in greater alpha power, was associated with greater cortical thinning, particularly in the right posterior cortex. No other published LHR studies to date have examined relations across brain imaging modalities.
4.1.2. Cross-dataset collaboration
Similar to our suggestion to combine modalities, cross-dataset collaboration also would advance the field as challenges to conducting LHR studies include the expense and dedication required to retain the cohort and the fact that onset of illness can occur a decade or longer after enrollment. These difficulties mean there are few well-characterized longitudinal studies of risk and that sample sizes is limited. The relatively small sample sizes of LHR cohorts in comparison to other studies of biological measures makes replication efforts even more important. Combining data across cohorts has been an underutilized technique to date. Recently, a composite dataset of 2307 youth from multiple studies (not all HR, therefore not reviewed above) was used to examine the specific symptoms of appetite and weight gain in adolescent depression (Cole et al., 2012). Analyses of this composite dataset revealed trends that could not have been detected in individual datasets, demonstrating the utility of this method as well as the feasibility of investigators cooperating on such a project.
4.1.3. Assess reliability of identified risk factors
One proposed criterion of putative biomarkers is reliability over time and reproducibility (Ritsner and Gottesman, 2009). Most LHR studies have not yet demonstrated that findings are reliable over time. Nonetheless, data collection within several of these cohorts is ongoing and this could be emphasized further.
4.1.4. Examine sex differences
One understudied area among LHR cohorts is the effect of sex or gender on identified putative biological mechanisms. Patterns of risk may differ between the sexes, and this is compounded by the increases in prevalence rates for depression among females compared to males that emerge during adolescents (Nolen-Hoeksema and Girgus, 1994). Moreover, stress-arousal circuitry in the brain includes sexually dimorphic regions which can be influenced by fetal programming (Goldstein, 2006), making the examination of sex differences in the transmission of depression particularly warranted.
4.1.5. Examine hormones
Decreased growth hormone (GH) secretion following pharmacologic stimulation has been observed among individuals with depression (Dinan, 1998). Birmaher and colleagues (Birmaher et al., 2000) examined GH within a HR population (excluded due to mixed methods in deriving HR sample). No differences were detected at baseline; however, after an infusion of growth hormone releasing hormone (GHRH), irregularities in GH secretion were detected. Both the HR and LR groups demonstrated increased GH secretion, but the HR group produced significantly less, consistent with findings among acutely depressed children and adolescents (Birmaher et al., 1997). These findings suggest a stable, trait-dependent marker for those at risk for developing depression, and as the authors suggest, warrant longitudinal study. Sex hormones such as estrogens and progestins influence individual neurons in addition to the structure and activity of the neuronal circuits and are worthy of more attention within LHR studies (for a review of pubertal neuromaturation please see (Walker et al., 2004).
4.1.6. Reanalyze data to study dimensionality
Many LHR studies gathered rich data regarding early life from multiple informants (family factors, prenatal health, etc.) that could be analyzed to fit dimensions of mood disorders. MDD is a broad phenotype that likely captures multiple and overlapping symptomatology deriving from distinct and overlapping biological mechanisms. This approach is in line with the NIMH's strategic plan supporting the study of psychopathology from a dimensional framework (Research Domain Criteria, RDoC; (Cuthbert and Insel, 2010)) and with a growing literature conceptualizing MDD as occurring along a spectrum of affective disorders (Cardoso de Almeida and Phillips, 2013). The tradeoff of studying domains of function across disorders is that specificity between findings and disorder is reduced. However, many LHR cohorts include dimensional measures of symptoms of psycho-pathology and rich measurement from early life that could be analyzed with genetic data. Perhaps efforts to combine dimensional data across cohorts for genetic studies that require larger sample sizes would aid in the examination of domains of function and dysfunction in mental illness.
4.1.7. Study networks underlying risk rather than Regions of Interest (ROIs)
Neuroimaging research conducted among LHR studies suggests that there are important ROIs for translational treatment studies. However, examinations of network level dysfunction have not been maximized to date. To this point, a recent meta-analysis documented the large proportion of studies documenting differences between healthy individuals and those with MDD in the default mode network (DMN) (Hamilton et al., 2011). As such, investigations of network dysfunction are likely to be more illustrative and descriptive in mapping risk at the level of the brain. One specific method for examining patterns across ROIs involves pattern recognition, which has recently been used to identify those at risk. For example, one study of youth at risk for bipolar disorder during an emotional faces paradigm was analyzed using Gaussian Process Classifiers (GPC) and was able to differentiate between those who would go on to develop bipolar disorder from those who would stay well with 75% accuracy (Mourão-Miranda et al., 2012).
4.1.8. Examine the biological substrates of positive affect
One way to utilize an RDoC approach in LHR studies is to examine domains such as positive valence systems. To date, the substrates of individual responses to negative affect has led to results implicating the salience of negative information for those at risk for depression. Many investigators have noted that low positive emotionality may increase risk of depression by decreasing reactivity to positive reinforcers and rewards (Durbin et al., 2005; Kovacs and Lopez-Duran, 2010); however, compared to the examination of negative affect, much less data has been gathered regarding the role of reduced positive affect in risk for depression. Disparities between HR and LR populations in positive affect have been documented in very young children (Forbes et al., 2004), but the biological underpinnings of these disparities have not yet been adequately explored. Examining positive affect within a LHR study could be helpful in designing prevention programs designed to enhance at-risk individual's ability to engage in and sustain positive emotion across development, particularly through the passage of periods of vulnerability.
4.1.9. Continue examining genetic mechanisms, particularly in combination with experience, especially life stress
In a pilot study, Weissman and colleagues examined the promoter region of the serotonin transporter and found that 5HTTLPR genotype varied with risk status, in that the observed rates of individuals with a ss genotype was more than four times higher in the HR offspring compared to LR offspring (Talati et al., 2013). In comparing these rates to that of the general population, however, evidence emerged that the LR offspring demonstrated lower rates of the ss genotype, rather than the HR group having a higher prevalence of the ss genotype. These findings lend support to the hypothesis that genetic risk alone is not sufficient to catapult a HR individual into disease; rather, it is the interaction of genetic susceptibility with greater exposure to life stressors that ultimately leads to illness.
4.1.10. Investigate tryptophan depletion, which offers a unique window into risk
Five-hydroxy-L-tryptophan (L-5HTP) depletion studies offer a unique opportunity to examine serotonergic precursors (e.g., (Delgado et al., 1994)). For example, Birmaher and colleagues (Birmaher et al., 1997) found that following L-5HTP infusion, HR children and depressed children had similar responses, secreting significantly less cortisol regardless of sex. These authors were not able to demonstrate differences specific to HR, but other cross-sectional HR and MDD studies show cognitive and affective changes with tryptophan depletion (Benkelfat et al., 1994; Neumeister et al., 2004).
4.1.11. Start with the second generation
Finally, we offer one practical recommendation. Rather than starting with one cohort and following offspring over several generations, a targeted sample of HR and LR individuals would benefit from beginning with a sample of adults in their thirties and seek to interview participants’ parents as well as their offspring as they are born, thereby shortening necessary follow-up in a time of relative fiscal restraint.
A final note highlights the importance of the developmental continuum in understanding mechanisms of risk in the intergenerational transmission of depression. The brain circuitry and networks underlying these biological mechanisms are often not fully instantiated prior to the development of the first depressive episode. As such, child and adolescent development may not ever reach prototypic levels of functioning observed in LR cohorts. This is both an opportunity and a challenge. LHR cohorts may be inherently different in many ways that underlie the disorder, risk for the disorder, as well as abnormal compensation to address HR environments or aberrant biological mechanisms. Therefore, differences between HR and LR may be highly variable and some of them may even be adaptive. The use of LHR designs, then, is one way of defining which biological mechanisms indicate risk and which may represent facets of possible resilience.
4.2. Limitations
Our review is based on relatively narrow inclusion and exclusion criteria. As such, many interesting studies were excluded, but this weakness is offset by strengths such as the increased reliability of findings. For example, we excluded studies that relied exclusively on family history methods of interview as this method is known to be less reliable (Orvaschel et al., 1982). Rao (Rao et al., 2009; Rao et al., 2009a, 2009b, 2010) has published several LHR findings that are consistent with results we describe here, which may render our exclusion criteria overly conservative. Nonetheless, we think that using family history methods can introduce many false negatives and underpowered studies, so we did not include these publications in the current review. In addition, we excluded studies that defined HR using stress and personality measurement, such as those by Goodyer and colleagues (Goodyer et al., 2010) who defined HR based on high levels of life-stress and neuroticism. Again, this stemmed from our desire to examine biological mechanisms in the intergenerational familial transmission of MDD as a more prescriptive phenotype, as opposed to a broader phenotype that may derive from life events or other environmental factors. This strategy can tend to minimize environmental contributions, enhancing study of genetic and heritable risks. Finally, our longitudinal follow-up criterion led to the exclusion of several cross-sectional studies, or studies that have not yet reported longitudinal follow-up (e.g. (Monk et al., 2008)). It is noteworthy that these studies generally confirm findings from LHR studies. We propose that our focus on biological measures in LHR provides the most reliable, homogeneous, and conservative markers on which to base future studies and link important clinical outcomes.
In addition to the strategies by which we included and excluded studies, the LHR studies we review are not without limitation. First, it is difficult to balance demographics when recruiting these highly selected samples. Some cohorts may be biased by significant differences in variables such as pubertal status, sex, or socioeconomic status (SES). Although these factors are often included as statistical covariates, these factors may importantly interact with biological risk and could, by themselves, offer fruitful avenues for mechanistic studies. In addition, some LR populations may actually represent “super-controls” or resilient individuals. For example, Gotlib's LR sample consists of adolescent girls who have no psychopathology at study entry, which may not be representative of a more generalizable LR cohort deriving from the general community. While these groups offer an acceptable contrast for exploratory findings, in order for identified risk factors to be used effectively for prevention, they must discriminate HR individuals from the broader spectrum of vulnerability to resilience, an important strength of LHR designs. Lastly, LHR studies are often limited by smaller sample sizes, making well-powered investigations challenging.
5. Conclusion and clinical applications
LHR studies have contributed much to the literature regarding risk, onset, and progression of MDD across the lifespan. The inclusion of biological methods in these studies has resulted in a strong foundation from which future studies can be built. The time is ripe for new investigations designed to further explore these biological mechanisms. Perhaps the most important future research direction involves targeting identified risk factors through prevention studies. Biomarkers of vulnerability are not deterministic (Beauchaine et al, 2008), rather these systems are often malleable and provide fruitful targets for prevention and intervention. Blanket prevention programs are inefficient and plagued by low effect sizes due to low rates of actual conversion to disorder. The inclusion of biomarkers of risk may lead to enhanced program efficiency by targeting those with greatest risk. For example, accurately being able to identify those at “ultra-high-risk,” or at least at more proximal risk, could protect a vulnerable adolescent from the lifelong negative sequalae of depression. It is noteworthy that the majority of groups conducting LHR studies have advanced to pilot novel interventions targeting identified risks. For example, Peterson and colleagues have launched a study recruiting adolescents at risk for depression by nature of parental depression as well as cortical thinning upon baseline scan. This group is testing whether delivering cognitive behavior therapy (CBT) to these “ultra-high-risk” individuals can ameliorate cortical thinning and/or protect adolescents from MDD onset. In addition, Gotlib's group has been testing the effectiveness of real-time neurofeedback. Here, HR girls are instructed to dampen their amygdala activity in response to negative emotion by focusing on positive experiences. Preliminary results indicate that successful neurofeedback reduces biological reactivity to external stressors as measured with psychophysiological outcomes such as heart rate and skin conductance one week later (Gotlib, 2013). These studies highlight the importance of studying the neural correlates of risk for depression among adolescence while the brain continues to develop, particularly in regions involved in the cognitive control of emotion including the prefrontal cortex (e.g., Luna et al., 2001).
Acknowledgments
This paper is based on the following symposium presented at the 2012 annual meeting of the Society of Biological Psychiatry: M.M. Weissman & R.H. Jacobs (Chairs), Endophenotypes for Major Depressive Disorder (MDD) using High-Risk Design. Dr. Weissman outlined the initial ideas for both the symposium and this paper and the authors are grateful for this collaboration. The authors would also like to thank Adi Talati for his invaluable feedback on this manuscript and Ms. Mary Kelly and Ms. Alyssa Barba for their administrative assistance.
Role of Funding Sources. This manuscript was funded in part by NIMH 5R01MH036197 (PI Weissman) and 1P50MH090966 (PI Gingrich). Preparation of this manuscript was funded by 5T32MH016434 (PI Peterson supporting RHJ), and UL1TR00050 (to UIC Center for Clinical and Translational Science supporting RHJ). SAL was supported by NIH MH091811 and MH101487 and EEF was supported through NIH MH093605 and DA033612. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Contributors: RHJ designed the study. RHJ and JLR conducted literature searches and provided summaries of previous research studies. RHJ wrote the first draft of the manuscript and all authors contributed to and have approved the final manuscript.
References
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Develop Psychopathol. 2002;14(2):333–349. doi: 10.1017/s0954579402002080. http://dx.doi.org/10.1017/S0954579402002080. [DOI] [PubMed] [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H. Trajectories of maternal depression over 7 years: relations with child psychophysiology and behavior and role of contextual risks. Develop Psychopathol. 2008;20(1):55–77. doi: 10.1017/S0954579408000035. http://dx.doi.org/10.1017/S0954579408000035. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Develop Psychopathol. 2008;20(3):745–774. doi: 10.1017/S0954579408000369. http://dx.doi.org/10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49(8):651–668. doi: 10.1001/archpsyc.1992.01820080059010. http://dx.doi.org/10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young SN. Mood-lowering effect of tryptophan depletion: enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994;51(9):687–697. doi: 10.1001/archpsyc.1994.03950090019003. http://dx.doi.org/10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Kaufman J, Brent DA, Dahl RE, Perel JM, al-Shabbout M, Nelson B, Stull S, Rao U, Waterman GS, Williamson DE, Ryan ND. Neuroendocrine response to 5-hydroxy-L-tryptophan in prepubertal children at high risk of major depressive disorder. Arch Gen Psychiatry. 1997;54(12):1113–1119. doi: 10.1001/archpsyc.1997.01830240073010. http://dx.doi.org/10.1001/archpsyc.1997.01830240073010. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Dahl RE, Williamson DE, Perel JM, Brent DA, Axelson DA, Kaufman J, Dorn LD, Stull S, Rao U, Ryan ND. Growth hormone secretion in children and adolescents at high risk for major depressive disorder. Arch Gen Psychiatry. 2000;57(9):867–872. doi: 10.1001/archpsyc.57.9.867. http://dx.doi.org/10.1001/archpsyc.57.9.867. [DOI] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39(6):411–418. doi: 10.1016/0006-3223(95)00188-3. http://dx.doi.org/10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Warner V, Nomura Y, Grillon C, Hille J, Leite P, Weissman MM. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biol Psychiatry. 2005;57(4):328–335. doi: 10.1016/j.biopsych.2004.11.015. http://dx.doi.org/10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Warner V, Weissman MM. Grandchildren at high and low risk for depression differ in EEG measures of regional brain asymmetry. Biol Psychiatry. 2007;62(11):1317–1323. doi: 10.1016/j.biopsych.2006.12.006. http://dx.doi.org/10.1016/j.biopsych.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Bansal R, Tenke CE, Liu J, Hao X, Warner V, Peterson BS, Weissman MM. Relationship of resting EEG with anatomical MRI measures in individuals at high and low risk for depression. Hum Brain Mapp. 2012;33(6):1325–1333. doi: 10.1002/hbm.21284. http://dx.doi.org/10.1002/hbm.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KC, Burke JD, Jr, Regier DA, Rae DS. Age at onset of selected mental disorders in five community populations. Arch Gen Psychiatry. 1990;47(6):511–518. doi: 10.1001/archpsyc.1990.01810180011002. http://dx.doi.org/10.1001/archpsyc.1990.01810180011002. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso de Almeida JR, Phillips ML. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biol Psychiatry. 2013;73(2):111–118. doi: 10.1016/j.biopsych.2012.06.010. http://dx.doi.org/10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. http://dx.doi.org/10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42(7):696–702. doi: 10.1001/archpsyc.1985.01790300064008. http://dx.doi.org/10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Chen MC, Burley HW, Gotlib IH. Reduced sleep quality in healthy girls at risk for depression. J Sleep Res. 2012;21(1):68–72. doi: 10.1111/j.1365-2869.2011.00934.x. http://dx.doi.org/10.1111/j.1365-2869.2011.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DA, Cho SJ, Martin NC, Youngstrom EA, March JS, Findling RL, Compas BE, Goodyer IM, Rohde P, Weissman MM, Essex MJ, Hyde JS, Curry JF, Forehand R, Slattery MJ, Felton JW, Maxwell MA. Are increased weight and appetite useful indicators of depression in children and adolescents? J Abnorm Psychol. 2012;121(4):838–851. doi: 10.1037/a0028175. http://dx.doi.org/10.1037/a0028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B, Insel T. The data of diagnosis: new approaches to psychiatric classification. Psychiatry. 2010;73(4):311–314. doi: 10.1521/psyc.2010.73.4.311. http://dx.doi.org/10.1521/psyc.2010.73.4.311. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology. 1998;35(5):607–614. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Osterling J, Hessl D. Infants of depressed mothers exhibit atypical frontal brain activity: a replication and extension of previous findings. J Child Psychol Psychiatry. 1997;38(2):179–186. doi: 10.1111/j.1469-7610.1997.tb01852.x. http://dx.doi.org/10.1111/j.1469-7610.1997.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Self J, Panagiotides H, Hessl D, Yamada E, Rinaldi J. Frontal brain electrical activity in infants of depressed and nondepressed mothers: relation to variations in infant behavior. Dev Psychopathol. 1999a;11(3):589–605. doi: 10.1017/s0954579499002229. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Yamada E, Hessl D, Osterling J. Infants of depressed mothers exhibit atypical frontal electrical brain activity during interactions with mother and with a familiar, nondepressed adult. Child Dev. 1999b;70(5):1058–1066. doi: 10.1111/1467-8624.00078. http://dx.doi.org/10.1111/1467-8624.00078. [DOI] [PubMed] [Google Scholar]
- Dawson G, Ashman SB, Hessl D, Spieker S, Frey K, Panagiotides H, Embry L. Autonomic and brain electrical activity in securely- and insecurely-attached infants of depressed mothers. Infant Behav Dev. 2001;24(2):135–149. http://dx.doi.org/10.1016/S0163-6383(01)00075-3. [Google Scholar]
- Dawson G, Ashman SB, Panagiotides H, Hessl D, Self J, Yamada E, Embry L. Preschool outcomes of children of depressed mothers: role of maternal behavior, contextual risk, and children's brain activity. Child Dev. 2003;74(4):1158–1175. doi: 10.1111/1467-8624.00599. [DOI] [PubMed] [Google Scholar]
- Debener S, Beauducel A, Nessler D, Brocke B, Heilemann H, Kayser J. Is resting anterior EEG alpha asymmetry a trait marker for depression? Findings for healthy adults and clinically depressed patients. Neuropsychobiology. 2000;41(1):31–37. doi: 10.1159/000026630. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Price LH, Miller HL, Salomon RM, Aghajanian GK, Heninger GR, Charney DS. Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Arch Gen Psychiatry. 1994;51(11):865–874. doi: 10.1001/archpsyc.1994.03950110025005. http://dx.doi.org/10.1001/archpsyc.1994.03950110025005. [DOI] [PubMed] [Google Scholar]
- Dinan TG. Psychoneuroendocrinology of depression: growth hormone. Psychiatr Clin N Am. 1998;21(2):325–339. doi: 10.1016/s0193-953x(05)70008-3. http://dx.doi.org/10.1016/S0193-953X(05)70008-3. [DOI] [PubMed] [Google Scholar]
- Dubin MJ, Weissman MM, Xu D, Bansal R, Hao X, Liu J, Warner V, Peterson BS. Identification of a circuit-based endophenotype for familial depression. Psychiatry Res: Neuroimaging. 2012;201(3):175–181. doi: 10.1016/j.pscychresns.2011.11.007. http://dx.doi.org/10.1016/j.pscychresns.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC. Temperamental emotionality in preschoolers and parental mood disorders. J Abnorm Psychol. 2005;114(1):28–37. doi: 10.1037/0021-843X.114.1.28. http://dx.doi.org/10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- Fanous A, Gardner CO, Prescott CA, Cancro R, Kendler KS. Neuroticism, major depression and gender: a population-based twin study. Psychol Med. 2002;32(04):719–728. doi: 10.1017/s003329170200541x. http://dx.doi.org/10.1017/S003329170200541X. [DOI] [PubMed] [Google Scholar]
- Feng X, Forbes EE, Kovacs M, George CJ, Lopez-Duran NL, Fox NA, Cohn JF. Children's depressive symptoms in relation to EEG frontal asymmetry and maternal depression. J Abnorm Child Psychol. 2012;40(2):265–276. doi: 10.1007/s10802-011-9564-9. http://dx.doi.org/10.1007/s10802-011-9564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Cohn JF, Allen NB, Lewinsohn PM. Infant affect during parent-infant interaction at 3 and 6 months: differences between mothers and fathers and influence of parent history of depression. Infancy. 2004;5(1):61–84. doi: 10.1207/s15327078in0501_3. http://dx.doi.org/10.1207/s15327078in0501_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Fox NA, Cohn JF, Galles SF, Kovacs M. Children's affect regulation during a disappointment: psychophysiological responses and relation to parent history of depression. Biol Psychol. 2006;71(3):264–277. doi: 10.1016/j.biopsycho.2005.05.004. http://dx.doi.org/10.1016/j.biopsycho.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biol Psychol. 2009;82(2):156–163. doi: 10.1016/j.biopsycho.2009.07.002. http://dx.doi.org/10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzler AL, Rottenberg J, Kovacs M, George CJ, Morey JN. Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Dev Psychobiol. 2012;54(5):556–567. doi: 10.1002/dev.20614. http://dx.doi.org/10.1002/dev.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM. Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Horm Behav. 2006;50(4):612–622. doi: 10.1016/j.yhbeh.2006.06.029. http://dx.doi.org/10.1016/j.yhbeh.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Croudace T, Dudbridge F, Ban M, Herbert J. Polymorphisms in BDNF (Val66Met) and 5-HTTLPR, morning cortisol and subsequent depression in at-risk adolescents. Br J Psychiatry. 2010;197(5):365–371. doi: 10.1192/bjp.bp.110.077750. http://dx.doi.org/10.1192/bjp.bp.110.077750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH. Understanding and Reducing Risk for Depression. Paper presented at the Association for Psychological Science; Washington, D.C.: 2013. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. http://dx.doi.org/10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67(4):380–387. doi: 10.1001/archgenpsychiatry.2010.13. http://dx.doi.org/10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grillon C, Warner V, Hille J, Merikangas KR, Bruder GE, Tenke CE, Nomura Y, Leite P, Weissman MM. Families at high and low risk for depression: a three-generation startle study. Biol Psychiatry. 2005;57(9):953–960. doi: 10.1016/j.biopsych.2005.01.045. http://dx.doi.org/10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. http://dx.doi.org/10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D, Hazel NA, Najman JM. Chronic and acute stress, gender, and serotonin transporter gene-environment interactions predicting depression symptoms in youth. J Child Psychol Psychiatry. 2010;51(2):180–187. doi: 10.1111/j.1469-7610.2009.02177.x. http://dx.doi.org/10.1111/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Hazel NA, Brennan PA, Najman J. Intergenerational transmission and continuity of stress and depression: depressed women and their offspring in 20 years of follow-up. Psychol Med. 2012;42(5):931–942. doi: 10.1017/S0033291711001978. http://dx.doi.org/10.1017/S0033291711001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. http://dx.doi.org/10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heller W, Etienne MA, Miller GA. Patterns of perceptual asymmetry in depression and anxiety: implications for neuropsychological models of emotion and psychopathology. J Abnorm Psychol. 1995;104(2):327–333. doi: 10.1037//0021-843x.104.2.327. http://dx.doi.org/10.1037/0021-843X.104.2.327. [DOI] [PubMed] [Google Scholar]
- Johnstone Tom, van Reekum Carien M, Urry Heather L, Kalin Ned H, Davidson Richard J. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. http://dx.doi.org/10.1523/jneurosci.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. J Abnorm Psychol. 2012;121(1):61–72. doi: 10.1037/a0025294. http://dx.doi.org/10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002–1014. doi: 10.1016/s0006-3223(01)01129-5. http://dx.doi.org/10.1016/S0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Borges G, Nock M, Wang PS. Trends in suicide ideation, plans, gestures, and attempts in the United States, 1990-1992 to 2001-2003. J Am Med Assoc. 2005;293(20):2487–2495. doi: 10.1001/jama.293.20.2487. http://dx.doi.org/10.1001/jama.293.20.2487. [DOI] [PubMed] [Google Scholar]
- Kimura M, Winkelmann J. Genetics of sleep and sleep disorders. Cell Molec Life Sci. 2007;64(10):1216–1226. doi: 10.1007/s00018-007-6532-1. http://dx.doi.org/10.1007/s00018-007-6532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Lewinsohn PM, Seeley JR, Rohde P. A family study of major depressive disorder in a community sample of adolescents. Arch Gen Psychiatry. 2001;58(1):13–20. doi: 10.1001/archpsyc.58.1.13. http://dx.doi.org/10.1001/archpsyc.58.1.13. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38(3):557–577. doi: 10.1017/s0048577201990559. http://dx.doi.org/10.1017/S0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Lopez-Duran N. Prodromal symptoms and atypical affectivity as predictors of major depression in juveniles: implications for prevention. J Child Psychol Psychiatry. 2010;51(4):472–496. doi: 10.1111/j.1469-7610.2010.02230.x. http://dx.doi.org/10.1111/j.1469-7610.2010.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Gatsonis C, Paulauskas SL, Richards C. Depressive disorders in childhood. IV. A longitudinal study of comorbidity with and risk for anxiety disorders. Arch Gen Psychiatry. 1989;46(9):776–782. doi: 10.1001/archpsyc.1989.01810090018003. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Nusslock R, George C, Kovacs M. Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology. 2012;49(4):510–521. doi: 10.1111/j.1469-8986.2011.01332.x. http://dx.doi.org/10.1111/j.1469-8986.2011.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. http://dx.doi.org/10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, III, Guardino M, Masten CL, McClure-Tone E, Fromm S, Blair RJ, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165(1):90–98. doi: 10.1176/appi.ajp.2007.06111917. http://dx.doi.org/10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Morehouse RL, Kusumakar V, Kutcher SP, LeBlanc J, Armitage R. Temporal coherence in ultradian sleep EEG rhythms in a never-depressed, high-risk cohort of female adolescents. Biol Psychiatry. 2002;51(6):446–456. doi: 10.1016/s0006-3223(01)01297-5. http://dx.doi.org/10.1016/S006-3223(01)01297-5. [DOI] [PubMed] [Google Scholar]
- Mourão-Miranda J, Almeida JRC, Hassel S, de Oliveira L, Versace A, Marquand AF, Sato JR, Brammer M, Phillips ML. Pattern recognition analyses of brain activation elicited by happy and neutral faces in unipolar and bipolar depression. Bipolar Disord. 2012;14(4):451–460. doi: 10.1111/j.1399-5618.2012.01019.x. http://dx.doi.org/10.1111/j.1399-5618.2012.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. http://dx.doi.org/10.1016/S0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61(8):765–773. doi: 10.1001/archpsyc.61.8.765. http://dx.doi.org/10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115(3):424–443. doi: 10.1037/0033-2909.115.3.424. http://dx.doi.org/10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Wickramaratne PJ, Pilowsky DJ, Newcorn JH, Bruder-Costello B, Davey C, Fifer WP, Brooks-Gunn J, Weissman MM. Low birth weight and risk of affective disorders and selected medical illness in offspring at high and low risk for depression. Compr Psychiatry. 2007;48(5):470–478. doi: 10.1016/j.comppsych.2007.04.005. http://dx.doi.org/10.1016/j.comppsych.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvaschel H, Thompson WD, Belanger A, Prusoff BA, Kidd KK. Comparison of the family history method to direct interview. Factors affecting the diagnosis of depression. J Affect Disord. 1982;4(1):49–59. doi: 10.1016/0165-0327(82)90019-2. http://dx.doi.org/10.1016/0165-0327(82)90019-2. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trend Neurosci. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. http://dx.doi.org/10.1016/j.tins.2008.06.006". [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Fox NA, Cohn JF, Kovacs M. Behavioral and electrophysiological markers of selective attention in children of parents with a history of depression. Biol Psychiatry. 2006;60(10):1131–1138. doi: 10.1016/j.biopsych.2006.02.036. http://dx.doi.org/10.1016/j.biopsych.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams PB, Wickramaratne P, Weissman MM. Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci USA. 2009;106(15):6273–6278. doi: 10.1073/pnas.0805311106. http://dx.doi.org/10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. http://dx.doi.org/10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen CL, London ED, Poland RE. Contribution of hypothalamic-pituitary-adrenal activity and environmental stress to vulnerability for smoking in adolescents. Neuropsychopharmacology. 2009;34(13):2721–2732. doi: 10.1038/npp.2009.112. http://dx.doi.org/10.1038/npp.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Poland RE. Mechanisms underlying the comorbidity between depressive and addictive disorders in adolescents: interactions between stress and HPA activity. Am J Psychiatry. 2009a;166(3):361–369. doi: 10.1176/appi.ajp.2008.08030412. http://dx.doi.org/10.1176/appi.ajp.2008.08030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Poland RE. Risk markers for depression in adolescents: sleep and HPA measures. Neuropsychopharmacology. 2009b;34(8):1936–1945. doi: 10.1038/npp.2009.27. http://dx.doi.org/10.1038/npp.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Hammen CL, Poland RE. Longitudinal course of adolescent depression: neuroendocrine and psychosocial predictors. J Am Acad Child Adolesc Psychiatry. 2010;49(2):141–151. doi: 10.1097/00004583-201002000-00008. http://dx.doi.org/10.1016/j.jaac.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem BS, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. J Am Med Assoc. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. http://dx.doi.org/10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner MS, Gottesman II. Where do we stand in the quest for neuropsychiatric biomarkers and endophenotypes and what next? In: Ritsner MS, editor. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes. Springer; Netherlands: 2009. pp. 3–21. Vol. I: Neuropsychological Endophenotypes and Biomarkers. [Google Scholar]
- Santucci AK, Silk JS, Shaw DS, Gentzler A, Fox NA, Kovacs M. Vagal tone and temperament as predictors of emotion regulation strategies in young children. Dev Psychobiol. 2008;50(3):205–216. doi: 10.1002/dev.20283. http://dx.doi.org/10.1002/dev.20283. [DOI] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164(1):300–330. doi: 10.1016/j.neuroscience.2009.03.082. http://dx.doi.org/10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32(4):407–410. doi: 10.1016/j.psyneuen.2007.01.009. http://dx.doi.org/10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. http://dx.doi.org/10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Talati A, Weissman MM, Hamilton SP. Using the high-risk family design to identify biomarkers for major depression. Philos Trans B. 2013;368(1615):20120–20129. doi: 10.1098/rstb.2012.0129. http://dx.doi.org/10.1098/rstb.2012.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36(1):144–147. doi: 10.1016/j.psyneuen.2010.07.003. http://dx.doi.org/10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Garber J, Simien C. Resting frontal brain activity: linkages to maternal depression and socio-economic status among adolescents. Biol Psychol. 2004;67(1–2):77–102. doi: 10.1016/j.biopsycho.2004.03.011. http://dx.doi.org/10.1016/j.biopsycho.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Walker EF, Sabuwalla Z, Huot R. Pubertal neuromaturation, stress sensitivity, and psychopathology. Dev Psychopathol. 2004;16(04):807–824. doi: 10.1017/s0954579404040027. http://dx.doi.org/10.1017/S0954579404040027. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Muhtadie L, Thompson RJ, Joormann J, Gotlib IH. Affective and physiological responses to stress in girls at elevated risk for depression. Dev Psychopathol. 2012;24(2):661–675. doi: 10.1017/S0954579412000235. http://dx.doi.org/10.1017/S0954579412000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM. Translating epidemiology in psychiatry: the future is here. Epidemiol Psychiatr Sci. 2012;21(2):167–169. doi: 10.1017/S2045796012000054. http://dx.doi.org/10.1017/S2045796012000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne P, Moreau D, Olfson M. Offspring of depressed parents: 10 years later. Arch Gen Psychiatry. 1997;54(10):932–940. doi: 10.1001/archpsyc.1997.01830220054009. http://dx.doi.org/10.1001/archpsyc.1997.01830220054009. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. Am J Psychiatry. 2006;163(6):1001–1008. doi: 10.1176/ajp.2006.163.6.1001. http://dx.doi.org/10.1176/appi.ajp.163.6.1001. [DOI] [PubMed] [Google Scholar]
- Wray Naomi R, Gottesman Irving I. Using summary data from the Danish national registers to estimate heritabilities for schizophrenia, bipolar disorder, and major depressive disorder. Front Genet. 2012;3(118) doi: 10.3389/fgene.2012.00118. http://dx.doi.org/10.3389/fgene.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Appendix A
- Anokhin AP, Heath AC, Myers E. Genetic and environmental influences on frontal EEG asymmetry: a twin study. Biol Psychol. 2006;71(3):289–295. doi: 10.1016/j.biopsycho.2005.06.004. http://dx.doi.org/10.1016/j.biopsycho.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Heath AC. Genetic and environmental influences on emotion-modulated startle reflex: a twin study. Psychophysiology. 2007;44(1):106–112. doi: 10.1111/j.1469-8986.2006.00486.x. http://dx.doi.org/10.1111/j.1469-8986.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJC. Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology. 2003;28(2):121–137. doi: 10.1016/s0306-4530(02)00003-3. http://dx.doi.org/10.1016/S0306-4530(02)00003-3. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, van Baal GCM. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol Psychol. 2002;61(1–2):111–138. doi: 10.1016/s0301-0511(02)00055-8. http://dx.doi.org/10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG: Int J Obstet Gynaecol. 2000;107(3):375–381. doi: 10.1111/j.1471-0528.2000.tb13234.x. http://dx.doi.org/10.1111/j.1471-0528.2000.tb13234.x. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Picchioni M, Ettinger U, Bramon E, Freedman R, Murray RM, Sham P. Heritability and reliability of P300, P50 and duration mismatch negativity. Behav Genet. 2006;36(6):845–857. doi: 10.1007/s10519-006-9091-6. http://dx.doi.org/10.1007/s10519-006-9091-6. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990;13(4):318–335. doi: 10.1093/sleep/13.4.318. [DOI] [PubMed] [Google Scholar]
- Smit DJA, Posthuma D, Boomsma DI, de Geus EJC. The relation between frontal EEG asymmetry and the risk for anxiety and depression. Biol Psychol. 2007;74(1):26–33. doi: 10.1016/j.biopsycho.2006.06.002. http://dx.doi.org/10.1016/j.biopsycho.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Snieder H, van Doornen LJP, Boomsma DI, Thayer JF. Sex differences and heritability of two indices of heart rate dynamics: a twin study. Twin Res Hum Genet. 2007;10(2):364–372. doi: 10.1375/twin.10.2.364. http://dx.doi.org/10.1375/twin.10.2.364. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, McClure C, Johnson BD, Sheps DS, Bittner V, Rutledge T, Shaw LJ, Sopko G, Olson MB, Krantz DS, Parashar S, Marroquin OC, Merz CN, Bairey CN. Depression, the metabolic syndrome and cardiovascular risk. Psychosom Med. 2007;70(1):40–48. doi: 10.1097/PSY.0b013e31815c1b85. http://dx.doi.org/10.1097/PSY.0b013e31815c1b85. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetic studies. Neuroimage. 2010;53(3):1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. http://dx.doi.org/10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


