Abstract
Objective
Overall survival (OS) in endometrial cancer (EC) is dependent on patient-, disease-, and treatment-specific risk factors. Comprehensive risk-scoring models were developed to estimate OS in low-grade and high-grade EC.
Methods
Patients undergoing primary surgery for EC from 1999 through 2008 were stratified histologically according to the International Federation of Gynecology and Obstetrics (FIGO) as either (i) low grade: grades 1 and 2 endometrioid EC or (ii) high grade: grade 3, including non-endometrioid EC. Associations between patient-, pathological-, and treatment-specific risk factors and OS starting on postoperative day 30 were assessed using multivariable Cox regression models. Factors independently associated with OS were used to construct nomograms and risk-scoring models.
Results
Eligible patients (N= 1281) included 925 low-grade and 356 high-grade patients; estimated 5-year OSs were 87.0% and 51.5%, respectively. Among patients alive at last follow-up, median follow-up was 5.0 (low grade) and 4.6 years (high grade), respectively. In low-grade patients, independent factors predictive of compromised OS included age, cardiovascular disease, pulmonary dysfunction, stage, tumor diameter, pelvic lymph node status, and grade 2 or higher 30-day postoperative complications. Among high-grade patients, age, American Society of Anesthesiologists score, stage, lymphovascular space invasion, adjuvant therapy, para-aortic nodal status, and cervical stromal invasion were independent predictors of compromised OS. The two risk-scoring models/nomograms had excellent calibration and discrimination (unbiased c-indices = 0.803 and 0.759).
Conclusion
Patients with low-grade and high-grade EC can be counseled regarding their predicted OS using the proposed risk-scoring models. This may facilitate institution of personalized treatment algorithms, surveillance strategies, and lifestyle interventions.
Keywords: Endometrial cancer, Low grade, High grade, Nomogram, Overall survival
Introduction
Although endometrial cancer is the most common gynecologic malignancy diagnosed in the United States, it is considered the most amenable to early diagnosis and definitive treatment, thus presupposing extended longevity [1]. Nevertheless, endometrial cancer consists of two patient populations differentiated by disparate risk factors and dissimilar long-term prognoses [2,3]. Uterine grades 1 and 2 endometrioid histologies encompass the majority of endometrial cancers, have excellent disease-free survival, and are associated with acquired risk factors [4]. These acquired risk factors, including obesity, diabetes, and metabolic syndrome, not only facilitate the pathogenesis of this disease but also, either directly or indirectly, impact overall survival [5–8]. On the contrary, grade 3 endometrioid, serous, and clear cell carcinomas are considered high risk and, while representing a minority of corpus cancers, they lack acquired risk factors and account for the majority of deaths from this disease [9]. Therefore, preoperative and postoperative counseling for these two disparate high-risk and low-risk populations must be sufficiently personalized to maximize treatment, surveillance, and lifestyle modifications.
Examination of overall survival as a function of time demonstrates dramatic differences between high-risk and low-risk endometrial cancer cohorts [10]. The former is characterized by marked attrition during the initial 2 to 3 years, while the latter exhibits a very gradual annual decline. Optimizing outcomes in these diverse cohorts will require individualized tailoring of care based on multiple clinical risk factors [11]. International Federation of Gynecology and Obstetrics (FIGO) staging incorporates disease-based stratification that estimates prognosis, thereby providing a standard for comparative evaluation of treatment outcomes [3]. However, for more effective counseling and tailoring of clinical decisions, patient- and treatment-specific parameters ideally should also be considered [12]. Statistical predictive outcome models and nomograms are clinically utilized in counseling and clinical decision-making in breast and other cancers [13–16]. In 2010, Abu-Rustum et al. [17] developed a nomogram to predict overall survival (OS) in endometrial cancer by combining five factors including age, number of negative nodes, 1988 FIGO stage, grade, and histology. Post-surgical treatment was not included in the modeling [12,17]. Considering the recognized demographic, pathological, and treatment differences between high-risk and low-risk endometrial cancer, models specifically targeting these two diverse populations would provide more patient-specific information, enabling personalized counseling and treatment. Thus, comprehensive risk-scoring models with enhanced discrimination were developed for the prediction of OS after 30 days post-surgery for both high-risk and low-risk endometrial cancer patients.
Methods
Study patients
This retrospective risk-adjusted outcome assessment was approved by the Mayo Clinic Institutional Review Board. Between January 1, 1999, and December 31, 2008, 1415 patients presenting with EC were counseled and elected to pursue primary surgical intervention. In compliance with the Minnesota Statue for Use of Medical Information in Research, 22 women who declined the use of their recorded medical information were excluded from the study. An additional 112 patients were excluded predominantly due to the presence of synchronous invasive cancers (n = 79) and neoadjuvant chemotherapy (n = 11), with the remaining exclusions distributed among non-epithelial carcinoma, death within 30 days of surgery, loss to follow-up within 30 days, or unknown date of death. Therefore, the eligible study population consisted of 1281 patients.
Treatment
The standardization of the Mayo Clinic surgical algorithm for EC evolved during the early phases of this study period, being formally implemented with prospective quality assessment in January 2004. Following hysterectomy and removal of the adnexal structures, prompt frozen section assessment was performed as previously described [18]. In the absence of extra-uterine disease and favorable intrauterine pathology (endometrioid, FIGO grade 1/2, primary tumor diameter ≤2 cm, myometrial invasion [MI] ≤50%, or noninvasive endometrioid regardless of grade or size), hysterectomy alone was deemed sufficient [19]. For specimens failing to meet these criteria, definitive surgical staging including lymphadenectomy up to the renal vessels was recommended, as well as cytoreduction in the presence of intra-abdominal disease [20].
Stage and architectural grade assignments were in accord with the 2009 FIGO classification system [21]. The World Health Organization's taxonomy principles were used to designate histologic subtypes [22]. Primary tumor diameter was defined as the largest of the three dimensions of the tumor. To ensure the accuracy of assigned diagnoses, pathology slides were reviewed by a single gynecologic oncology pathologist (G.L.K.).
In the presence of lymph node metastases, irradiation was delivered in standard doses of 45.0 to 50.4 Gy to the pelvis, and 45.0 Gy to the paraaortic fields when indicated. Systemic therapy with or without radiotherapy was administered when patients harbored advanced disease or were perceived to be at high risk for occult dissemination. Platinum-based combination chemotherapy, predominantly using paclitaxel or doxorubicin or both, was the adjuvant systemic treatment of choice. In the presence of grade 3 histology or lymph-vascular space involvement, adjuvant brachytherapy was generally administered alone or in combination with other regional or systemic therapies.
Data collection
More than 130 patient-, disease-, and treatment-specific variables were abstracted from medical records by a dedicated registered nurse using a modification of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) platform [23,24]. Patient and tumor registry records were periodically reviewed to ascertain current information regarding complications, disease progression, and vital status. When information detailing disease status was insufficient, death certificates were reviewed, letters were sent to patients and/or personal physicians, and telephone interviews were conducted to garner additional information.
Patient-specific risk factors including demographic variables, patient comorbidities, and American Society of Anesthesiologist (ASA) scores were recorded. Pulmonary disease was defined by the presence of at least one of the following: dyspnea, history of severe chronic obstructive pulmonary disease (COPD), current pneumonia, history of sleep apnea, or past/current continuous use of positive airway pressure (CPAP). Cardiovascular disease included a history of congestive heart failure (CHF) within 30 days of surgery, angina within 30 days of surgery, myocardial infarction within 6 months of surgery, cardiac stenting, cardiac surgery, revascularization, or amputation for peripheral vascular disease and/or rest pain/gangrene.
Clinical and surgical variables pertinent to this study included surgical approach (laparotomy vs minimally invasive surgery), type and extent of lymphadenectomy, number of lymph nodes harvested, and adjuvant therapy. An adequate systematic lymphadenectomy was defined as removal of at least 10 pelvic and 5 para-aortic lymph nodes. Postoperative complications within the first 30 days of surgery were abstracted and graded using the modified Accordion Severity Grading System [25]. The grades were collapsed for analysis purposes as none or grade 1, grade 2 or 3, and grade 4, 5, or, 6.
Pathology variables included FIGO grade, peritoneal cytology, presence of macroscopic extrauterine disease, cervical stromal invasion, lymphovascular space invasion, primary tumor diameter, and depth of myometrial invasion as a percentage of myometrial thickness. Patients were ultimately stratified histologically for the purpose of this study according to: (i) FIGO grades 1 and 2 endometrioid endometrial cancer (low-risk) and (ii) grade 3, including non-endometrioid endometrial cancer (high-risk).
Statistical analysis
The demographic, clinical, and pathologic characteristics of patients classified as low-risk versus high-risk were contrasted and compared using the two-sample t test for age and BMI (body mass index), the Wilcoxon rank sum test for the extent of myometrial invasion and number of nodes removed, and the χ2 test for all other variables. The primary outcome examined in this study was OS (defined as death occurring ≥30 days post-surgery). Duration of follow-up was calculated starting at 30 days post-surgery to the date of death or to the last follow-up for patients who were alive.
The following analyses were conducted separately for patients in the low-risk and high-risk strata. Clinical and pathologic variables were assessed for an association with OS by fitting univariable Cox proportional hazard regression models. Parsimonious multivariable models were identified using stepwise and backward variable selection methods and variables with a P value less than 0.05 were retained in the final model. Associations were summarized using the hazard ratio, and corresponding 95% confidence intervals (CIs) were estimated from the models. In order to identify the best fit, continuous variables were evaluated univariately as non-transformed, log-transformed, or using restricted cubic splines. Risk-scoring models and nomograms were created using R Software (version 2.14.0) for the two final models.
Predictive discrimination was assessed using the concordance (c-index), which is a measure of a model's overall predictive ability. The c-index varies from 0 to 1, and a value of 0.5 denotes no predictive discrimination. The c-index derived from the original model will be overly-optimistic, therefore a nearly unbiased estimate was derived using 300 bootstrap resamples of the same size as the original sample. Each bootstrap resample consists of a random sample of patients from the original sample, with replacement, such that some patients may be represented in the new sample multiple times and other patients not at all. As outlined by Harrell et al., for each bootstrap sample, a Cox regression model was fitted using the variables identified in the final model and the c-index was calculated. The model estimates derived from the bootstrap sample were then applied to the original sample of patients and the corresponding c-index was calculated; the difference in the two c-indices represents the optimism in the fit for that bootstrap sample [26]. This process was repeated for each of the 300 bootstrap resamples to obtain the average optimism. This optimism estimate was then subtracted off the original c-index to obtain the bootstrap corrected performance of the model. Calibration was assessed graphically by examining how far the 5-year predicted OS probabilities are from the actual 5-year probability of OS as estimated by the Kaplan–Meier method, based on patients grouped into quintiles according to their 5-year OS probability predicted by the final model. Statistical analyses were performed using the SAS version 9.2 software package (SAS Institute Inc) and R Software.
Results
Among the 1281 included patients, 925 patients with FIGO grade 1 or 2 endometrioid histology were classified as low-risk, and 356 patients with FIGO grade 3 or non-endometrioid histology were classified as high-risk. A total of 329 deaths have been documented after 30 days post-surgery (152/925 in low-risk and 177/356 in high-risk patients). The 5-year OS was 87.0% and 51.5%, respectively, for low-risk and high-risk patients. Among the patients alive at last follow-up, the median duration of follow-up was 5.0 (IQR, 3.1–7.6) and 4.6 (IQR, 2.7–7.3) years, respectively.
High-risk patients were older (mean age, 67.3 vs 63.5 years, P < 0.001) and had a lower mean BMI (mean, 30.7 vs 34.4 kg/m2, P < 0.001) in comparison to low-risk patients (Table 1), but there was no difference in the prevalence of comorbidities. Advanced-stage disease (stage III or IV) was prevalent in 46.9% of high-risk patients compared to 8.2% of low-risk patients. Of note, 80.9% of low-risk patients did not receive any form of adjuvant therapy, and of those receiving adjuvant treatment, nearly three-fourths were treated with vaginal brachytherapy or external beam radiation therapy with or without brachytherapy. A minority of patients underwent minimally invasive surgery, 5.6% high-risk compared to 17.5% low-risk patients. Pelvic and para-aortic lymphadenectomies were performed in 83.7% and 69.4% of high-risk patients, respectively, compared to 57.8% and 42.2%, respectively, in low-risk patients.
Table 1.
Characteristics of low-grade and high-grade endometrial cancer patients.
| Characteristic | Low-risk∞ (N = 925) |
High-risk∞ (N = 356) |
P value |
|---|---|---|---|
| Age at surgery (years), mean (SD) | 63.5 (11.3) | 67.3 (11.4) | <0.001 |
| BMI (kg/m2), mean (SD) | 34.4 (9.8) | 30.7 (7.8) | <0.001 |
| BMI classification | <0.001 | ||
| Underweight/normal/overweight (BMI < 30.0) | 350/924 (37.9) | 185/354 (52.3) | |
| WHO class I/II (BMI 30.0–39.9) | 336/924 (36.4) | 126/354 (35.6) | |
| WHO class III/super obese (BMI ≥ 40.0) | 238/924 (25.8) | 43/354 (12.1) | |
| Pulmonary disease† | 156 (16.9) | 54 (15.2) | 0.46 |
| Cardiovascular disease‡ | 72 (7.8) | 17 (4.8) | 0.06 |
| Diabetes | 205 (22.2) | 67 (18.8) | 0.19 |
| Smoking history | 314 (33.9) | 122 (34.3) | 0.91 |
| ASA score >2 | 367 (39.7) | 147/353 (41.6) | 0.52 |
| 2009 FIGO stage | <0.001 | ||
| Ia | 748 (80.9) | 151 (42.4) | |
| Ib or II | 101 (10.9) | 38 (10.7) | |
| IIIa, IIIb, IIIc1, or IIIc2 | 56 (6.1%) | 73 (20.5) | |
| IV | 20 (2.2) | 94 (26.4) | |
| LVSI | 75 (8.1) | 139 (39.0) | <0.001 |
| Residual disease | 4/923 (0.4) | 55 (15.4) | <0.001 |
| Primary tumor diameter >2 cm | 596/903 (66.0) | 262/344 (76.2) | <0.001 |
| Cervical stromal invasion | 28 (3.0) | 36/354 (10.2) | <0.001 |
| Myometrial invasion (%), median (IQR) | 12.0 (3.7, 33.3) | 25.0 (8.7, 71.4) | <0.001 |
| Gross extrauterine disease | 16 (1.7) | 73 (20.5) | <0.001 |
| Adnexal involvement | 19 (2.1) | 80 (22.5) | <0.001 |
| Serosal involvement | 13 (1.4) | 67 (18.8) | <0.001 |
| Positive peritoneal cytology | 77/788 (9.8) | 108/335 (32.2) | <0.001 |
| Laparotomy | 763 (82.5) | 336 (94.4) | <0.001 |
| Pelvic nodes removed, median (IQR) | 18 (0, 35) | 29 (19, 38) | <0.001 |
| Paraaortic nodes removed, median (IQR) | 0 (0, 13) | 10 (0, 17) | <0.001 |
| Pelvic lymph node status | <0.001 | ||
| No LND or inadequate/negative LND | 411 (44.4) | 63 (17.7) | |
| Adequate/negative LND | 467 (50.5) | 201 (56.5) | |
| Positive pelvic nodes | 47 (5.1) | 92 (25.8) | |
| Paraaortic lymph node status | <0.001 | ||
| No LND or inadequate/negative LND | 555 (60.0) | 118 (33.2) | |
| Adequate/negative LND | 343 (37.1) | 177 (49.7) | |
| Positive para-aortic nodes | 27 (2.9) | 61 (17.1) | |
| Adjuvant therapy | <0.001 | ||
| No adjuvant therapy | 722/892 (80.9) | 97/323 (30.0) | |
| Vaginal BT or EBRT ± BT | 121/892 (13.6) | 98/323 (30.3) | |
| Chemo ± BT or chemo and EBRT ± BT | 49/892 (5.5) | 128/323 (39.6) | |
| 30-day postoperative complications | <0.001 | ||
| None or grade 1 | 721 (77.9) | 215 (60.4) | |
| Grade 2 or 3 | 183 (19.8) | 125 (35.1) | |
| Grade 4, 5, or 6 | 21 (2.3) | 16 (4.5) |
Abbreviations: LND, lymphadenectomy; FIGO, International Federation of Gynecology and Obstetrics; ASA, American Society of Anesthesiologists, PA, para-aortic; EBRT, external beam radiotherapy; BT, brachytherapy.
Pulmonary disease is defined as a report of at least one of the following: dyspnea, history of severe chronic obstruction pulmonary disease (COPD), current pneumonia, history of sleep apnea, or past/current CPAP use.
Cardiovascular disease is defined as a report of at least one of the following: congestive heart failure (CHF) within 30days, history of myocardial infarction (MI) within 6months, previous cardiac stenting, previous cardiac surgery, history of angina within 30days, history of revascularization/amputation for peripheral vascular disease (PVD), or rest pain/gangrene.
Within low-risk patients, factors evaluated for an association with OS are summarized in Table 2. Among these variables, older age at surgery, cardiovascular disease, pulmonary dysfunction, advanced stage, primary tumor diameter greater than 2 cm, pelvic lymph node status, and 30-day postoperative complications were independently predictive of compromised OS. Specifically, a 10-year increase in age at surgery, presence of pulmonary disease, and presence of cardiovascular disease (as defined in the Methods section) each independently conferred nearly a two-fold increased risk of death. Furthermore, patients with stage IV disease (2.2% of low-risk patients) were approximately 16 times more likely to die of any cause (P < 0.001). Primary tumor diameter was the only pathology variable associated with poor OS in low-risk patients (adjusted hazard ratio 2.22 [95% CI 1.38, 3.58], P = 0.001). Noteworthy, among the 925 low-risk patients, 152 deaths have been documented of which 37 (24.3%) were due to disease.
Table 2.
Univariable and multivariable analysis of factors associated with overall survival in low-risk patients.
| Characteristic | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Age at surgery* | 1.89 (1.62, 2.21) | <0.001 | 1.80 (1.52, 2.12) | <0.001 |
| BMI* | 1.06 (0.98, 1.15) | 0.16 | ||
| Pulmonary disease† | <0.001 | 0.003 | ||
| No | Reference | Reference | ||
| Yes | 2.13 (1.48, 3.05) | 1.78 (1.21, 2.61) | ||
| Cardiovascular disease‡ | <0.001 | 0.001 | ||
| No | Reference | Reference | ||
| Yes | 3.65 (2.45, 5.42) | 2.04 (1.32, 3.15) | ||
| Diabetes | 0.026 | |||
| No | Reference | |||
| Yes | 1.51 (1.05, 2.16) | |||
| Smoking history | 0.11 | |||
| No | Reference | |||
| Yes | 1.31 (0.94, 1.81) | |||
| ASA score | <0.001 | |||
| ≤2 | Reference | |||
| >2 | 2.32 (1.68, 3.20) | |||
| 2009 FIGO stage | <0.001 | <0.001 | ||
| Ia | Reference | Reference | ||
| Ib or II | 2.34 (1.51, 3.64) | 1.70 (1.05, 2.75) | ||
| IIIa, IIIb, IIIc1, or IIIc2 | 7.38 (4.72, 11.51) | 4.80 (2.31, 9.99) | ||
| IV | 12.46 (7.03, 22.09) | 16.33 (8.24, 32.35) | ||
| LVSI | 0.022 | |||
| No | Referent | |||
| Yes | 1.72 (1.08, 2.73) | |||
| Residual disease | <0.001 | |||
| No | Reference | |||
| Yes | 21.94 (7.92, 60.75) | |||
| Primary tumor diameter | <0.001 | 0.001 | ||
| ≤2 cm | Reference | Reference | ||
| >2 cm | 3.07 (2.02, 4.66) | 2.22 (1.38, 3.58) | ||
| Cervical stromal invasion | <0.001 | |||
| No | Reference | |||
| Yes | 5.06 (2.96, 8.66) | |||
| Myometrial invasion* | 1.23 (1.17, 1.29) | <0.001 | ||
| Gross extrauterine disease | <0.001 | |||
| No | Reference | |||
| Yes | 9.61 (5.30, 17.45) | |||
| Adnexal involvement | <0.001 | |||
| No | Reference | |||
| Yes | 9.23 (5.29, 16.11) | |||
| Serosal involvement | <0.001 | |||
| No | Reference | |||
| Yes | 9.07 (4.89, 16.86) | |||
| Peritoneal cytology | <0.001 | |||
| Negative | Reference | |||
| Positive | 2.77 (1.72, 4.46) | |||
| Laparotomy | 0.37 | |||
| No | Reference | |||
| Yes | 0.82 (0.54, 1.26) | |||
| Pelvic lymph node status | <0.001 | 0.001 | ||
| No or inadequate/negative LND | 1.10 (0.77, 1.56) | 2.01 (1.36, 2.98) | ||
| Adequate/negative LND | Reference | Reference | ||
| Positive pelvic nodes | 6.41 (4.01, 10.27) | 1.15 (0.54–2.49) | ||
| Paraaortic lymph node status | <0.001 | |||
| No or inadequate/negative LND | 0.80 (0.55, 1.16) | |||
| Adequate/negative LND | Reference | |||
| Positive para-aortic nodes | 4.50 (2.37, 8.54) | |||
| Adjuvant therapy | <0.001 | |||
| No adjuvant therapy | Reference | |||
| Vaginal BT or EBRT ± BT | 1.94 (1.33, 2.84) | |||
| Chemo ± BT or chemo and EBRT ± BT | 2.52 (1.26, 503) | |||
| 30-day postoperative complications | <0.001 | <0.001 | ||
| None or grade 1 | Reference | Reference | ||
| Grade 2 or 3 | 2.83 (2.01, 3.99) | 2.03 (1.42, 2.91) | ||
| Grade 4, 5, or 6 | 3.17 (1.46, 6.91) | 3.97 (1.76, 8.98) | ||
Abbreviations: LND, lymphadenectomy; FIGO, International Federation of Gynecology and Obstetrics; ASA, American Society of Anesthesiologists, PA, para-aortic; EBRT, external beam radiotherapy; BT, brachytherapy.
Hazard ratio per 10-year increase in age, 5-kg/m2 increase in body mass index (BMI), and 10-unit increase in percent of myometrial invasion.
Pulmonary disease is defined as a report of at least one of the following: dyspnea, history of severe chronic obstruction pulmonary disease (COPD), current pneumonia, history of sleep apnea, or past/current CPAP use.
Cardiovascular disease is defined as a report of at least one of the following: congestive heart failure (CHF) within 30 days, history of myocardial infarction (MI) within 6 months, previous cardiac stenting, previous cardiac surgery, history of angina within 30 days, history of revascularization/amputation for peripheral vascular disease (PVD), or rest pain/gangrene.
By contrast, 177 deaths were documented among the 356 patients in the high-risk cohort of which 128 (72.2%) were due to disease. Notwithstanding the progressive incremental increase in the hazard ratio associated with advancing FIGO stage, the adverse impact on OS of older age at surgery, ASA score >2, lymphovascular space invasion (LVSI), cervical stromal invasion, metastatic involvement of para-aortic nodes, and adjuvant therapy retained independent significance in the multivariable modeling for the high-risk cohort (Table 3). Independent of other factors, the risk of death nearly doubled when para-aortic nodes were positive. Conversely, the administration of adjuvant therapy portended an improvement in OS; adjuvant chemotherapy with or without radiotherapy was associated with more than a two-fold extension of longevity. The independent benefit of radiotherapy (predominantly external beam ± vaginal brachytherapy) on OS in this cohort was unanticipated, and was essentially equivalent to that garnered with chemotherapy ± external beam radiotherapy. Nevertheless recent randomized and retrospective studies addressing the efficacy of chemotherapy and radiotherapy in grade 3 and/or type II endometrial cancer have yielded similar observations in early and advanced stage disease [27–30].
Table 3.
Univariable and multivariable analysis of factors associated with overall survival in high-risk patients.
| Characteristic | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Age at surgery* | 1.43 (1.25, 1.64) | <0.001 | 1.33 (1.14, 1.54) | <0.001 |
| BMI* | 1.01 (0.92, 1.11) | 0.86 | ||
| Pulmonary disease† | 0.031 | |||
| No | Reference | |||
| Yes | 1.54 (1.04, 2.27) | |||
| Cardiovascular disease‡ | 0.67 | |||
| No | Reference | |||
| Yes | 1.16 (0.59, 2.26) | |||
| Diabetes | 0.48 | |||
| No | Reference | |||
| Yes | 1.15 (0.78, 1.69) | |||
| Smoking history | 0.65 | |||
| No | Reference | |||
| Yes | 0.93 (0.68, 1.28) | |||
| ASA score | <0.001 | 0.005 | ||
| ≤2 | Reference | Reference | ||
| >2 | 1.87 (1.39, 2.53) | 1.61 (1.16, 2.23) | ||
| 2009 FIGO stage | <0.001 | <0.001 | ||
| Ia | Reference | Reference | ||
| Ib or II | 2.09 (1.17, 3.76) | 2.11 (1.11, 4.01) | ||
| IIIa, IIIb, IIIc1, or IIIc2 | 3.23 (2.08, 5.02) | 3.69 (2.17, 6.26) | ||
| IV | 7.65 (5.14,11.39) | 7.40 (4.50, 12.14) | ||
| LVSI | <0.001 | 0.002 | ||
| No | Reference | Reference | ||
| Yes | 2.56 (1.90, 3.45) | 1.71 (1.22, 2.42) | ||
| Residual disease | <0.001 | |||
| No | Reference | |||
| Yes | 4.85 (3.48, 6.78) | |||
| Primary tumor diameter | 0.05 | |||
| ≤2 cm | Reference | |||
| >2 cm | 1.44 (0.99–2.09) | |||
| Cervical stromal invasion | <0.001 | 0.020 | ||
| No | Reference | Reference | ||
| Yes | 3.18 (2.14, 4.73) | 1.71 (1.09, 2.69) | ||
| Myometrial invasion* | 1.18 (1.13, 1.23) | <0.001 | ||
| Gross extrauterine disease | <0.001 | |||
| No | Reference | |||
| Yes | 4.14 (3.02, 5.69) | |||
| Adnexal involvement | <0.001 | |||
| No | Reference | |||
| Yes | 3.55 (2.59, 4.86) | |||
| Serosal involvement | <0.001 | |||
| No | Reference | |||
| Yes | 3.69 (2.68, 5.08) | |||
| Peritoneal cytology | <0.001 | |||
| Negative | Reference | |||
| Positive | 3.38 (2.46, 4.65) | |||
| Laparotomy | 0.62 | |||
| No | Reference | |||
| Yes | 0.85 (0.44, 1.63) | |||
| Pelvic lymph node status | <0.001 | |||
| No or inadequate/negative LND | 2.91 (1.97, 4.29) | |||
| Adequate/negative LND | Reference | |||
| Positive pelvic nodes | 3.57 (2.54, 5.01) | |||
| Paraaortic lymph node status | <0.001 | 0.015 | ||
| No or inadequate/negative LND | 1.90 (1.34, 2.69) | 1.37 (0.94, 2.01) | ||
| Adequate/negative LND | Reference | Reference | ||
| Positive para-aortic nodes | 4.38 (2.99, 6.42) | 1.91 (1.23, 2.97) | ||
| Adjuvant therapy | <0.001 | <0.001 | ||
| No adjuvant therapy | Reference | Reference | ||
| Vaginal BT or EBRT ± BT | 0.36 (0.23, 0.55) | 0.41 (0.25, 0.67) | ||
| Chemo ± BT or chemo and EBRT ± BT | 0.95 (0.67, 1.35) | 0.44 (0.29, 0.67) | ||
| 30-day postoperative complications | 0.002 | |||
| None or grade 1 | Reference | |||
| Grade 2 or 3 | 1.64 (1.20, 2.23) | |||
| Grade 4, 5, or 6 | 2.16 (1.12, 4.14) | |||
Abbreviations: SD, standard deviation; IQR, interquartile range; BMI, body mass index; LND, lymphadenectomy; FIGO, International Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion; EBRT, external beam radiotherapy; BT, brachytherapy.
Hazard ratio per 10-year increase in age, 5-kg/m2 increase in BMI, and 10-unit increase in percent of myometrial invasion.
Pulmonary disease is defined as a report of at least one of the following: dyspnea, history of severe chronic obstructive pulmonary disease (COPD), current pneumonia, history of sleep apnea, or past/current CPAP use.
Cardiovascular disease defined as a report of at least one of the following: congestive heart failure (CHF) within 30 days, history of myocardial infarction (MI) within 6 months, previous cardiac stenting, previous cardiac surgery, history of angina within 30 days, history of revascularization/amputation for peripheral vascular disease (PVD), or rest pain/gangrene.
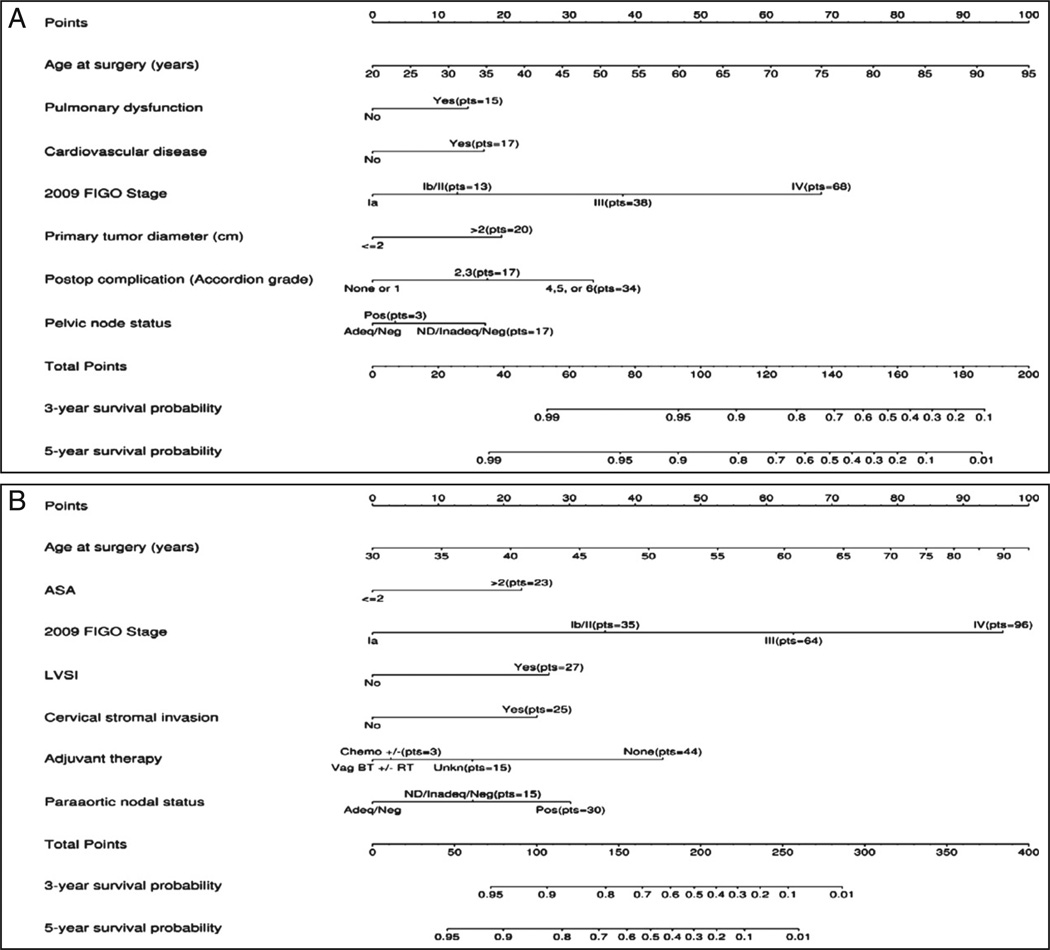
The two parsimonious multivariable models were re-fitted using a restricted cubic spline to accommodate a potential non-linear relationship for age and improve the model fit. Nomograms for each of the final models are depicted in Fig. 1A and B. For a given patient, points are assigned based on the patient's clinical and pathological profile, and a total score is derived. The bottom three axes are then used to determine the predicted 3-year and 5-year OS probability based on the patient's total points. The predicted probabilities for five hypothetical low-risk patients and five hypothetical high-risk patients with varying levels of risk are illustrated in Table 4A and B. Clinical scenario 1,with an estimated 5-year OS of 95%, fulfills the criteria for Mayo Clinic low-risk cases and, as noted, foregoes lymphadenectomy, but an added history of cardiac stenting and dyspnea on exertion would project a 5-year estimated OS of 84% (clinical scenario 2). In the absence of pulmonary dysfunction, cardiovascular disease, and postoperative complications, clinical scenarios 3, 4, and 5 illustrate decreasing OS rates with progressive increments in surgical stage. Clinical scenarios 6 through 10 provide a spectrum of hypothetical high-risk cases from one adequately staged with limited disease and a favorable ASA score (scenario 6) to another presenting with advanced disease and an ASA score of 3 or more in whom the merits of lymphadenectomy was not warranted and the patient elected to forego adjuvant therapy (scenario 10); corresponding 5-year OS estimates range from 91% to less than 1%.
Fig. 1.
A. Overall survival nomogram for low-risk patients (grades 1–2, endometrioid histology). B. Overall survival nomogram for high-risk patients (grade 3, non-endometrioid histology). Pelvic nodal status defined as ND, not done; Adeq, adequate (≥10 nodes removed); Inadeq, inadequate (<10 nodes removed); Neg, negative; and Pos, positive. Para-aortic nodal status defined as ND, not done; Adeq, adequate (≥5 nodes removed); Inadeq, inadequate (<5 nodes removed); Neg, negative; and Pos, positive. Vag BT denotes vaginal brachytherapy; RT, radiation therapy; Chemo±, chemotherapy ± radiation therapy ± vaginal brachytherapy.
Table 4.
Hypothetical low-risk case scenarios using the corresponding nomogram to determine the total risk score and estimated 5-year overall survival based on applicable values for each independent variable.
| A. Clinical scenarios for low-risk endometrial cancer | |||||
|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
| Age (years) | 67 | 67 | 67 | 67 | 67 |
| Pulmonary disease | No | Yes | No | No | No |
| Cardiac disease | No | Yes | No | No | No |
| Stage | IA | IA | IB | III | IV |
| Primary tumor diameter (cm) | ≤2 | ≤2 | >2 | >2 | >2 |
| 30-day complications | None or 1 | None or 1 | None or 1 | None or 1 | None or 1 |
| Pelvic lymphadenectomy | Not done | Not done | Adequate/negative | Positive | Positive |
| Total score | 73 | 105 | 89 | 117 | 147 |
| Predicted 5-year OS (%) | 95 | 84 | 91 | 76 | 39 |
| Abbreviation: OS, overall survival. | |||||
| B. Clinical scenarios for high-risk endometrial cancer | |||||
| Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | |
| Age (years) | 67 | 67 | 67 | 67 | 67 |
| ASA score | ≤2 | ≤2 | ≤2 | ≤2 | >2 |
| Stage | IA | IA | IB | III | IV |
| LVSI | No | No | Yes | Yes | Yes |
| Cervical stromal invasion | No | Yes | Yes | Yes | Yes |
| Adjuvant therapy | Vag BT ± RT | Vag BT ± RT | Vag BT ± RT | Vag BT ± RT | No |
| Para-aortic lymphadenectomy | Adequate/negative | Adequate/negative | Adequate/negative | Positive | Not done |
| Total score | 75 | 100 | 162 | 221 | 305 |
| Predicted 5-year OS (%) | 91 | 85 | 55 | 13 | <1 |
Abbreviations: ASA, American Society of Anesthesiologists: LVSI, lymphovascular space invasion; OS, overall survival; RT, radiation therapy; Vag BT, vaginal brachytherapy.
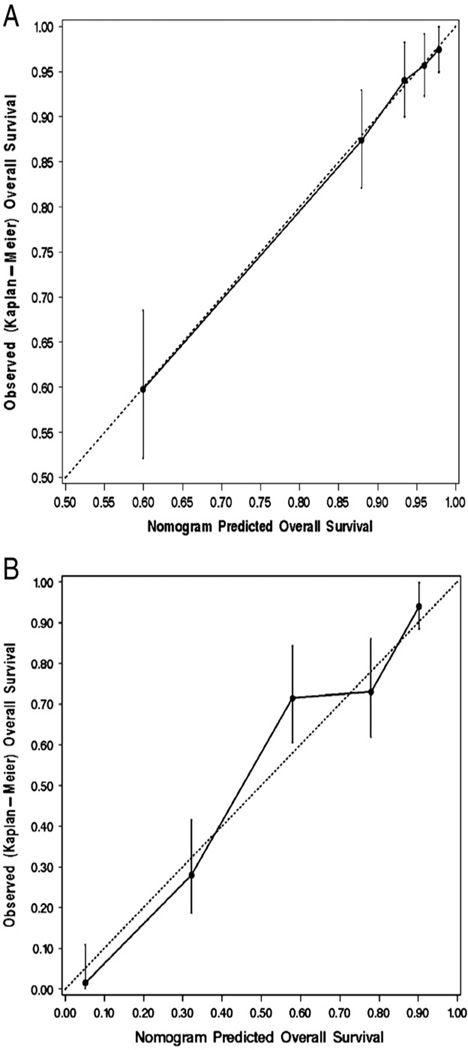
The performance of the two final models was assessed through calibration and discrimination. A graphical assessment of calibration was made by determining how far the predicted probabilities are from the actual observed 5-year probability of OS as estimated by the Kaplan–Meier method. The models had excellent calibration, as illustrated in the calibration plots in Fig. 2A and B. Discrimination was measured using the c-index. The unbiased estimate of the c-index derived from bootstrap resamples was 0.803 and 0.759 for the models for the low-risk and high-risk patients, respectively; both of these estimates indicate that the models have substantial predictive discrimination.
Fig. 2.
A. Calibration plot for 5-year overall survival (OS) probability based on the multivariable models for low-risk patients. B. Calibration plot for 5-year overall survival (OS) probability based on the multivariable models for high-risk patients. The dashed line indicates the ideal reference line where the predicted 5-year OS probabilities estimated from each model would match the Kaplan–Meier estimates of 5-year OS. Patients were grouped into quintiles according to their 5-year OS probability predicted by the final model. The vertical bars denote the 95% confidence interval for the Kaplan–Meier OS estimate derived for the patients in each quintile.
Discussion
Clinicopathological characteristics, as well as molecular profiles, readily distinguish low-grade endometrioid from type II histology, while high-grade endometrioid simulates and frequently coexists with serous or clear cell carcinomas, affording stratification into low-risk and high-risk subpopulations [9]. Low-risk endometrial cancers are associated with acquired risk factors such as obesity, diabetes, and/or metabolic syndrome, which often impact OS more than the patient's cancer [5,6,8]. Hence, the low-risk endometrial cancer diagnosis is representative of a generalized health dilemma among patients who are often cured of their endometrial cancer. On the contrary, the high-risk cohort represents a minority of endometrial cancers but accounts for the greater majority of cancer-related deaths; this is a continuing oncologic quandary [9]. These disparate outcomes illustrate a need for more individualized counseling regarding prognosis, therapeutic options, surveillance strategies, and lifestyle modifications. To facilitate more personalized care, comprehensive risk-scoring models, incorporating patient, disease, and treatment-specific variables, were developed for both low-risk and high-risk endometrial cancer patients to predict 3- and 5-year OS with high levels of accuracy.
In this study, low-risk patients accounted for 72.2% of the endometrial cancer cohort with an associated 5-year OS of 87%. Seven independent risk factors were identified as being predictive of poor OS. Of these risk factors, four are potentially modifiable, including pulmonary dysfunction, cardiovascular disease, pelvic lymphadenectomy, and postoperative complications. While BMI and diabetes mellitus were not identified as independent risk factors, their ultimate effect may be on pulmonary and cardiovascular biology. Comparison of case scenarios 1 and 2 (Table 4A) illustrates an 11% diminution on OS when both comorbidities are present. The omission of an adequate pelvic lymphadenectomy is considered modifiable; the intentional lymphadenectomy omission in case scenario 1 is warranted with an OS of 95%. Likewise, reduction in postoperative complications can be anticipated with judicious use of prophylactic guidelines and minimally invasive surgery [31–34]. While adjuvant therapy, including both radiotherapy and chemotherapy, was significant on univariate analysis, it failed to reach independence on multivariate analysis, possibly suggesting under-utilization, lack of efficacy, and/or insufficient numbers of at-risk patients. Non-modifiable variables, as illustrated in case scenarios 3, 4, and 5, were age, advanced stage, and primary tumor diameter, which either solely or collectively adversely affected OS. Utilizing age, stage, primary tumor diameter and pelvic node status, the nomogram estimates the impact of these disease-specific variables on OS thereby facilitating prognosticating, counseling and treatment planning particularly regarding the potential need for additional surgery or adjuvant therapy. The addition of pulmonary dysfunction, cardiovascular disease and post-operative complications will provide the incremental contribution of these factors to OS for counseling regarding potential lifestyle alterations and surveillance.
By comparison, 27.8% of the endometrial cancer population was deemed high-risk attested to by the prevalence of extrauterine disease (47%) and an estimated 5-year OS of 51.5%. Considering OS approximated disease-related survival in this high-risk cohort, it was again judged to be the most appropriate metric for use in counseling high-risk patients and treatment planning. Seven independent variables influencing OS were identified and comprise the HR nomogram, risk score calculation, and estimation of longevity. Of the seven risk variables, age and the four pathology parameters (stage, LVSI, cervical stromal invasion, and positive para-aortic node status) are non-modifiable but represent cogent references for treatment planning and counseling, as illustrated via case scenarios 6 through 10.With specialized medical intervention, the ASA score is potentially modifiable pre- and/or postoperatively. Likewise, the administration of adjuvant therapy is considered a modifiable component of care, but the elements that encompass selection and acceptance are multifactorial. While adjuvant therapy portended a definitive survival advantage, the roles of radiotherapy or chemotherapy, or a combination of both, were independently efficacious. The value of both modalities in treatment planning and counseling should not be underestimated.
It is evident from the proposed risk-scoring models that the independent risk factors are sufficiently diverse between low-risk endometrial cancer and high-risk endometrial cancer and separate models are required for use in prognosticating, treatment planning, and counseling. The development of risk-scoring models for estimating OS should encompass patient-, disease-, and treatment-specific parameters extending from diagnosis through sufficient surveillance. OS nomograms restricted to pathological variables and age at diagnosis, as proposed by Abu-Rustum et al. [16], omit pertinent clinical risk factors and adjuvant therapy status [12,17]. This may account for the slightly lower c-index attained in the Abu-Rustum nomogram (0.746) compared to our nomograms (c-index 0.803 and 0.759 for low-risk and high-risk patients, respectively).
The Adjuvant!™ early breast cancer computer calculator, which is in use worldwide, considers patient factors (age, menopausal status, and comorbidities) in addition to tumor-specific characteristics (tumor size, number of positive axillary lymph nodes, and estrogen receptor status) similar to our risk-scoring model [14,15]. Further, outcome risks are projected based on type of adjuvant therapy administered (endocrine therapy vs polychemotherapy). Post-surgical treatment was carefully incorporated into our analysis and is taken into account in determining final OS risk in high-risk patients. That is, a difference of 44 points between high-risk patients who received no adjuvant therapy and those who received chemotherapy, in addition to vaginal brachytherapy or external beam therapy and vaginal brachytherapy, has significant impact on 5-year OS. However, it must be acknowledged that the use of our nomogram may be limited in situations where clinical data may be missing.
Once externally validated, our risk-scoring models will be developed into risk calculators that should serve as important tools in patient counseling, disease management, and potentially endometrial cancer clinical trials. Incorporation of patient- and disease-specific risks in management and counseling is the cornerstone of individualized medicine and allows for informed patient and provider decisions.
HIGHLIGHTS.
Endometrial cancer consists of two disparate patient populations.
Risk scoring models were developed to predict overall survival in endometrial cancer.
Enhanced discrimination of risk scoring models enables personalized counseling and treatment.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983 Feb;15(1):10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006 Nov;95(Suppl. 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 4.Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol. 2012 Aug;126(2):176–179. doi: 10.1016/j.ygyno.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol. 2011 Dec;205(6):518–525. doi: 10.1016/j.ajog.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012 Nov;35(11):2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arem H, Irwin ML. Obesity and endometrial cancer survival: a systematic review. Int J Obes (Lond) 2013 May;37(5):634–639. doi: 10.1038/ijo.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013 Jul 10;31(20):2607–2618. doi: 10.1200/JCO.2012.48.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006 Mar 13;94(5):642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prat J. Prognostic parameters of endometrial carcinoma. Hum Pathol. 2004 Jun;35(6):649–662. doi: 10.1016/j.humpath.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Meyer JM, Ginsburg GS. The path to personalized medicine. Curr Opin Chem Biol. 2002 Aug;6(4):434–438. doi: 10.1016/s1367-5931(02)00340-x. [DOI] [PubMed] [Google Scholar]
- 12.Polterauer S, Zhou Q, Grimm C, Seebacher V, Reinthaller A, Hofstetter G, et al. External validation of a nomogram predicting overall survival of patients diagnosed with endometrial cancer. Gynecol Oncol. 2012 Jun;125(3):526–530. doi: 10.1016/j.ygyno.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008 Mar 10;26(8):1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 14.Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001 Feb 15;19(4):980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 15.Michaelson JS, Moore R, Garland A, Satija S, Halpern EF, Kopans DB. Computer simulation estimates of the consequences of various breast cancer screening strategies. Radiology. 1999 Nov;213P:240–241. [Google Scholar]
- 16.Kattan MW, Stapleton AMF, Wheeler TM, Scardino PT. Evaluation of a nomogram used to predict the pathologic stage of clinically localized prostate carcinoma. Cancer. 1997 Feb 1;79(3):528–537. [PubMed] [Google Scholar]
- 17.Abu-Rustum NR, Zhou Q, Gomez JD, Alektiar KM, Hensley ML, Soslow RA, et al. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: toward improving individualized cancer care. Gynecol Oncol. 2010 Mar;116(3):399–403. doi: 10.1016/j.ygyno.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreiro JA, Myers JL, Bostwick DG. Accuracy of frozen section diagnosis in surgical pathology: review of a 1-year experience with 24,880 cases at Mayo Clinic Rochester. Mayo Clin Proc. 1995 Dec;70(12):1137–1141. doi: 10.4065/70.12.1137. [DOI] [PubMed] [Google Scholar]
- 19.Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008 Apr;109(1):11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000 Jun;182(6):1506–1519. doi: 10.1067/mob.2000.107335. [DOI] [PubMed] [Google Scholar]
- 21.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009 May;105(2):103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Scully R, Bonfiglio T, Kurman R, Silverberg S, Wilkinson E. WHO—histological typing of female genital-tract tumors. Ann Pathol. 1995;15(4):296–297. [Google Scholar]
- 23.Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American college of surgeons national surgical quality improvement program an evaluation of all participating hospitals. Ann Surg. 2009 Sep;250(3):363–376. doi: 10.1097/SLA.0b013e3181b4148f. [DOI] [PubMed] [Google Scholar]
- 24.Khuri SF, Daley J, Henderson WG. The comparative assessment and improvement of quality of surgical care in the Department of Veterans Affairs. Arch Surg. 2002 Jan;137(1):20–27. doi: 10.1001/archsurg.137.1.20. [DOI] [PubMed] [Google Scholar]
- 25.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009 Aug;250(2):177–186. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996 Feb 28;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Bakkum-Gamez JN, Mariani A, Dowdy SC, Weaver AL, McGree ME, Martin JR, et al. Efficacy of contemporary chemotherapy in stage IIIC endometrial cancer: a histologic dichotomy. Gynecol Oncol. 2014;132(1):194–202. doi: 10.1016/j.ygyno.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Secord AA, Geller MA, Broadwater G, Holloway R, Shuler K, Dao NY, et al. A multicenter evaluation of adjuvant therapy in women with optimally resected stage IIIC endometrial cancer. Gynecol Oncol. 2013 Jan;128(1):65–70. doi: 10.1016/j.ygyno.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006 Jan 1;24(1):36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 30.Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer—results from two randomised studies. Eur J Cancer. 2010 Sep;46(13):2422–2431. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowdy SC, Borah BJ, Bakkum-Gamez JN, Kumar S, Weaver AL, McGree ME, et al. Factors predictive of postoperative morbidity and cost in patients with endometrial cancer. Obstet Gynecol. 2012 Dec;120(6):1419–1427. doi: 10.1097/aog.0b013e3182737538. [DOI] [PubMed] [Google Scholar]
- 32.Bakkum-Gamez JN, Dowdy SC, Borah BJ, Haas LR, Mariani A, Martin JR, et al. Predictors and costs of surgical site infections in patients with endometrial cancer. Gynecol Oncol. 2013 Jul;130(1):100–106. doi: 10.1016/j.ygyno.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141(2 Suppl.):e227S–e277S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander JW, Solomkin JS, Edwards MJ. Updated recommendations for control of surgical site infections. Ann Surg. 2011 Jun;253(6):1082–1093. doi: 10.1097/SLA.0b013e31821175f8. [DOI] [PubMed] [Google Scholar]