Abstract
Objective
Insomnia is commonly co-morbid with obstructive sleep apnea. Among patients reporting insomnia symptoms, sleep misperception occurs when self-reported sleep duration under-estimates objective measures. Misperception represents a clinical challenge since insomnia management is based entirely on patient self-report. We tested the hypothesis that misperception occurring in sleep apnea patients would improve with subsequent treatment.
Methods
We compared subjective sleep-wake reports with objective sleep architecture in a cohort of adults with obstructive sleep apnea (n=405) who had two nights of polysomnography (diagnostic and treatment) within a median interval of 92 days.
Results
Sleep latency was generally over-estimated, while wake after sleep onset and number of awakenings were under-estimated. None of these estimations differed between diagnostic and treatment polysomnography. We observed a large spectrum of total sleep time misperception values during the diagnostic polysomnogram, with one third of the cohort under-estimating their total sleep time by at least 60 minutes. Of those with >60 minute misperception, we observed improved total sleep time perception during treatment polysomnography. Improved perception correlated with improvements in self-reported sleep quality and response confidence. We found no polysomnogram or demographic predictors of total sleep time misperception for the diagnostic polysomnogram, nor did we find objective correlates of improved perception during titration.
Conclusion
Our results suggest that misperception may improve with treatment of obstructive sleep apnea in patients who also exhibit misperception. Within subject changes in misperception is consistent with misperception being, at least to some extent, a state characteristic, which has implications for management of patients with comorbid insomnia and sleep apnea.
Keywords: paradoxical insomnia, subjective, self-report
Introduction
Sleep misperception, the mismatch between objective laboratory polysomnogram (PSG) data and subjective self-reported patient accounts, has been reported most commonly in patients with insomnia, but can also occur in other sleep disorders such as obstructive sleep apnea (OSA)[1, 2]. The diagnostic category of “paradoxical” insomnia refers to an extreme form of misperception, in which the sleep is objectively normal yet the patient reports little or no sleep[3]. However, there is no accepted clinical framework for phenotyping patients with misperception with less severe manifestations, or in whom misperception occurs concurrently with another sleep disorder such as OSA. In our prior retrospective study of misperception, the range of sleep misperception among patients with OSA (with or without insomnia symptoms) was quite large during diagnostic PSG nights[4]. Vanable and colleagues showed no difference in total sleep time (TST) misperception among patients with OSA, and they too observed a large variation in that group[5].
The mechanisms underlying sleep misperception remain to be conclusively elucidated, in part because misperception is likely influenced by various psychological, cognitive, and physiological factors. For example, physiological arousal[6], alpha-delta sleep[7] (but not all studies have found a relation with alpha-delta sleep [8]), cyclic alternating pattern[9], rapid eye movement (REM) sleep or slow wave sleep content[10-12], high frequency EEG content[13, 14] and personality traits[15] have been associated with sleep misperception. Misperception can occur in healthy adults without sleep problems during routine laboratory and home conditions[16, 17], and with extended time in bed[18]. One compelling hypothesis regarding sleep misperception is that it relates to fragmentation of sleep architecture, in which light stages or high frequency awakenings might predict decreased perception of sleep. However, we have not been able to definitively link the degree of misperception to the amount of stage N1, the degree of fragmentation, or other sleep-wake stage composition metrics[4, 18].
Understanding misperception is important for several reasons: 1) objective short sleep may carry the preponderance of risk associated with insomnia[19]; 2) feedback techniques may improve the sleep of those with insomnia and misperception[20, 21]; and 3) patients who underestimate their sleep may develop increased arousal related to anxiety about insomnia, which could then result in objective sleep disturbance as a perpetuating factor[2, 22].
The approach to insomnia symptoms, and in particular sleep misperception, may be of particular interest for patients with comorbid OSA. Several cohort studies have suggested that those with insomnia may be at higher risk for OSA[23, 24]. Specifically, the prevalence of OSA (using various respiratory event rate cutoffs of 5, 10 or 15 per hour) ranged from 15-75%[25-31]. Similarly, patients with OSA are more likely to report insomnia symptoms, and concomitant treatment of both disorders may be mutually beneficial[24, 32]. In particular, treatment of OSA may improve insomnia symptoms, but it may also be the case that positive airway pressure (PAP) treatment itself represents disturbance disruptive stimulus in the susceptible insomnia patient.
Methods
This retrospective database study was approved by the Partners Institutional Review Board without requiring additional consent for use of clinically acquired data. Inclusion criteria were: age >16 who underwent clinical polysomnography in our sleep center between January 2009 and June 2013, exhibited a respiratory disturbance index (RDI) or apnea-hypopnea index (AHI) of greater than 5, had a subsequent titration PSG within 12 months of the diagnostic PSG. We excluded those with missing questionnaires (although partially completed subjective data was allowed) or who had <2 hours of sleep in either diagnostic or titration study. The final cohort included 405 patients. The reason for referral was not an inclusion criteria, but most were referred for OSA, and most were referred by non-sleep specialists. The median time between diagnostic and titration was 92 days. Our lab employs criteria for converting a diagnostic PSG into a titration study (split-night), based on severity, and thus the cohort studied here is enriched for mild to moderate severity OSA, although some severe cases were present. CPAP titrations were performed in accordance with the recommendations of the American Academy of Sleep Medicine. We separated those with AHI >10 during the titration study (n=74) as a pre-specified marker for incomplete titrations, meaning that substantial apnea persisted. Since our goal was to investigate potential impact of treatment in the sense that apneas and hypopneas were reduced on the titration night, it was important to avoid inclusion of patients with ongoing apneas and hypopneas (even if their titration was eventually successful in the later hours of the study). Therefore, we pre-specified an AHI value of 10 during titration as a cutoff in this regard; this subset represented approximately the top 20% of AHI values during titrations. The remainder of PSG metrics were specified according to routine clinical reporting. The spontaneous arousal index was used (instead of total arousal index) to distinguish arousals not related to breathing pauses, which are the dominant form of arousal in OSA patients.
Self-report data was obtained through pre-sleep and post-sleep questionnaires that are routinely administered for all clinical PSGs in our lab. Pre-sleep questionnaire responses were taken from the diagnostic PSG nights, while post-sleep questionnaires were used from both diagnostic and titration PSGs. Pre-sleep questionnaires were used to assess self reported insomnia symptoms. Formal insomnia diagnostic categorization was not possible because the majority of patients did not undergo evaluation by a sleep specialist. We used insomnia symptom data to pre-specify division of the OSA patients into four categories: onset insomnia, maintenance insomnia, both, or neither. Onset insomnia was defined as reporting taking 30-60 or more minutes to fall asleep and/or choosing “I have trouble falling sleep” as a complaint from a list of check-boxes. Maintenance insomnia was defined as selecting “When I wake up at night, it takes me a long time to fall back asleep”, and/or “I have trouble staying asleep”, and/or reporting waking up 3 or more times at night.
Post-sleep questionnaires were obtained on the morning after both the diagnostic and titration studies. Patients made subjective assessments of their sleep onset latency (Lat), total sleep time (TST), wake after sleep onset (WASO), number of awakenings, and overall quality of sleep. Each of these subjective queries was accompanied by a 7-point Likert scale rating the confidence of their response.
Statistics
Statistics were performed using Prism (GraphPad software, La Jolla, CA). Since most of the sleep measures being considered (TST, Latency, WASO, number of awakenings) were distributed non-normally, we used the non-parametric Kruskal-Wallis ANOVA (with Dunn's multiple comparison post hoc testing) for group comparisons, or Friedman's test for Likert Scale values. For the Tables, the pre-specified groupings within which to use these ANOVA tests were diagnostic versus titration groups (two groups), and misperception groups (three groups). When the paired ANOVA (Friedman test) was used, we removed subjects with incomplete data from the questionnaires (which amounted to <5% of the cohort). Correlation analysis was done using non-parametric methods. We pre-specified categorization of misperception based on a cutoff value of 60 minutes of under-estimation, based in part on our prior work showing underestimation of total sleep time in patients with insomnia of 45-80 minutes depending on the statistical methods. This pre-specified choice of cutoff does not cause undue imbalance of population size regarding subsequent statistical comparisons between groups.
Results
Table 1 shows the clinical characteristics and PSG metrics of patients with apneahypopnea index (AHI) values of <10 during their treatment PSG, also known as the CPAP “titration” PSG. The equivalent measures for the smaller subset with titration AHI values >10 are given in the supplement (Table S1). As expected, the median body mass index (BMI) values were high in our cohort. The median Epworth Sleepiness Scale (ESS) was in the normal range of ≤10, consistent with prior work in which even severe OSA patients generally score in the normal range[33]. Paired comparisons of diagnostic versus titration PSG did not reveal significant differences in any PSG metrics (Table 1). The relatively higher percentage of N1 and reduced REM and N3 percentages may be a combination of the presence of apnea-related fragmentation and the laboratory environment.
Table 1.
Demographics and PSG metrics for diagnostic and treatment PSGs
| n | 331 | |
| sex | 54% male | |
| age | 54 (16, 89) | |
| BMI | 31 (27, 35) | |
| ESS | 8 (5, 11) | |
| Time between studies (days) | 93 (47, 161) | |
| Dx | PAP | |
| Subj Lat (min) | 30 (15, 60) | 20 (15, 45) |
| Subj TST (min) | 360 (300, 420) | 360 (300, 420) |
| Subj # wakes | 3 (2, 5) | 3 (2, 5) |
| Subj WASO (min) | 20 (10, 60) | 20 (10, 45) |
| S-O Lat (min) | 15 (3, 34.4) | 10 (-3, 23.3) |
| S-O TST (min) | −34 (−91, 14) | −19 (−69, 36)* |
| S-O # wakes | −17 (−26, −10) | −16 (−23, −10) |
| S-O WASO (min) | −16 (−46, 5) | −22 (−49, −0) |
| TST (min) | 383 (345,417) | 374 (326,402) |
| Lat (min) | 5 (2,10) | 4 (1,10) |
| LPS (min) | 12 (4,27) | 13 (5,29) |
| REM Lat | 124 (84,205) | 104 (73,182) |
| N1% | 14 (10,23) | 12 (8,19) |
| N2% | 55 (48,62) | 52(45,62) |
| N3% | 12 (5,19) | 16 (8,24) |
| R% | 14 (9,19) | 16 (11,22) |
| Eff (%) | 88 (80,94) | 87 (78,93) |
| #30sW | 22 (14,30) | 20 (14,27) |
| #60sW | 11 (6,16) | 11 (7,16) |
| Sp AI (hr−1) | 3 (2,5) | 5 (3,7) |
| LMAI (hr−1) | 1 (0,4) | 2 (0,6) |
| PLMI (hr−1) | 4 (1,17) | 7 (1,24) |
| AHI (hr−1) | 13 (8,20) | 2 (1,4)* |
| AHI Supine | 17 (10,30) | 2 (1,5)* |
| AHI REM | 26 (12,44) | 2 (0,5)* |
| RDI (hr−1) | 27 (17,37) | 4 (2,7)* |
| O2 nadir REM | 85 (80,89) | 92 (89,94) |
| O2 nadir NR | 85 (82,88) | 90 (88,92) |
Median values, with 25-75% range shown in parentheses, separated by commas.
Significant differences using Kruskal-Wallis with Dunn's correction are given by.
AHI, apnea-hypopnea index; BMI, body mass index; Dx, diagnostic PSG; Eff, efficiency; ESS; Epworth Sleepiness Scale; Lat, latency; LMAI, limb movement arousal index; LPS, latency to persistent sleep; min, minutes; N1-N3, NREM stages of sleep; O2, oxygen; REM, rapid eye movement; PAP, positive airway pressure treatment PSG; PLMI, periodic limb movement index; S-O, subjective-objective difference; Sp AI, spontaneous arousals; subj, subjective; TST, total sleep time; WASO, wake after sleep onset; #30sW, number of wakes ≥30 seconds long; #60sW, number of wakes ≥60 seconds long.
In this cohort, based on the self-reporting of symptoms in pre-sleep questionnaires, insomnia symptoms were indicated in 310 patients (76.5%), with the 1majority of these also reporting one or more OSA symptoms. A minority of the cohort (n=41; 10.1%) reported insomnia symptom(s) but no OSA symptoms. By contrast, 125 (30.9%) reported OSA symptom(s) but none of the insomnia symptoms.
Misperception in diagnostic and titration PSGs
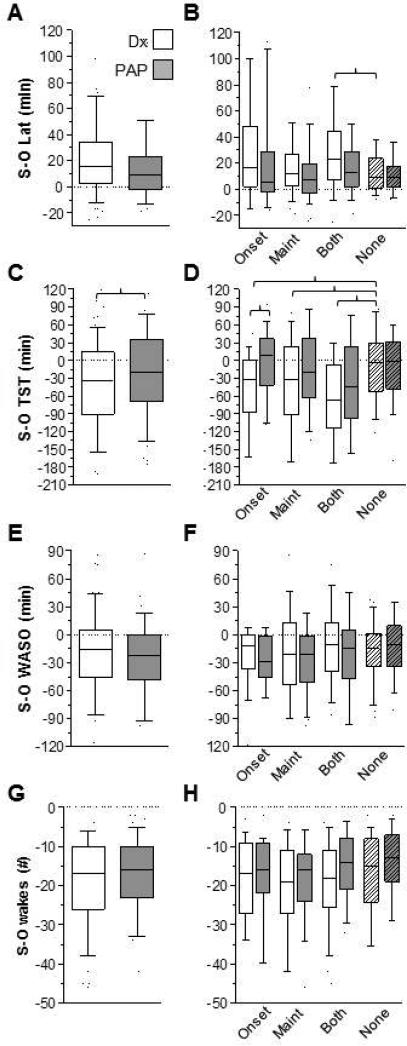
We quantified sleep-wake misperception by subtracting the objective PSG data from subjective durations obtained by post-sleep questionnaires. Negative values thus indicate that the subjective response was an under-estimate and positive values indicate the subjective response was an over-estimate. Figure 1 shows mismatch calculations from diagnostic and titration studies, including total sleep time (TST), sleep onset latency (Lat), wake after sleep onset (WASO), and number of awakenings. Misperception of TST was predominantly an underestimation, while misperception of latency was predominantly an over-estimation. In contrast, both WASO and the number of awakenings were predominantly under-estimated.
Figure 1. Mismatch between subjective and objective sleep-wake metrics.
The distribution of values for mismatch (subjective – objective) on diagnostic PSG nights (open) and titration PSG nights (gray) is given for latency (panel A), TST (panel C), WASO (panel E), and # of awakenings (panel G). Adjacent to each of these metrics, the distribution of mismatch values is sub-categorized according to the self-reported insomnia symptoms. Brackets indicate statistical significance based on Kruskal-Wallis with Dunn's correction. Box and whisker plots show the median and the 25th and 75th percentile, with the error bars indicating the 10-90% range of the data. Latency, WASO, and # awakenings were all significantly different than zero in both diagnostic and titration studies.
Of the measures studied, only TST misperception showed a significant difference between diagnostic and titration studies, after correction for multiple comparisons. We further subdivided each measurement of mismatch according to categories of self-reported insomnia: onset, maintenance, both, or none (see methods). Each of the insomnia symptom groups had a greater degree of TST under-estimation than the group that did not report any insomnia symptoms.
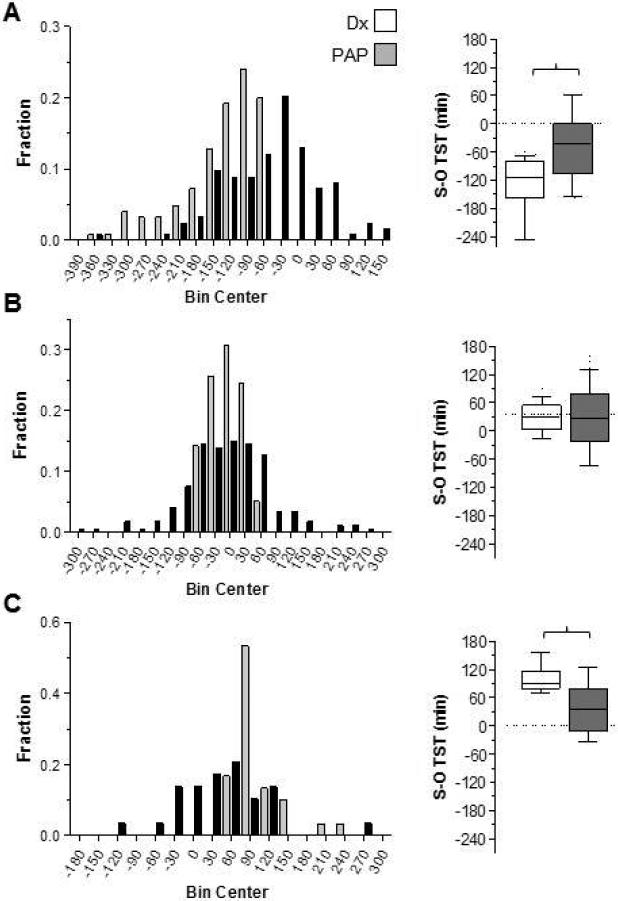
To investigate the apparent improvement in TST perception during the titration studies, we generated histograms for three subgroups according to the degree of TST mismatch: greater than 60 minutes under-estimation (Figure 2A), within 60 minutes (positive or negative) (Figure 2B), and greater than 60 minutes TST over-estimation (Figure 2C). In the group with more than 60 minutes under-estimation in the diagnostic studies, improved perception accuracy was observed during the titration studies (Figure 2A; p<0.05, Friedman paired test).
Figure 2. Distribution of TST mismatch grouped by the degree of misperception in the diagnostic PSG.
Frequency histogram plots of TST mismatch (subjective - objective; X-axis) is shown according to the degree of misperception during the diagnostic PSG (gray bars). The groups are separately shown according to those with >60 minutes of TST under-estimation (panel A), within 60 minutes of objective TST (panel B), or >60 minutes of over-estimation (panel C). In each panel, the distribution of TST mismatch during the titration PSG is also shown (black bars). Next to each panel, the box-and-whiskers plot of the same data is shown for visual comparison. The differences between diagnostic and titration studies were statistically significant for panels A and C (p<0.05, Friedman paired test; brackets).
In the smaller subset with >60 minutes of TST over-estimation in the diagnostic PSGs, we noted less over-estimation during the titration PSGs (Figure 2A; p<0.05, Friedman paired test). The pattern of improvement, regardless of whether under-or over-estimation occurred during the diagnostic PSG, raises the possibility of a “regression to the mean” phenomenon. Regression can occur with random processes, but it can also occur with deterministic processes that have sufficient unmeasured factors as to appear random (for example, a fair coin toss simply appears random because one cannot routinely assess the determinants). There is of course no reason to believe that patients randomly guess their subjective sleep duration; rather, the heuristics patients use to estimate sleep duration are likely influenced by multiple factors. We thus sought additional clues that treating OSA improves state misperception.
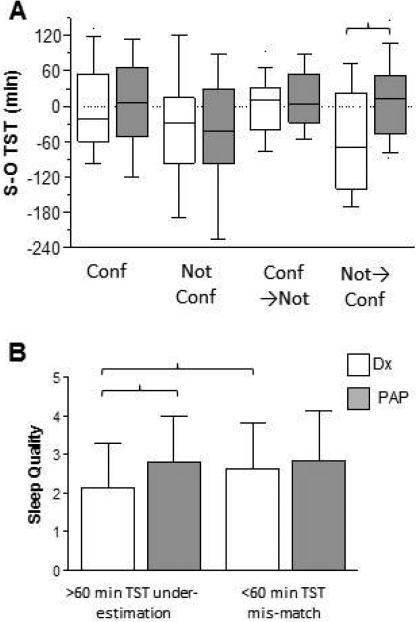
First we investigated whether the confidence in self-reported TST improved from diagnostic to titration studies, which argue against a simple regression to the mean phenomenon. We subdivided patients as “not confident” (Likert score of 1 or 2), versus “confident” (Likert score of 6 or 7), to look for patterns of change in perception between diagnostic and titration studies (Figure 3A). Improvement in misperception was seen only in those who were not confident during the diagnostic PSG then changed to report being confident during their titration PSG (p<0.005, Wilcoxin rank sum test). These results suggest that, although some regression to the mean may be occurring, this cannot by itself explain our observation of improvement during titration studies.
Figure 3. Improved confidence and sleep quality during titration PSGs among those with misperception.
Categorical patterns of confidence emphasizing extreme values (confidence = values of 6-7 on the 7-point scale; not confident = values of 1-2) indicates that only the subset who were not-confident in the diagnostic PSG and confident in the titration PSG showed a significant improvement in TST misperception (brackets; Kruskal-Wallis with Dunn's correction) (panel A). Panel B shows that pre-treatment sleep quality was lower in the group with misperception (>60 minute under-estimation of TST) than the group with <60 minutes of mismatch, and only the group with >60 minutes under-estimation showed significantly increased quality on PAP treatment (brackets; p<0.05, Kruskal-Wallis with Dunn's correction).
We also investigated whether self-reported sleep quality differed after treatment depending on the presence of misperception. Sleep quality was assessed after each PSG using a 5-point scale where higher is better quality. Sleep quality was lower during the diagnostic PSG in those with >60 minutes of TST under-estimation than those who perceived within 60 minutes of the objective TST (Figure 3B; p<0.05, Kruskal-Wallis with Dunn's post-hoc test). Importantly, sleep quality only improved during the titration night among those who showed >60 minutes of misperception during the diagnostic night (Figure 3B; p<0.05, Kruskal-Wallis with Dunn's post-hoc test). Taken together, the results suggest that treating OSA with CPAP improved misperception.
We also compared patients with >60 minutes of TST misperception with those who perceived TST within 60 minutes (Table 2). We did not find any demographic or PSG metrics that differed by this categorization, for diagnostic or titration PSG nights. In addition to this analysis based on a cutoff value of 60 minutes of under-estimation, we investigated whether TST misperception was correlated with various PSG metrics (sleep stage content, arousal index, PLMS) or demographics (age, sex, BMI, ESS). In this correlation analysis, we were not limited to pre-defined categories of under-estimation as described for the previous analysis. We did not find significant correlation coefficients between TST misperception and the PSG and demographic metrics shown in Table 2.
Table 2.
Demographics and PSG metrics during diagnostic and treatment PSGs by misperception group
| MP>60 | MP<60 | |||
|---|---|---|---|---|
| n | 125 | 175 | ||
| sex | 49.6% male | 58.5% male | ||
| age | 53 (28,89) | 54 (16,78) | ||
| BMI | 31 (27,36) | 30 (27,35) | ||
| ESS | 8 (5,12) | 7 (4,11) | ||
| Time between studies (days) | 93 (42,167) | 93 (48,160) | ||
| Dx | PAP | Dx | PAP | |
| S-Lat (min) | 45 (20,90) | 15 (30,60) | 25 (15,40) | 10 (20,45) |
| S-TST (min) | 270 (180,300) | 240 (318,360) | 360 (330,420) | 300 (360,420) |
| S-# wake | 4 (2,6) | 2 (3,5) | 3 (2,5) | 2 (3,5) |
| S-WASO (min) | 40 (15,90) | 10 (30,60) | 18 (10,30) | 10 (20,46) |
| S-O Lat (min) | 27 (10,54) | 2 (12,27) | 14 (1,27) | −1 (10,22) |
| S-O TST (min) | −114 (−158,−80) | −107 (−42,0) | −5 (−32,20) | −58 (−9,43) |
| S-O # wake | −20 (−27,−11) | −23 (−14,−9) | −15 (−23,−8) | −22 (−16,−10) |
| S-O WASO | −6 (−34,29) | −46 (−17,8) | −18 (−44,0) | −46 (−22,−3) |
| TST (min) | 392 (355,426) | 327 (377,404) | 382 (346,416) | 332 (371,403) |
| Lat (min) | 5 (2,9) | 1 (4,10) | 4 (2,9) | 1 (4,10) |
| LPS (min) | 15 (5,29) | 5 (13,29) | 10 (3,24) | 4 (12,26) |
| REM Lat | 135 (85,215) | 74 (106,197) | 122 (83,202) | 74 (107,169) |
| N1% | 17 (10,24) | 7 (13,18) | 13 (9,19) | 8 (12,19) |
| N2% | 54 (49,61) | 46 (52,60) | 56 (49,63) | 45 (52,62) |
| N3% | 11 (4,18) | 9 (16,22) | 13 (6,20) | 8 (16,24) |
| R% | 16 (9,20) | 12 (16,22) | 14 (9,19) | 11 (16,21) |
| Eff | 89 (81,93) | 79 (88,94) | 90 (81,95) | 79 (87,93) |
| #30sW | 24 (15,33) | 13 (18,28) | 19 (11,28) | 13 (20,27) |
| #60sW | 11 (7,17) | 7 (10,16) | 10 (5,14) | 7 (11,15) |
| Sp AI (hr−1) | 3 (2,5) | 3 (4,7) | 3 (2,6) | 3 (5,7) |
| LMAI (hr−1) | 2 (0,5) | 0 (2,7) | 1 (0,3) | 0 (2,6) |
| PLMI (hr−1) | 6 (1,21) | 1 (6,28) | 4 (1,14) | 1 (7,21) |
| AHI (hr−1) | 14 (8,21) | 1 (2,3) | 13 (7,19) | 1 (2,4) |
| AHI Supine | 20 (11,29) | 1 (2,4) | 15 (10,29) | 1 (2,5) |
| AHI REM | 28 (10,42) | 0 (2,5) | 26 (13,46) | 0 (2,4) |
| RDI (hr−1) | 27 (21,38) | 2 (4,6) | 26 (17,37) | 2 (4,8) |
| O2 nadir REM | 85 (80,89) | 89 (92,94) | 85 (80,88) | 89 (92,94) |
| O2 nadir NR | 86 (83,89) | 88 (90,92) | 85 (81,88) | 88 (90,92) |
Median values, with 25-75% range shown in parentheses, separated by commas. There were no significant differences between misperception groups for diagnostic or treatment nights, using Kruskal-Wallis with Dunn's correction. Abbreviations are as in Table 1.
Discussion
In this study, patients with OSA and misperception during their diagnostic PSG showed perceptual improvement during the titration PSG. Self reported confidence in perception as well as sleep quality showed parallel improvements with treatment. The degree of misperception was not related to demographics or to standard PSG metrics, including degree of OSA during the diagnostic or during titration. Although the basis for perception improvement remains uncertain, our findings support the view that, at least for some insomnia patients, treating comorbid OSA results in improvements of clinically important parameters.
Clinical Implications
The occurrence of misperception remains a major challenge in the clinical management of insomnia patients. Self-reported sleep-wake times, obtained during clinical history and/or by sleep diaries, are the foundation for evaluation of the insomnia patient, but this data is confounded by the potential for misperception. Even the time frame over which patients are asked to recall their sleep-wake durations may impact the response. For example, retrospective “summary” estimates after 7-days of monitoring showed even more misperception than individual night-after estimates[34]. If the degree of misperception is not known, it becomes challenging for example to weigh the risks of the insomnia against and the risks and benefits of pharmacological treatments. Although objective data is not currently required for the clinical diagnosis of insomnia, recent work suggests that the subset of patients reporting short sleep duration who actually exhibit short TST during PSG carry the preponderance of health risks previously published using only self-report assessments[19].
Another implication of PSG not routinely being performed in patients with insomnia relates to the presence of occult OSA in this population. Despite perhaps commonly held beliefs that the clinical history should direct testing for OSA, the relationship of even “classic” symptoms such as snoring, sleepiness, and witnessed apneas have only modest utility[35], and thus many patients with OSA remain undiagnosed[36]. There is a growing literature indicating that a surprisingly high portion of patients with insomnia may have occult OSA[23, 24]. The treatment of these patients with certain sedating medications may increase the risk and/or severity of occult OSA. Pharmacological treatment in such patients may give the impression of improved sleep, masking occult OSA that will remain undiagnosed without formal sleep testing. Among patients known to have both OSA and insomnia, especially if there is a misperception component, CPAP treatment may be beneficial for the insomnia symptoms as well as the OSA.
Sleep misperception can be improved with OSA treatment
Although misperception may have both state and trait components contributing, it is important to recognize the possibility of improving perception, especially in patients with comorbid OSA, who may view the use of CPAP headgear as potentially disruptive. Our results show that, at least for some patients, improvement in perception, sleep quality, and confidence in self-assessment improved with CPAP treatment. That misperception appears to be malleable at least for some individuals under some circumstances may have important implications for patients with comorbid insomnia and OSA, which have a prominent overlap[24]. Also arguing in favor of state-dependent mechanisms, we showed previously that healthy adults with no objective sleep disturbance and accurate subjective perception developed misperception under laboratory conditions (extended time in bed and lack of time cues)[18]. Night-to-night variability and patterns of insomnia have been reported and emphasize the limitations of short duration measurements in this population[37, 38]. Without objective repeated measures over multiple nights, the extent to which state versus trait factors are relevant for individual patients remains uncertain.
Objective and Subjective Correlates of Misperception
Many studies have attempted to find connections between misperception and objective PSG metrics or subjective patient self reports. As described in the introduction, the literature regarding misperception suggests that a variety of physiological factors may be involved. Here we did not identify PSG metrics that predicted misperception during the diagnostic study, consistent with our prior work. It is possible that more advanced analytics such as transition probabilities[39], cyclic alternating pattern[40], or spectral analysis of the EEG may prove more sensitive for potential correlates. In addition, there is undoubtedly variability in the heuristics patients may use to estimate the passage of sleep-wake times. Understanding how patients estimate how much time they have been asleep or awake is not a trivial task. For example, a patient who is unsure of their sleep duration but feels tired upon awakening may infer that sleep must have been fragmented or of limited duration. However, we did not find a correlation between the degree of misperception and the Likert Scale rating of sleep quality (admittedly this may not be surprising in a heterogeneous group with potentially many factors influencing sleep quality). Altered time perception has been proposed, but the results are mixed[12, 34, 41-43].
The basis for misperception in patients with OSA in particular also remains uncertain. However, in such patients, there may be different mechanisms involved than in patients without OSA who have misperception. For example, it is well known that OSA is associated with autonomic fluctuations and in particular sympathetic surges[44]. Recently it was shown that autonomic hyper-arousal occurred in patients with primary insomnia associated with over-estimation of sleep latency[13], consistent with the hyper-arousal theory of insomnia pathophysiology[6].
Limitations
Our study has several limitations. First, the retrospective cohort was heterogeneous in regards to symptoms and PSG findings. Although heterogeneity may reduce the chance of identifying objective correlates of misperception, the relatively large size of the cohort speaks to the generality of the findings of misperception among those with OSA. In addition, changes in health, such as weight and medication intake, could have contributed to differences between diagnostic and titration PSGs, with inter-study times ranging from 1 day to 365 days (median 92 days). Patients who reviewed their diagnostic PSG data in sufficient detail with their referring providers could have been made aware of their misperception, and the feedback could result in improvement during their titration. As with any study based on clinical PSG, the lack of accommodation nights and repeated measures under fixed conditions is limiting in terms of nightly variability and first-night effect, each of which may also vary between patients. Heterogeneity in the clinical features of this population, as well as in the objective measures, emphasizes the importance of large sample sizes and the need in future work for repeated measurements over multiple nights to tease apart some of these uncertainties.
Supplementary Material
Acknowledgments
Dr Bianchi received support from the department of Neurology, Massachusetts General Hospital, the Young Clinician Award from the Center for Integration of Medicine and Innovative Technology, and the Harvard Catalyst KL2 Medical Research Investigator Fellowship.
Footnotes
Disclosure: Dr Bianchi is co-inventor on pending patent related to a sleep monitoring device, is a consultant for Sunovion, and is on the advisory board of Foramis.
References
- 1.McCall WV, et al. Subjective estimates of sleep differ from polysomnographic measurements in obstructive sleep apnea patients. Sleep. 1995;18(8):646–50. doi: 10.1093/sleep/18.8.646. [DOI] [PubMed] [Google Scholar]
- 2.Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychol Bull. 2011 doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Classification of Sleep Disorders: Diagnostic & Coding Manual. Second ed. American Academy of Sleep Medicine; Westchester: 2005. [Google Scholar]
- 4.Bianchi MT, et al. The subjective-objective mismatch in sleep perception among those with insomnia and sleep apnea. J Sleep Res. 2013 doi: 10.1111/jsr.12046. [DOI] [PubMed] [Google Scholar]
- 5.Vanable PA, et al. Sleep latency and duration estimates among sleep disorder patients: variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics. Sleep. 2000;23(1):71–9. [PubMed] [Google Scholar]
- 6.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14(1):9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Martinez D, Breitenbach TC, Lenz Mdo C. Light sleep and sleep time misperception - relationship to alpha-delta sleep. Clin Neurophysiol. 2010;121(5):704–11. doi: 10.1016/j.clinph.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Schneider-Helmert D, Kumar A. Sleep, its subjective perception, and daytime performance in insomniacs with a pattern of alpha sleep. Biol Psychiatry. 1995;37(2):99–105. doi: 10.1016/0006-3223(94)00162-V. [DOI] [PubMed] [Google Scholar]
- 9.Parrino L, et al. Paradoxical insomnia: the role of CAP and arousals in sleep misperception. Sleep Med. 2009;10(10):1139–45. doi: 10.1016/j.sleep.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Feige B, et al. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res. 2008;17(2):180–90. doi: 10.1111/j.1365-2869.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 11.Riemann D, et al. REM sleep instability--a new pathway for insomnia? Pharmacopsychiatry. 2012;45(5):167–76. doi: 10.1055/s-0031-1299721. [DOI] [PubMed] [Google Scholar]
- 12.Aritake-Okada S, et al. Diurnal fluctuations in subjective sleep time in humans. Neurosci Res. 2010;68(3):225–31. doi: 10.1016/j.neures.2010.07.2040. [DOI] [PubMed] [Google Scholar]
- 13.Maes J, et al. Sleep misperception, EEG characteristics and Autonomic Nervous System activity in primary insomnia: A retrospective study on polysomnographic data. Int J Psychophysiol. 2014;91(3):163–71. doi: 10.1016/j.ijpsycho.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Perlis ML, et al. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Mendoza J, et al. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosom Med. 2011;73(1):88–97. doi: 10.1097/PSY.0b013e3181fe365a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manconi M, et al. Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J Sleep Res. 2010 doi: 10.1111/j.1365-2869.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 17.Means MK, et al. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med. 2003;4(4):285–96. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 18.Bianchi MT, Wang W, Klerman EB. Sleep misperception in healthy adults: implications for insomnia diagnosis. J Clin Sleep Med. 2012;8(5):547–54. doi: 10.5664/jcsm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vgontzas AN, et al. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med Rev. 2013 doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang NK, Harvey AG. Altering misperception of sleep in insomnia: behavioral experiment versus verbal feedback. J Consult Clin Psychol. 2006;74(4):767–76. doi: 10.1037/0022-006X.74.4.767. [DOI] [PubMed] [Google Scholar]
- 21.Downey R, 3rd, Bonnet MH. Training subjective insomniacs to accurately perceive sleep onset. Sleep. 1992;15(1):58–63. doi: 10.1093/sleep/15.1.58. [DOI] [PubMed] [Google Scholar]
- 22.Mercer JD, Bootzin RR, Lack LC. Insomniacs' perception of wake instead of sleep. Sleep. 2002;25(5):564–71. [PubMed] [Google Scholar]
- 23.Luyster FS, Buysse DJ, Strollo PJ., Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6(2):196–204. [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Jawder SE, Bahammam AS. Comorbid insomnia in sleep-related breathing disorders: an under-recognized association. Sleep Breath. 2012;16(2):295–304. doi: 10.1007/s11325-011-0513-1. [DOI] [PubMed] [Google Scholar]
- 25.Lichstein KL, et al. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67(3):405–10. doi: 10.1037//0022-006x.67.3.405. [DOI] [PubMed] [Google Scholar]
- 26.Stone J, et al. Neuropsychological functioning in older insomniacs with or without obstructive sleep apnea. Psychol Aging. 1994;9(2):231–6. doi: 10.1037//0882-7974.9.2.231. [DOI] [PubMed] [Google Scholar]
- 27.Krakow B, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49(11):948–53. doi: 10.1016/s0006-3223(00)01087-8. [DOI] [PubMed] [Google Scholar]
- 28.Guilleminault C, et al. Chronic insomnia, postmenopausal women, and sleep disordered breathing: part 1. Frequency of sleep disordered breathing in a cohort. J Psychosom Res. 2002;53(1):611–5. doi: 10.1016/s0022-3999(02)00445-2. [DOI] [PubMed] [Google Scholar]
- 29.Gooneratne NS, et al. Consequences of comorbid insomnia symptoms and sleep- related breathing disorder in elderly subjects. Arch Intern Med. 2006;166(16):1732–8. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- 30.Krakow B, Ulibarri VA, Romero E. Persistent insomnia in chronic hypnotic users presenting to a sleep medical center: a retrospective chart review of 137 consecutive patients. J Nerv Ment Dis. 2010;198(10):734–41. doi: 10.1097/NMD.0b013e3181f4aca1. [DOI] [PubMed] [Google Scholar]
- 31.Krakow B, Ulibarri VA, Romero EA. Patients with treatment-resistant insomnia taking nightly prescription medications for sleep: a retrospective assessment of diagnostic and treatment variables. Prim Care Companion J Clin Psychiatry. 2010;12(4) doi: 10.4088/PCC.09m00873bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wickwire EM, Collop NA. Insomnia and sleep-related breathing disorders. Chest. 2010;137(6):1449–63. doi: 10.1378/chest.09-1485. [DOI] [PubMed] [Google Scholar]
- 33.Gottlieb DJ, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159(2):502–7. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 34.Fichten CS, et al. Time estimation in good and poor sleepers. J Behav Med. 2005;28(6):537–53. doi: 10.1007/s10865-005-9021-8. [DOI] [PubMed] [Google Scholar]
- 35.Skomro RP, Kryger MH. Clinical presentations of obstructive sleep apnea syndrome. Prog Cardiovasc Dis. 1999;41(5):331–40. doi: 10.1053/pcad.1999.0410331. [DOI] [PubMed] [Google Scholar]
- 36.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 37.Edinger JD, et al. The empirical identification of insomnia subtypes: a cluster analytic approach. Sleep. 1996;19(5):398–411. [PubMed] [Google Scholar]
- 38.Vallieres A, et al. Predictability of sleep in patients with insomnia. Sleep. 2011;34(5):609–17. doi: 10.1093/sleep/34.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bianchi MT, et al. Obstructive sleep apnea alters sleep stage transition dynamics. PLoS One. 2010;5(6):e11356. doi: 10.1371/journal.pone.0011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terzano MG, Parrino L. Evaluation of EEG cyclic alternating pattern during sleep in insomniacs and controls under placebo and acute treatment with zolpidem. Sleep. 1992;15(1):64–70. doi: 10.1093/sleep/15.1.64. [DOI] [PubMed] [Google Scholar]
- 41.Rioux I, Tremblay S, Bastien CH. Time estimation in chronic insomnia sufferers. Sleep. 2006;29(4):486–93. doi: 10.1093/sleep/29.4.486. [DOI] [PubMed] [Google Scholar]
- 42.Tang NK, Harvey AG. Time estimation ability and distorted perception of sleep in insomnia. Behav Sleep Med. 2005;3(3):134–50. doi: 10.1207/s15402010bsm0303_2. [DOI] [PubMed] [Google Scholar]
- 43.Harrow L, Espie C. Applying the quarter-hour rule: can people with insomnia accurately estimate 15-min periods during the sleep-onset phase? J Sleep Res. 2010;19(1 Pt 1):19–26. doi: 10.1111/j.1365-2869.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 44.Harper RM, et al. Functional neuroanatomy and sleep-disordered breathing: implications for autonomic regulation. Anat Rec (Hoboken) 2012;295(9):1385–95. doi: 10.1002/ar.22514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.