Abstract
Background/Aim
Obesity is associated with changes in adiponectin and pro-inflammatory adipokines. Sodium intake can affect adipokine secretion suggesting a role in cardiovascular dysfunction. We tested if long-term dietary sodium restriction modifies the expression of adiponectin and ameliorates the pro-inflammatory profile of obese, diabetic
Methods/Results
Db/db mice were randomized to high sodium (HS 1.6% Na+, n=6) or low sodium (LS 0.03% Na+, n=8) diet for 16 weeks and compared with lean, db/+ mice on HS diet (n=8). Insulin levels were 50% lower in the db/db mice on LS diet when compared with HS db/db (p <0.05). LS diet increased cardiac adiponectin mRNA levels in db/db mice by 5-fold when compared with db/db mice on HS diet and by 2-fold when compared with HS lean mice (both p < 0.01). LS diet increased adiponectin in adipose tissue compared with db/db mice on HS diet, achieving levels similar to those of lean mice. MCP-1, IL-6 and TNF-α expression were reduced more than 50% in adipose tissue of db/db mice on LS diet when compared with HS db/db mice (all p < 0.05), to levels observed in the HS lean mice. Further, LS db/db mice had significantly reduced circulating MCP-1 and IL-6 levels when compared with HS db/db mice (both p < 0.01).
Conclusion
In obese-diabetic mice, long-term LS diet increases adiponectin in heart and adipose tissue and reduces pro-inflammatory factors in adipose tissue and plasma. These additive mechanisms may contribute to the potential cardioprotective benefits of LS diet in obesity-related metabolic disorders.
Keywords: sodium intake, obesity, inflammation, adiponectin, diabetes, insulin resistance
Introduction
Obesity is associated with increased prevalence of type 2 diabetes (T2DM), hypertension, metabolic syndrome (MetS) and cardiovascular disease (CVD). Human and rodent obesity are characterized by reduced adiponectin levels and elevated levels of proinflammatory cytokines, which both contribute to the development of associated metabolic disorders [1]. According to a recent-meta-analysis, lower adiponectin levels are amongst the strongest biochemical predictors of insulin resistance and T2DM [2]. Interestingly, adiponectin is not only secreted by adipocytes but also is produced by cardiomyocytes and several studies report a cardioprotective effect of adiponectin on hypertension, heart failure and endothelial dysfunction [3] [4].
Obesity-related inflammation and cardiometabolic disorders have been associated with increased aldosterone levels and excess mineralocorticoid receptor (MR) activation [5]. We showed previously that MR blockade with eplerenone increases adiponectin and reduces inflammatory cytokines in obese db/db mice [6]. Studies examining the adverse cardiovascular effects of MR demonstrate an important influence of dietary sodium intake—a high sodium diet increases and a low sodium diet reduces MR-mediated cardiovascular and renal damage [7-10]. In addition, an animal model of aldosterone-induced injury showed that treatment with a MR antagonist or dietary sodium restriction has similar cardioprotective effects [9]. To date there is sparse and controversial information regarding a potential protective effect of low sodium diet on the obesity-related pro-inflammatory adipokine profile [4] [11].
The aim of the present study was to test the hypothesis that long-term dietary sodium restriction improves the expression of adiponectin in heart and adipose tissue, and reduces inflammatory cytokines of obese, diabetic db/db mice.
Methods
Animal protocol
We studied three groups of male rodents with dietary sodium intervention for 16 weeks from age 9 weeks until age 25 weeks. We selected lean, db/+ littermates on high sodium (HS) diet as a control group since high sodium intake is the most frequent diet worldwide.
The study group design was as follows: 1) lean, non-diabetic control group db/+ mice (HS, 1.6% NaCl, n=8); and obese, diabetic db/db mice randomized to either 2) a high sodium diet (HS, 1.6% NaCl, n=6), or 3) a low sodium diet (LS, 0.03% NaCl, n=8). The obese db/db mice (Jackson Laboratory, Bar Harbor, ME) are generated by mating male and female db/+ mice and thus are littermates with similar genetic backgrounds except for the number of mutated leptin receptors [12].
To confirm appropriate sodium intake, mice were placed in metabolic cages for 24h urinary sodium determination, considered the gold standard measurement [13]. Systolic blood pressure was measured in conscious animals by tail-cuff plethysmography at age 25 weeks (Blood Pressure Analyzer, Model 179, IITC Life Science). Animals were kept in a room lighted 12 h/day at an ambient temperature of 22 ± 1°C. At 25 weeks of age, blood samples, visceral adipose tissue from the retroperitoneum, and hearts were harvested. The Institutional Animal Care and Use Committee at Harvard University approved experimental procedures.
Biochemical parameters
Plasma aldosterone levels and plasma renin activity (PRA) were determined using radioimmunoassay techniques as previously described [14].
Plasma glucose was measured using Cobas Integra 400 chemistry analyzer (Roche Diagnostics, Indianapolis, IN) via a hexokinase enzymatic reaction. Plasma insulin was measured using the LincoPlex mouse insulin ELISA assay (LINCO Research, St. Charles, MO). To estimate insulin resistance, the homeostatic model assessment (HOMA) index was calculated. This method, despite its limitation compared to euglycemic glucose clamp, has been validated in rodents [15].
Plasma cytokine concentrations
Cytokine protein concentrations of interleukin (IL)-6, IL-10, monocyte chemotactic protein-1 (MCP-1), interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) were measured in four animals per group using the Cytometric Bead Array System (mouse inflammation CBA kit, Cat #552364, BD Biosciences, San Jose, CA). Cytokine concentrations were determined by flow cytometry (BD FACScan, BD biosciences). Results take into account the total protein concentration of the plasma and were expressed as pg/ml. Intra-assay variability was 2% and inter-assay variability was 5%.
Quantitative Real-Time PCR
Total mRNA was extracted from visceral adipose tissue using the RNeasy Lipid Tissue Mini Kit (Qiagen Sciences, Germantown, MD) and from heart tissue using the RNeasy Mini Kit (Qiagen Sciences). PCR amplification reactions were performed with TaqMan gene expression assays in duplicate using the ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). MRNA levels, calculated by the ΔΔCT method, were normalized to 18S rRNA.
Western blot analysis
High molecular weight (HMW) plasma adiponectin was determined using anti-adiponectin antibody (Chemicon, Temecula, CA) as previously described [6]. We chose HMW adiponectin levels as evidence supports strong inverse correlations with T2DM, coronary disease and CVD [16].
Analysis of heart tissue was performed using the following antibodies: MR (sc-11412, Santa Cruz, CA, dilution 1:1000) and AT1R (sc-1173, Santa Cruz, dilution 1:1000).
Immunohistochemical staining
Immunohistochemical staining was performed by the avidin biotin complex method according to the manufacturer's protocol (Vector) as previously described [17]. Adiponectin was detected by mouse primary antibody to adiponectin (Chemicon, Temecula, CA) and secondary goat anti-mouse antibody (dilution 1:200; Vector). Negative control sections were incubated with either secondary antibody alone or an irrelevant goat IgG (Vector).
Data Analysis
Data were analyzed using one-way ANOVA, followed by Tukey's post-hoc when comparing three groups or Student t-test when data was only available in two groups. To account for differences in the weight of the db/db animals on LS and HS, a linear regression model with the outcome of interest was performed using the animal groups as a categorical predictor and weight as a continuous covariate. Differences in means with p values ≤ 0.05 were considered statistically significant. All analyses were performed using SPSS 15.0 and GraphPad Prism 6 software. Values are expressed as mean ± standard deviation (SD).
Results
Over the 16 week study, lean db/+ mice gained less weight from baseline (8.5 g ± 0.8) when compared with HS db/db (19.9 g ± 1.7, p <0.01) or LS db/db mice (18.4 g ± 2.1, p <0.01). The characteristics at 25 weeks of lean nondiabetic db/+ mice on HS and of obese diabetic db/db mice on HS and LS are summarized in Table 1. As expected, db/db mice at age 25 weeks were significantly heavier and had severe hyperglycemia. Systolic blood pressure was similar across all three groups. Db/db mice on LS diet had a slightly lower weight as compared with db/db mice on HS diet (49.1 ± 1.8 g db/db LS group vs. 51.8 ± 2.4 g db/db HS group, p <0.05). Low sodium intake increased both aldosterone and PRA by roughly two fold in db/db mice when compared with lean HS controls, as was anticipated. Also db/db mice in the LS group had significantly higher PRA than db/db mice in the HS group (p <0.01). Aldosterone levels also increased in obese mice on HS diet as compared with the lean controls on HS diet (p < 0.01).
Table 1. Characteristics of lean HS db/+, obese HS db/db and obese LS db/db mice at 25 weeks.
| Lean control High sodium diet | Obese db/db High sodium diet | Obese db/db Low sodium diet | |
|---|---|---|---|
| n=8 | n=6 | n=8 | |
| Total body weight (g) | 30.1 ± 2 | 51.8 ± 2A | 49.1 ± 2 A,D |
| Heart weight (mg) | 176 ± 33 | 158 ± 17 | 148 ± 29 |
| Kidney weight (mg) | 254 ± 24 | 276 ± 20 | 281 ± 40 |
| Systolic blood pressure (mmHg) | 126 ± 19 | 118 ± 7 | 115 ± 15 |
| Aldosterone (ng/dl) | 22 ± 5 | 39 ± 21 A | 49 ± 9 A |
| Plasma Renin Activity (ng/ml/hr) | 13.1 ± 6 | 6.7 ± 4 | 20.7 ± 3 B, C |
| Glucose (mg/dl) | 138 ± 15 | 410 ± 42 A | 460 ± 24 A,D |
| Insulin (uU/ml) | 6.9 ± 4.1 | 19.4 ± 8 A | 10.2 ± 2 D |
| HOMA-IR | 2.3 ± 1 | 19.7 ± 9 A | 11.5 ± 3 A, D |
p<0.01,
p<0.05 vs. lean control;
p<0.01,
p<0.05 vs. obese db/db on HS.
Data given as mean ± SD.
HOMA-IR: homeostatic model assessment index of insulin resistance
2) Effects of obesity and dietary sodium restriction on insulin resistance
Insulin levels were increased in diabetic db/db mice versus nondiabetic db/+ mice, irrespective of diet (Table 1). Obese db/db mice on LS diet had about 50% lower insulin levels than those in db/db animals on HS diet and comparable insulin levels to lean control mice on HS (p <0.05). LS db/db mice had a mild 10% increase in glycemia compared with HS db/db mice. Insulin resistance, estimated by HOMA-IR, was significantly improved in the db/db mice on LS (Table 1). To account for the possibility that the improvement in insulin levels in the LS group may be due to weight loss, we performed a linear regression analysis adjusted by weight and observed that insulin levels remained significantly lower in LS group (p <0.05) when compared with HS db/db animals. The beneficial effect of LS versus HS diet on HOMA-IR in db/db mice was not statistically significant when adjusting for weight.
3) Effect of sodium intake on AT1R and MR protein expression in db/db heart tissue
Consistent with our previous reports in nondiabetic rodent models13, db/db animals on HS diet as compared with db/db animals on LS diet had a roughly 2-fold increase in both AT1R (1.2 vs 0.6 optical density, p <0.05) and MR cardiac protein levels (0.4 vs 0.2 optical density, p <0.05).
4) Effects of dietary sodium restriction on adiponectin
On the HS diet, cardiac adiponectin mRNA levels were markedly decreased in the heart tissue of obese diabetic db/db mice when compared with lean nondiabetic db/+ mice (p <0.05). With dietary sodium restriction, cardiac expression of adiponectin was significantly increased in db/db mice as compared with db/db mice on HS (Figure 1), with and without adjustment for weight. Furthermore, LS diet increased cardiac adiponectin mRNA levels in the obese db/db mice to levels that were two-fold higher than those observed in the lean nondiabetic db/+ mice on HS diet (p <0.01, Figure 1) even when adjusted by weight. There was no detectable leptin mRNA in cardiac tissue suggesting that these samples were not contaminated with fat. Additionally, immunohistochemistry studies confirmed the presence of adiponectin protein in cardiomyocytes from db/db mice (Figure 2).
Figure 1. Expression in heart, adipose tissue and plasma levels of adiponectin in studied groups.
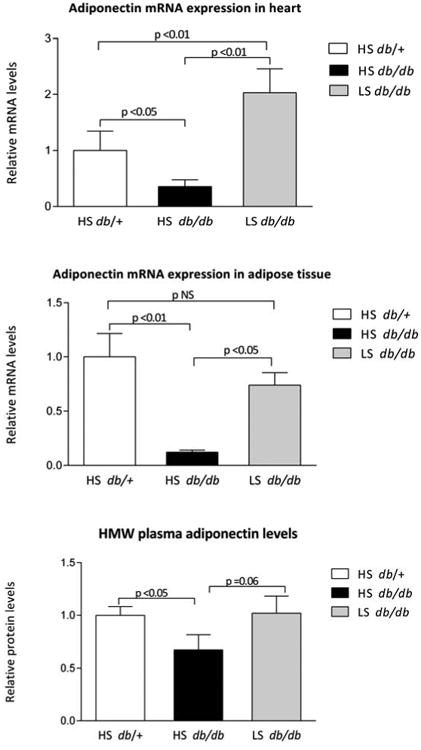
Adiponectin mRNA levels in heart, visceral adipose tissue and plasma levels of high molecular weight adiponectin in 25-week old lean high sodium (HS) diet db/+ mice, obese db/db mice treated with HS diet and obese db/db mice treated with low sodium (LS) diet. mRNA levels are expressed relative to 18S mRNA. Data are mean ± SD.
Figure 2. Cardiomyocyte-specific adiponectin expression confirmed by immunohistochemistry.
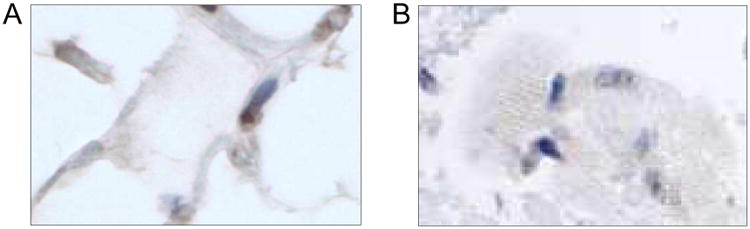
Expression of adiponectin (40×) in cytoplasm was detected by immunohistochemistry staining in db/db mouse adipose tissue (A) and cardiomyocyte of db/db mouse heart (B).
Regarding visceral adipose tissue expression, obese diabetic HS db/db mice again had reduced levels of adiponectin mRNA when compared with lean nondiabetic HS db/+ mice (p <0.01). In addition, db/db mice on LS diet had higher adiponectin mRNA levels in adipose tissue compared to db/db mice on HS (p <0.01), and this statistical difference remained even after adjusting by weight. Low sodium intake in db/db mice increased adipose tissue expression of adiponectin to levels observed in the lean animals (Figure 1).
Obese HS db/db mice had significantly lower levels of circulating HMW adiponectin compared with lean nondiabetic HS db/+ mice (p <0.05). Obese db/db mice on LS diet tended to have higher levels of HMW adiponectin as compared with db/db animals on HS diet (p= 0.06). Moreover, plasma levels of HMW adiponectin of obese LS db/db mice were similar to levels in the lean HS db/+ group, consistent with adiponectin expression in adipose tissue (Figure 1).
5) Effects of dietary sodium restriction on pro-inflammatory cytokines
The expression of several cytokines was measured in adipose tissue in the three animal groups. Adipose tissue expression of the chemokine MCP-1 and the pro-inflammatory cytokine IL-6 were both increased by roughly 5-fold in obese db/db mice on HS diet as compared with HS lean nondiabetic control animals (both p<0.01). Db/db mice on a LS diet had significantly lower mRNA levels of MCP1 and IL-6 when compared to HS db/db mice (both p <0.05). Moreover, LS db/db mice had similar expression of MCP-1 and IL-6 to levels observed in the lean HS control group, even when adjusted by weight (Figure 3). Regarding TNF-α, obese db/db mice on HS diet had roughly an 8-fold increase in adipose tissue expression when compared with the HS lean control group (p <0.01). Also, TNF-α transcript expression was reduced in db/db mice on LS diet as compared to db/db mice on HS diet (p <0.05), and this effect remained significant when adjusted by weight differences between LS and HS groups. However, TNF-α mRNA in db/db LS mice were elevated when compared with the HS lean control group (Figure 3). In addition, mRNA levels of PAI-1 and the macrophage marker CD 68 expression were significantly increased in HS db/db mice versus HS lean mice. Visceral fat from LS obese animals tended to have lower levels of CD68 and PAI-1 mRNA as compared with HS obese mice, although this did not reach statistical significance.
Figure 3. Adipose tissue expression of inflammatory cytokines in db/db mice.
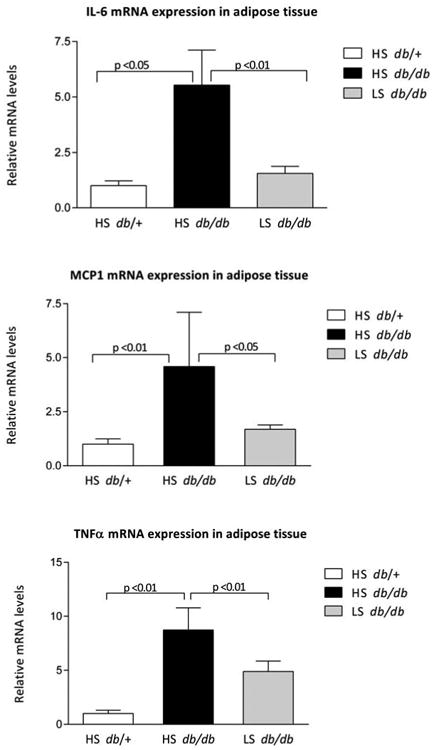
IL-6, MCP-1 and TNF-á mRNA levels in visceral adipose tissue of 25-week old lean db/+ mice, obese db/db mice treated with high sodium diet and obese db/db mice treated with low sodium diet mRNA levels are expressed relative to 18S rRNA. Data are mean ± SD.
Consistent with the gene expression studies in adipose tissue, plasma levels of MCP-1 and IL-6 were increased when comparing HS obese db/db mice to HS lean nondiabetic mice (both p <0.01). Additionally, obese LS db/db mice had significantly reduced plasma MCP-1 and IL-6 levels when compared with the levels seen in obese HS db/db mice (both p ≤0.01), even when adjusting for weight (both p <0.01). LS in db/db mice led to a decrease in MCP-1 to levels that were even significantly lower than those observed in the non-diabetic, lean nondiabetic HS db/+ mice (Figure 4). Plasma levels of IL-10, TNF-α, and interferon γ were similar in the three groups (Data not shown).
Figure 4. Plasma levels of circulating inflammatory cytokines in db/db mice.
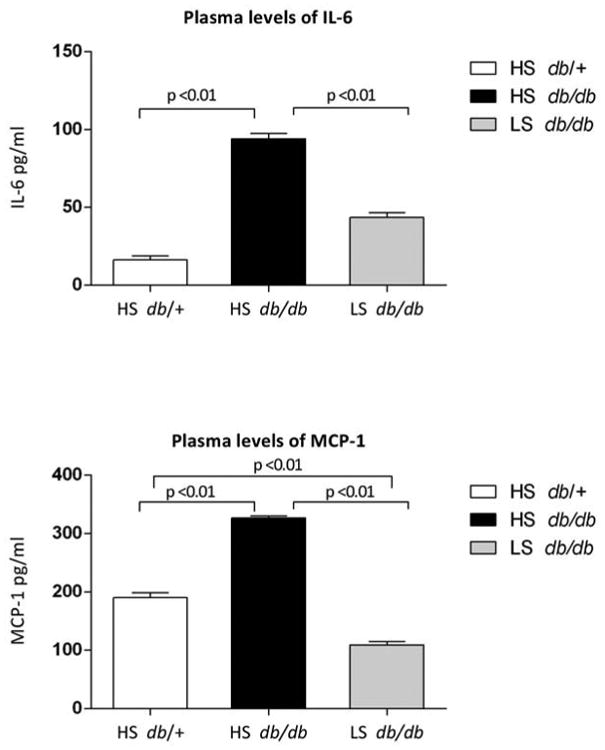
Circulating levels of IL-6 and MCP-1 in plasma of 25-week old lean db/+ mice, obese db/db mice treated with high sodium diet and obese db/db mice treated with low sodium diet. Data are mean ± SD.
Discussion
The present study demonstrates that long-term reduction of sodium intake increases the expression of adiponectin in both heart and adipose tissue and also reduces the expression of pro-inflammatory factors in visceral adipose tissue of obese-diabetic mice. Also, restricting dietary sodium improves plasma levels of the pro-inflammatory cytokines (MCP-1, IL-6, TNF-α) and reduces insulin levels.
To our knowledge, this is the first report demonstrating that adiponectin expression is increased in heart tissue of obese rodents on a long-term low sodium diet. Adiponectin, secreted by cardiomyocytes, is involved in the regulation of cardiac metabolism and function [3], has a protective effect against ischemia-reperfusion injury through activation of adiponectin receptors and improves cardiomyocyte contractile function [18, 19]. Herein, we confirmed cardiac expression of adiponectin by RT-PCR and demonstrated cardiomyocyte-specific adiponectin expression by immunohistochemistry
We also observed that LS diet, compared to HS, increased adiponectin in visceral adipose tissue and tended to increase plasma levels of HMW adiponectin in obese mice. The importance of adiponectin in obesity-related disorders is highlighted by the adiponectin KO rodent model whose phenotype includes insulin resistance, dyslipidemia, hypertension, and endothelial dysfunction [20]. Moreover, adiponectin replacement therapy both lowers blood pressure and corrects the eNOS levels by blocking superoxide production [4].
In regards to the effects of LS diet on inflammation, we observed that sodium restriction in obese mice decreased MCP-1, IL-6, and TNF-α mRNA expression in adipose tissue as well as decreased plasma MCP-1 and IL-6 to levels observed in the lean control group on HS. Furthermore, human studies have shown that central obesity correlates with plasma pro-inflammatory markers like MCP-1, IL-6 and C reactive protein [21] [22]. With respect to sodium loading and insulin, our results showed that a low sodium diet decreases insulin levels. This finding is consistent with studies demonstrating that a HS diet increases insulin levels and worsens insulin-mediated glucose disposal [23] and with a recent report showing that HS diet increases HOMA-IR and decreases adiponectin [24]. In the current study, the 50% reduction in insulin levels with LS diet was accompanied by an unexpected mild 10% increase in glycemia. The reason for this increase in glucose is unclear, but may be related to LS diet affecting multiple factors that regulate glucose homeostasis and warrants further investigation.
Interestingly, several studies have shown that high sodium intake, probably in conjunction with an activated MR, contributes to cardiometabolic diseases [25] Moreover, our present results, with a restricted sodium diet, resemble the effects of an MR antagonist observed in the same rodent model. We reported that eplerenone increases adiponectin expression in both heart and adipose tissue and decreases MCP-1 and CD 68 expression in adipose tissue of obese diabetic db/db mice [6]. Thus, it is possible that the protective effects of dietary sodium restriction are mediated, at least in part, through decreases in tissue MR levels. First, we confirmed a decrease in cardiac MR levels with sodium restriction in obese db/db mice, similar to what we have reported previously in healthy rats on LS diet [7]. Second, restricted sodium intake and an MR antagonist were shown to have similar cardioprotective effects in a rodent model of cardiovascular injury with excess aldosterone [9]. Third, nuclear or membrane proteins like lysine-specific demethylase (LSD1), Rac1 and AT1R can modify MR activity. These factors are modified by dietary sodium intake and thus could potentially mediate some of the effects of a restricted sodium diet on MR activity [10] [26].
Recently, the overall health benefits of reducing dietary sodium intake have been questioned [27]. The mechanisms involved in the discrepant results have not been elucidated, but changes in metabolic and inflammatory parameters could be dependent on the period of time and the quantity of dietary sodium restriction, the comorbidities, the genetics of the population studied and/or other environmental factors. A recent meta-analysis that included only long term studies (at least 4 weeks of intervention) showed beneficial effects of sodium restriction despite aldosterone levels being mildly elevated [28]. Unfavorable effects of dietary sodium restriction, such as insulin resistance and an increase in inflammatory cytokines, are observed with short term highly restricted sodium intake (< 20 mEq/day) resulting in a five to seven fold increase in aldosterone levels [29]. Further, a recent report showed a J-shaped effect regarding sodium intake and cardiovascular outcomes, suggesting that very low sodium restriction could increase cardiovascular mortality [30]. It should be noted that the obese LS group in our study had only a two fold increase in aldosterone, raising the possibility that a 0.03 Na% diet in mice has an effect comparable to a moderately sodium restricted diet in humans. Also, it is possible that dietary sodium restriction may be particularly beneficial in specific clinical scenarios associated with excess MR activation. Human obesity is probably associated with increased MR activation secondary to higher aldosterone levels [5], corroborated in this study when comparing aldosterone levels of obese versus lean mice both on HS diet. Future studies are needed to define the beneficial effects of different degrees of sodium restriction in specific clinical conditions.
Our study has limitations as the results obtained from this specific rodent model may lack generalizability to other populations. However, this model was studied as obesity and diabetes are highly prevalent worldwide and our previous work showed a beneficial effect of an MR antagonist in this model [6]. Another potential limitation includes our relatively small sample size, which could lead to an inability to detect significant differences between groups. Further, we did not assess protein levels; however, we previously reported a very good correlation between transcript and protein expression in the same rodent model [6]. This study did not include a group of lean animals on a low sodium diet so we cannot generalize our results to non obese rodents. A strength of the study was the long term diet intervention (16 weeks), which is roughly equivalent to one fifth of the db/db's standard life span. In conclusion, long-term low sodium diet has beneficial effects in obese diabetic mice by increasing the expression of adiponectin in both heart and adipose tissue and by ameliorating the expression of pro-inflammatory factors in adipose tissue. Further, we demonstrated that a sodium restricted diet reduces both plasma pro-inflammatory cytokines and insulin levels, even when adjusting by weight changes. Future human studies are needed to ascertain whether dietary sodium restriction is of benefit in cardiac dysfunction and obesity-related metabolic disorders, potentially independent of changes in weight.
Acknowledgments
We thank Dr. Kilmer McCully for his critical review of this manuscript.
Sources of funding: This work was supported by grants from the National Institutes of Health: K24 HL103845 (Dr Adler), HL063423 (Dr Adler), 5T32HL007609 (Dr CG Lian) and DK064841 (Dr Williams) and from the Chilean National Science and Technology Research Fund (Fondecyt) 1130427 to Dr. Baudrand.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamamoto Y, Hirose H, Saito I, Nishikai K, Saruta T. Adiponectin, an adipocyte-derived protein, predicts future insulin resistance: two-year follow-up study in Japanese population. J Clin Endocrinol Metab. 2004;89:87–90. doi: 10.1210/jc.2003-031163. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–88. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 3.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163–9. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 4.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47:1108–16. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 5.Ronconi V, Turchi F, Appolloni G, di Tizio V, Boscaro M, Giacchetti G. Aldosterone, mineralocorticoid receptor and the metabolic syndrome: role of the mineralocorticoid receptor antagonists. Curr Vasc Pharmacol. 2012;10:238–46. doi: 10.2174/157016112799304969. [DOI] [PubMed] [Google Scholar]
- 6.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–61. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricchiuti V, Lapointe N, Pojoga L, Yao T, Tran L, Williams GH, et al. Dietary sodium intake regulates angiotensin II type 1, mineralocorticoid receptor, and associated signaling proteins in heart. J Endocrinol. 2011;211:47–54. doi: 10.1530/JOE-10-0458. [DOI] [PubMed] [Google Scholar]
- 8.Waanders F, de Vries LV, van Goor H, Hillebrands JL, Laverman GD, Bakker SJ, et al. Aldosterone, from (patho)physiology to treatment in cardiovascular and renal damage. Curr Vasc Pharmacol. 2011;9:594–605. doi: 10.2174/157016111796642689. [DOI] [PubMed] [Google Scholar]
- 9.Martinez DV, Rocha R, Matsumura M, Oestreicher E, Ochoa-Maya M, Roubsanthisuk W, et al. Cardiac damage prevention by eplerenone: comparison with low sodium diet or potassium loading. Hypertension. 2002;39:614–8. [PubMed] [Google Scholar]
- 10.Fujita T. Mineralocorticoid receptors, salt-sensitive hypertension, and metabolic syndrome. Hypertension. 2010;55:813–8. doi: 10.1161/HYPERTENSIONAHA.109.149062. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, Mu J, Yuan Z, Wu G, Liu E, Zheng S, et al. High salt intake fails to enhance plasma adiponectin in normotensive salt-sensitive subjects. Nutrition. 2012;28:422–5. doi: 10.1016/j.nut.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 13.Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813. doi: 10.1093/ije/dyp139. [DOI] [PubMed] [Google Scholar]
- 14.Raji A, Williams GH, Jeunemaitre X, Hopkins PN, Hunt SC, Hollenberg NK, et al. Insulin resistance in hypertensives: effect of salt sensitivity, renin status and sodium intake. J Hypertens. 2001;19:99–105. doi: 10.1097/00004872-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Muniyappa R, Yan X, Chen H, Yue LQ, Hong EG, et al. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab. 2008;294:E261–70. doi: 10.1152/ajpendo.00676.2007. [DOI] [PubMed] [Google Scholar]
- 16.Kizer JR, Arnold AM, Benkeser D, Ix JH, Djousse L, Zieman SJ, et al. Total and high-molecular-weight adiponectin and risk of incident diabetes in older people. Diabetes Care. 2012;35:415–23. doi: 10.2337/dc11-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo C, Martinez-Vasquez D, Mendez GP, Toniolo MF, Yao TM, Oestreicher EM, et al. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology. 2006;147:5363–73. doi: 10.1210/en.2006-0944. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li R, et al. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2010;298:E663–70. doi: 10.1152/ajpendo.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong F, Ren J. Adiponectin improves cardiomyocyte contractile function in db/db diabetic obese mice. Obesity (Silver Spring) 2009;17:262–8. doi: 10.1038/oby.2008.545. [DOI] [PubMed] [Google Scholar]
- 20.Sam F, Duhaney TA, Sato K, Wilson RM, Ohashi K, Sono-Romanelli S, et al. Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology. 2010;151:322–31. doi: 10.1210/en.2009-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahlman I, Kaaman M, Olsson T, Tan GD, Bickerton AS, Wahlen K, et al. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab. 2005;90:5834–40. doi: 10.1210/jc.2005-0369. [DOI] [PubMed] [Google Scholar]
- 22.Kraja AT, Province MA, Arnett D, Wagenknecht L, Tang W, Hopkins PN, et al. Do inflammation and procoagulation biomarkers contribute to the metabolic syndrome cluster? Nutr Metab (Lond) 2007;4:28. doi: 10.1186/1743-7075-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yatabe MS, Yatabe J, Yoneda M, Watanabe T, Otsuki M, Felder RA, et al. Salt sensitivity is associated with insulin resistance, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am J Clin Nutr. 2010;92:77–82. doi: 10.3945/ajcn.2009.29028. [DOI] [PubMed] [Google Scholar]
- 24.Baudrand R, Campino C, Carvajal CA, Olivieri O, Guidi G, Faccini G, et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clinical endocrinology. 2013 doi: 10.1111/cen.12225. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.He FJ, Macgregor GA. Salt intake, plasma sodium, and worldwide salt reduction. Ann Med. 2012;44(Suppl 1):S127–37. doi: 10.3109/07853890.2012.660495. [DOI] [PubMed] [Google Scholar]
- 26.Pojoga LH, Williams JS, Yao TM, Kumar A, Raffetto JD, do Nascimento GR, et al. Histone demethylase LSD1 deficiency during high-salt diet is associated with enhanced vascular contraction, altered NO-cGMP relaxation pathway, and hypertension. Am J Physiol Heart Circ Physiol. 2011;301:H1862–71. doi: 10.1152/ajpheart.00513.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review) Am J Hypertens. 2012;25:1–15. doi: 10.1038/ajh.2011.210. [DOI] [PubMed] [Google Scholar]
- 28.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 29.Garg R, Williams GH, Hurwitz S, Brown NJ, Hopkins PN, Adler GK. Low-salt diet increases insulin resistance in healthy subjects. Metabolism. 2011;60:965–8. doi: 10.1016/j.metabol.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–38. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]


