Abstract
Background
Peanut allergy is a major public health problem that affects 1% of the population and has no effective therapy.
Objective
To examine the safety and efficacy of oraldesensitization in peanut allergic children in combination with a brief course of anti-IgE monoclonal antibody (omalizumab, Xolair).
Methods
We performed oral peanut desensitization in peanut allergic children at high risk for developing significant peanut-induced allergic reactions. Omalizumab was administered prior to and during oral peanut desensitization.
Results
We enrolled 13 children (median age, 10 years), with a median peanut-specific IgE of 229 kUA/L and a median total serum IgE of 621 kU/L, who failed an initial double-blind placebo controlled food challenge at doses 100 mg peanut flour. After pre-treatment with omalizumab, all subjects tolerated the initial 11 desensitization doses given on the first day, including the maximum dose of 500 mg peanut flour (cumulative dose, 992 mg, equivalent to >2 peanuts), requiring minimal or no rescue therapy. 12 subjects then reached the maximum maintenance dose of 4,000 mg peanut flour/day in a median time of 8 weeks, at which point omalizumab was discontinued. All 12 subjects continued on 4,000 mg peanut flour/day and subsequently tolerated a challenge with 8,000 mg peanut flour (equivalent to about 20 peanuts), or 160 to 400 times the dose tolerated before desensitization. During the study, 6 of the 13 subjects experienced mild or no allergic reactions; 6 subjects had Grade 2, and 2 subjects Grade 3 reactions, all of which responded rapidly to treatment.
Conclusions
Among children with high-risk peanut allergy, treatment with omalizumab may facilitate rapid oral desensitization, and qualitativelyimprove the desensitization process.
Keywords: oral immunotherapy, desensitization, food allergy, peanut allergy, omalizumab
Introduction
Food allergy is a major public health problem that affectsa large proportion of the general population in industrialized countries, estimated to include 4% of the US population1,2. While many different foods cause allergy, peanut is one of the more common foods causing allergy3–5. Further, reactions to peanuts and tree nuts account for a disproportionate number of severe reactions (94% of fatalities) from food allergy3,6. In addition, accidental ingestion of peanuts occurs in up to 25–75% of patients over a 5-year period, despite strict dietary avoidance measures, resulting in significant anxiety for many patients and families of children with peanut allergy7. Moreover, while sensitivity to other common foods such as milk and soy often resolves spontaneously over time, sensitivity to peanut more commonly fails to diminish8. Unfortunately for patients with food allergyno effective treatment is currently available except to avoid offending foods and to have ready access to self-injectable epinephrine1.
Recently, there have been reports of success in several clinical trials of oral food allergen immunotherapy/desensitization for milk9–11, egg12,13, peanut14–16 and hazelnut17. The protocols for desensitization are varied, involving rush therapy phases11, weekly increases in dose over many months9 or both10,12, and using oral and/or sublingual approaches17,18. Double blind, placebo-controlled food challenges (DBPCFC) at the conclusion of these studies demonstrated that most patients tolerated more food protein than at study onset, and that long term, safe daily intake of the food could be achieved in many patients19,20. However, mild to severe clinical symptoms including anaphylaxis occurred in most patients during the desensitization, greatly limiting the utility of this procedure. In addition, 10–25% of patients had severe reactions, particularly those with high peanut-specificIgE, and may be refractory to oral Further, many of the studies focused on reducing the severity of reactions on accidental ingestion rather than on adding normal dietary quantities of the food to the diet. Nevertheless, these studies demonstrate that oral food desensitization might be a useful method for treating food allergic patients to increase the threshold for food tolerance and possibly to hasten the resolution of food allergy.
We hypothesized that oral desensitization might occur more rapidly and with greater success using anti-IgE monoclonal antibody (mAb) (omalizumab, Xolair®, Genentech Inc) as pretreatment prior to and during oral food desensitization. Omalizumab is a humanized monoclonal antibody that binds free IgE thereby inhibiting allergic reactions, and is FDA approved for use in older children and adults with moderate to severe allergic asthma24. Omalizumab and a related anti-IgE mAb, TNX-901, have been used in patients with peanut allergy, and have been shown to significantly increase the threshold of sensitivity to peanut on oral peanut challenge25,26; however, these tudies did not assess the role of anti-IgE mAb therapy on enhancing oral desensitization to peanuts. Recently, we showed in a pilot safety study that omalizumab pretreatment prior to rapid oral desensitization in children with significant milk allergy was safe, and may have facilitated oral desensitization27–29. These results suggested that such an approach might be effective for oral desensitization in patients with peanut allergy at high risk for developing allergic reactions even with trace amounts of peanuts. Indeed, we herein demonstrate that a short 20-week course of omalizumab in peanut allergic children at high risk for developing significant peanut-induced allergic reactions was effective in facilitating rapid and successful oral peanut desensitization.
Methods
Study Population
The inclusion criteria for this study were as follows: patients with peanut allergy, between the ages of 7–25 years with a history of significant clinical symptoms (generalized urticaria, vomiting and/or anaphylaxis) within 1 hour of peanut ingestion; peanut-specific IgE >20 kU/L; total IgE >50 kU/L but <2,000 kU/L; positive skin prick test (SPT) to peanut extract >6 mm (wheal). Patients also had to fail a DBPCFC with peanut at a dose of ≤100 mg peanut flour (cumulative dose of ≤186 mg) (light roasted peanut flour, Golden Peanut Company, Alpharetta, GA), with no reactions to the placebo challenge. The exclusion criteria included the presence of significant medical disease such as infections, autoimmune disease, cardiac disease, and treatment with beta-adrenergic antagonistic drugs. Subjects having a history of anaphylaxis to peanut requiring intubation, chronic urticaria, severe eczema, poorly controlled persistent asthma, gastrointestinal or gastroesophageal disease and non-IgE mediated food allergy (eosinophilic esophagitiseosinophilic enteritis, proctocolitis, food protein induced enterocolitis syndrome (FPIES)) were also excluded.
Thirteen patients with a history of IgE-mediated peanut allergy with a high risk for developing significant peanut-induced allergic reactions were enrolled. The median age of the patients at enrollment was 10 years (range 8–16 years) (Table 1). The subjects included eight boys and five girls. Six of the 13 subjects had a past or current history of eczema, asthma, or both, six had a history of at least one other food allergy, and four had two or more additional food allergies. All of the children had skin prick test (SPT) wheal responses to peanut extract >8.5 mm (mean of the longest diameter and the longest orthogonal width); the median peanut-specific IgE level was 229 kUA/L (Phadea ImmunoCAP System, Portage, MI). The Institutional Review Board at Boston Children’s Hospital approved the clinical protocol, and all participants and their parents provided written informed assent and consent, respectively.
Table 1.
Characteristics of Enrolled Subjects
| Subject No. | Age yrs | sex | Total IgE (kU/L) | Peanut Specific IgE (kUA/L)1 | Peanut Skin Test Wheal (mm)/2 Erythema (mm) | Dose (peanut flour) Failed DBPCFC3 | Omalizumab dose and frequency | Total doses of omalizumab |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | M | 1786 | 436 | 12.5/37 | 100 mg | 450 mg every 2 wk | 10 |
| 2 | 8 | M | 276 | 58 | 20.5/46 | 50 mg | 225 mg every 4 wk | 6 |
| 3 | 9 | F | 1524 | 617 | 15/25.5 | 100 mg | 600 mg every 2 wk | 10 |
| 4 | 15 | M | 485 | 84 | 18/37.5 | 1 mg | 300 mg every 2 wk | 9 |
| 5 | 14 | M | 621 | 150 | 16.5/47.5 | 100 mg | 300 mg every 2 wk | 10 |
| 6 | 14 | M | 981 | 229 | 10.5/38.5 | 100 mg | 525 mg every 2 wk | 11 |
| 7 | 8 | M | 698 | 378 | 19/42.5 | 50 mg | 225 mg every 2 wk | 11 |
| 8 | 14 | F | 571 | 290 | 24/47.5 | 100 mg | 300 mg every 2wk | 13 |
| 9 | 7 | M | 169 | 65 | 9.5/25 | 20 mg | 150 mg every 4 wk | 6 |
| 10 | 10 | F | 735 | 327 | 9.5/35.5 | 50 mg | 300 mg every 2 wk | 11 |
| 11 | 11 | F | 106 | 21 | 8.5/30 | 50 mg | 150 mg every 4 wk | 6 |
| 12 | 12 | F | 630 | 307 | 24.5/57 | 100 mg | 225 mg every 2 wk | 10 |
| 13 | 8 | M | 389 | 172 | 18/37 | 20 mg | 225 mg every 4wk | 6 |
| median | 10 | 621 | 229 | 16.5/37.5 | 50 mg | n/a | 10 |
None of the subjects carried a diagnosis of eosinophilic esophagitis at screening and during the course of the study.
Entry criteria: Children 7 to 25 yrs of age with peanut specific IgE >20 kUA/L, total IgE <2,000 kU/L; with significant clinical history of IgE mediated peanut allergy, but without history of intubation, severe asthma, previous immunotherapy or biologic therapy, and without a medical diagnosis of non-IgE mediated eosinophilic disease.
Normal value, <0.35 kUA/L.
Mean of the longest diameter and the longest orthogonal width.
Peanut flour is approximately 50% peanut protein.
Design
Patients were treated with omalizumab using European dosing guidelines based on weight and serum total IgE30 for 12 weeks to minimize IgE bound to FcsR1 on mast cells and basophils31 (Figure 1). On week 12, patients were admitted to the Clinical and Translational Study Unit (CTSU) for initiation of oral desensitization. Eleven doses 30, 60, 125, 250, 500 mg; cumulative dose 992 mg peanut flour) were administered over a period of 6 hrs. Clinical research pharmacists prepared all doses. After the rush desensitization, all subjects returned the next day to the CTSU to start the slower up-dosing escalation phase, beginning at 500 mg peanut flour. Subjects received subsequent doses at home for the next 6 days, and were instructed not to exceed the specifically assigned doses at home, not to consume other peanut containing products and not to introduce any new foods to the child’s diet. Premeasured doses were provided to all participants, who were required to have diphenhydramine and self-injectable epinephrine available at all times. Home diary forms were provided to record the dose date, time taken, symptoms occurring after the dose or any other time, and medications taken each day. For the next 8 weeks (weeks 12–20), the subjects returned weekly for each increase in the daily oral dose to a dose of 4,000 mg peanut flour (doses: 750 mg, 1,000 mg, 1,250 mg, 1,600 mg, 2,000 mg, 2,600 mg, 3,250 mg, 4,000 mg peanut flour). At week 20, after subjects reached the 4,000 mg daily dose, omalizumab was discontinued, but daily oral peanut dosing continued.
Figure 1.
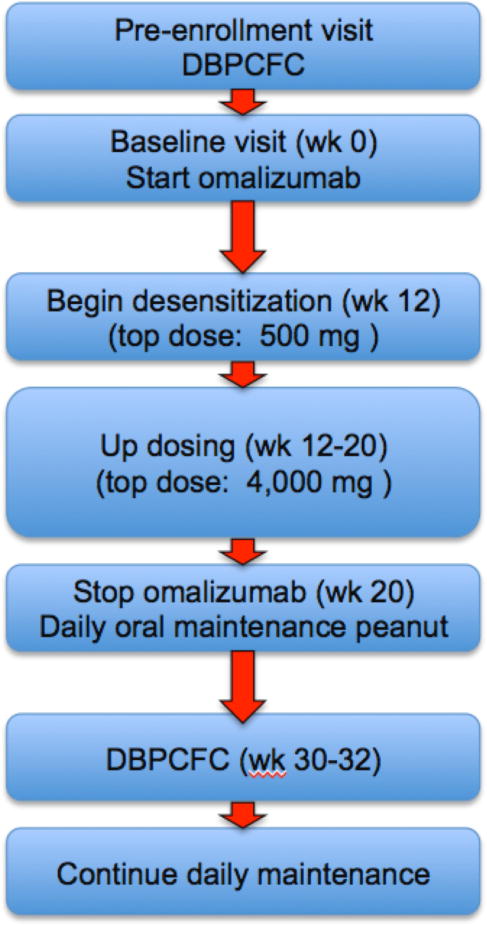
Protocol flow chart
A second DBPCFC was conducted 12 weeks after discontinuing the omalizumab (approximately week 32 of the study, and after four elimination half-lives of omalizumab). The challenge consisted of five doses (peanut or placebo, administered orally every 15 minutes: 500 mg, 750 mg, 1,250 mg, 2,000 mg and 3,500 mg (cumulative dose 8,000 mg peanut flour, equivalent to about 20 peanuts). If the subject passed the DBPCFC, an open challenge of 8,000 mg of peanut flour was given 16 hrs later. Allergic reactions occurring during the protocol were scored using the system developed by Bock32 (Supplemental Tables). After the DBPCFC, subjects continued on 10–20 daily peanuts orally/day.
Registration
This trial was registered on ClinicalTrials.gov (NCT01290913).
Statistical analysis
Statistical analysis is largely descriptive. Two-sided 95% confidence intervals (CI) for percentages are calculated using the exact binomial method. When the observed percentage is 100%, we report a one-sided 97.5% CI instead.
Results
Pre-treatment DBPCFC
During the screening DBPCFC, all 13 subjects who eventually enrolled developed significant allergic symptoms including anaphylax(six requiring epinephrine). During this initial DBPCFC, the patients who enrolled developed symptoms at a dose of peanut flour ≤100 mg (approximately ¼ peanut) (median dose, 50 mg peanut flour) (Table 1). Peanut flour contains approximately 50% peanut protein.
Oral desensitization
Upon enrollment, all subjects received omalizumab every 2 or 4 weeks for 12 weeks, based on modified Genentech dosing guidelines. Subjects were then admitted to the CTSU for oral desensitization, starting at 0.1 mg peanut flour and reaching a top dose of 500 mg peanut flour within 6 hrs. All 13 subjects (100%) reached the 500 mg peanut flour dose on the first day (cumulative dose, 992 mg), which was the primary outcome of the study, with minimal or no symptoms (97.5% confidence interval (CI), 75.3% to 100%). Over the next 7–12 weeks, the daily oral peanut dose was increased from 500 mg to 4,000 mg. Twelve of the 13 children (92%) reached the 4,000 mg dose which was a secondary outcome of the study, requiring a median time of 8 weeks to reach this dose. One subject withdrew at Week 15 after developing persistent nausea and vomiting associated with increased oral mucus production after reaching the 1,250 mg dose. In the remaining 12 subjects, omalizumab treatment was discontinued after the 4,000 mg dose of peanut was achieved. All 12 subjects continued daily peanut dosing (≥4,000 mg peanut flour/day) for the rest of the study.
Twelve weeks after stopping omalizumab (Week 32), the 12 subjects underwent a DBPCFC (cumulative dose 8,000 mg peanut flour). Eleven of the subjects (85%) (95% CI, 54.6% to 98.1%) tolerated this challenge, but one subject vomited once after receiving the highest dose (3,500 mg; cumulative dose 8,000 mg). However, this subject later passed an open challenge of 8,000 mg peanut flour. Therefore, 12 of 13 subjects (92%) tolerated an 8,000 mg dose of peanut flour (equivalent to approximately 20 peanuts). Following the DBPCFC, these subjects continued taking 10-20 peanuts daily until the end of the study at week 52.
Reactions/safety data
All symptoms including minor ones that occurred during the course of the desensitization were recorded, using the scoring system of Bock32, although in many instances the exact cause of the symptoms was not clear. For example, symptoms such as vomiting or wheezing were thought in several instances to be due to viral infection. Viral gastroenteritis was diagnosed clinically in at least three patients (as deduced by the presence of gastroenteritis symptoms in multiple family and community contacts), although symptoms may have been worsened by peanut ingestion. During this time, the dose of peanut was reduced or held for 1–2 days, since tolerance to peanut might be reduced during viral infection33. Difficulty in tolerating the taste of the peanut flour was also common in our patients, likely contributing to some of the nausea and vomiting, particularly with higher doses. In two subjects, swallowing the peanut flour in capsules rather than eating it mixed in with other foods alleviated some of these symptoms, suggesting that aversion to the taste of peanut flour was a significant issue in these patients. Finally, anxiety from having to ingest a food that was assiduously avoided in the past may have exacerbated allergy symptoms in many of our patients.
We recorded a total of 72 reactions during the study (2.0%0–25 reactions/subject) (Table 2). Twenty-five of the reactions occurred in one subject (patient 006), who also had a history of supraventricular tachycardia (SVT); most of these reactions were episodes of nausea and hypersalivation lasting 15–20 seconds and did not require treatment. In the other subjects, most of the reactions were also mild (Grade 1 or 2), and easily treated by observation or with oral H1 and H2 antihistamines. Importantly, six of the 13 subjects (46%) had no or a single Grade 1 allergic reaction; three subjects (23%) had no allergic reaction during the study (Table 3). Six of the 13 patients (39%) experienced a Grade 2 or 3 adverse event (Table 3); two Grade 3 reactions occurred during the maintenance phase (see below), and all reactions responded rapidly to treatment. All subjects tolerated omalizumab without adverse reactions, except for occasional injection site pain and swelling.
Table 2.
Overall Safety Data
| Total peanut doses | 3502 | |
|---|---|---|
|
| ||
| Peanut doses per child, mean (range) | 269 (31–295) | |
|
| ||
| Symptom | No. (% of total doses) | No. of reactions per child, mean (range) |
| Total reactions | 72 (2.0%) | 5.53 (0–25) |
|
| ||
| Grade 1 (Mild) symptoms | 63 (1.8%) | 4.8 (0–25) |
| On rush desensitization day | 6 | 0.46 (0–1) |
| During weekly dosing Escalation phase | 47 | 3.6 (0–18) |
| During maintenance dosing | 10 | 0.83 (0–5) |
|
| ||
| Grade 2 (Moderate) symptoms | 7 (0.2%) | 0.53 (0–7) |
| On rush desensitization day | 0 | 0 |
| During weekly dosing Escalation phase | 2 | 0.15 (0–1) |
| During maintenance dosing | 5 | 0.42 (0–2) |
|
| ||
| Grade 3 (Severe) symptoms | 2 (0.06%) | 0.17 (0–1) |
| On rush desensitization day | 0 | 0 |
| During weekly dosing Escalation phase | 0 | 0 |
| During maintenance dosing | 2 | 0.17 (0–1) |
Total number of subjects=13.
Reactions were graded using scores defined by Bock32.
Table 3.
Safety Data for Individual Subjects
| 1st Day Desensitization | Updosing | Maintenance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject Number | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 |
| 001 | No Reactions | No Reactions | No Reactions | ||||||
| 003 | No Reactions | No Reactions | No Reactions | ||||||
| 005 | No Reactions | No Reactions | No Reactions | ||||||
| 011 | No Reactions | 1* | No Reactions | ||||||
| 010 | 1 | No Reactions | No Reactions | ||||||
| 009 | 1 | 1 | No Reactions | ||||||
| 002 | 1 | No reactions | 1 | ||||||
| 012 | No Reactions | No Reactions | 2 | 1 | |||||
| 008 | No Reactions | 2 | 1 | 1 | 1 | ||||
| 013 | 1 | 6 | 1 | 1 | 1 | ||||
| 007 | No Reactions | 11 | 1 | 2 | |||||
| 006 | 1 | 18 | 1 | 5 | |||||
| 004 | 1 | 8 | ** | ||||||
| % of subjects not having reactions | 54% | 100% | 100% | 46% | 85% | 100% | 58% | 67% | 83% |
Number of reactions of the indicated Grade during the desensitization protocol. All patients, except 004 (left study at Week 15) and 007 (left study at Week 42), completed 52 weeks of the study. The DBPCFC occurred at Week 32.
The light and dark grey shaded boxes indicate no reactions were observed during the indicated time period.
Patient 004 dropped out at week 15 and was never on maintenance treatment.
During the first day of desensitization, seven of the 13 subjects (54%) tolerated a cumulative peanut dose, 992 mg with no reactions; the remaining six subjects developed Grade 1 reactions (two of these patients were treated with antihistamines) (Table 2 and 3). During this first day of desensitization, no subject developed a Grade 2 or Grade 3 reaction. During the up-dosing phase, when the dose was increased weekly from 500 mg to 4,000 mg peanut flour, 49 reactions were recorded (96% of these were Grade 1, and 4% were Grade 2), and most reactions were easily controlled with antihistamines. The most common reactions were nausea and salivation, or abdominal pain, occurring in seven patients(54%). Because of these symptoms, five patients (42%) were placed on maintenance H1 and H2 antihistamines. One patient (006), who had the prior history of SVT, developed nausea, lightheadedness and hypersalivation after a 1,250 mg dose and received epinephrine at home for a Grade 2 reaction. Another patient (004) withdrew from the study at the 15-week time point because of persistent nausea, vomiting and hypersalivation after reaching the 1,250 mg dose.
After reaching the 4,000 mg maintenance dose, and after discontinuing omalizumab, the subjects were followed for an additional six months maintenance period, during which time there were 17 recorded reactions (ten Grade 1, five Grade 2 and two Grade 3), with two subjects receiving epinephrine at home. Patient 008 received epinephrine for vomiting, diarrhea and wheezing (Grade 3 reaction) 2.5 hours after peanut dosing, possibly worsened by known triggers of reactions, including concomitant infection, menstruation, naproxen (NSAID) use, or school associated stress33. Patient 007 received epinephrine on three occasions for Grade 2 reactions: the first time, several days after passing the second DBPCFC, the second time on Week 36, and a third time on Week 42. On all of these occasions, the reactions of coughing, hives and wheezing were associated with exercise, which is known to trigger symptoms following ingestion of a previously tolerated dose20,23. Because these episodes were unpredictable and resulted in considerable anxiety, the family decided to withdraw from the study.
Discussion
The results of our study suggest that pretreatment with omalizumab may facilitate rapid oral desensitization in peanut allergic subjects at high risk for developing significant peanut-induced allergic reactions on exposure to even small amounts of peanut. Significant peanut allergy was confirmed by failing an initial DBPCFC after a median dose of 50 mg peanut flour. After receiving omalizumab, 13 of 13 subjects tolerated the first day desensitization to peanut, reaching the maximum first day desensitization dose of 500 mg peanut flour (cumulative dose 992 mg peanut flour), with minimal or no symptoms, thus achieving the primary end point of the study. Twelve of the 13 subjects (92%) then reached the maximum 4,000 mg dose over a 7–12 week time period (median time, 8 weeks), at which point omalizumab treatment was discontinued. All 12 subjects continued to tolerate daily oral dosing of peanut even after discontinuing omalizumab, and then tolerated an open challenge dose of 8,000 mg peanut flour, a dose approximately times the dose tolerated before desensitization.
Our current results extend those of a previous small study of oral desensitization with omalizumab of high-risk, milk allergic patients, in which nine of 11 of subjects (82%) were rapidly and successfully desensitized, and were able to pass an oral challenge of ≥8,000 mg of milk without symptoms. However, because an initial DBPCFC was not performed, we could not formally assess the degree of improvement in milk tolerance, although all of the patients had high initial levels of milk-specific IgE (median 50 kUA/L, range 42–342 (normal level <0.32 kUA/L)) and were unlikely to have spontaneously outgrown their milk allergy. Nevertheless, these studies together strongly suggest that omalizumab might facility oral desensitization not only with a food allergy that is frequently outgrown (milk), but also with a food allergy that does not normally resolve spontaneously and is associated with most of the fatalities (peanut).
In our current study, we intentionally focused on patients with high levels of peanut-specific IgE, who were at high risk for developing significant peanut-induced allergic reactions. Such patients with high peanut-specific IgE likely have very robust peanut-specific immunity that resists change, and therefore may benefit more from omalizumab treatment. Indeed, during desensitization without omalizumab, children with higher food-specific IgE levels were more likely to fail egg desensitization22 or were unable to tolerate high doses of peanut after rush desensitization21. Nevertheless, oral desensitization without omalizumab can be successful in patients with peanut, milk, egg, and hazelnut allergy12,14–17,34–36. However, in all of these studies a common theme was the high rate of significant reactions, with 10–30% having severe reactions and being refractory to oral desensitization, particularly in those with high food-specific IgE10,21–23, resulting in lengthy desensitization periods, varying from many months to years.
In contrast, after treatment with omalizumab, our patients, who had the highest median peanut-specific IgE level of any previous study of peanut desensitization that we know of, were all able to tolerate much higher doses of peanut in much shorter periods of time. Thus, 13 of 13 patients progressed within one day to a maximum dose of 500 mg peanut flour (cumulative dose 992 mg). By comparison, in one oral desensitization study without omalizumab, on the initial day of desensitization, only ten of 39 subjects (26%) tolerated a cumulative dose equivalent to 200 mg peanut flour34; in another study, only six of 28 subjects (21%) tolerated a cumulative dose equivalent to 200 mg peanut flour16. A post-hoc comparison showed that these two rates were significantly different from our results, with a P-value <0.0001 (Fisher’s exact test), even though the maximum cumulative dose in our study was three times higher than in the previous two studies. Because of the high frequency of allergic reactions associated with oral desensitization without omalizumab, more recent studies have limited the maximum dose on the first day to a cumulative dose equivalent to 24 mg peanut flour36,37. Finally, with omalizumab treatment, our patients required a median time of only 8 weeks to reach a maintenance dose of 4,000 mg peanut flour, whereas in a previous peanut desensitization study without omalizumab the median time for 14 subjects to reach doses of 500–2000 mg of peanut was 30 weeks21, and in another study the median time for 19 subjects to reach a dose equivalent to 1,600 mg peanut flour was 20 wks23. The rapidity of reaching maintenance desensitization dosing in our study compared to previous studies is remarkable, particularly given the much higher peanut-specific IgE levels in our patient population and our low failure rate.
Over the course of our study, 2.0% of the doses were associated with reactions, and most of these were Grade 1 or Grade 2, and easily controlled with antihistamines. As noted above, on the first day of desensitization 100% of our patients tolerated a cumulative dose of 992 mg peanut flour, with seven having no reactions at all and six patients having, Grade 1 reactions (two treated with antihistamines) (Table 3). Although the goal of our protocol was to allow peanut allergic patients to tolerate ingestion of 10–20 peanuts, the fact that all of our subjects tolerated 992 mg of peanut flour after only one day of desensitization, suggests that one day of desensitization might protect a patient against anaphylaxis associated with accidental exposure, a major concern among patients and their families. In addition, since omalilzumab neutralizes IgE of all specificities, our result with peanut suggests that rapid desensitization to other foods, either singly or simultaneously, may be achievable in a short period of time.
During the weekly up-dosing phase, six of the 13 patients (46%) had no reactions and two additional patients (15%) had only minor reactions that did not require treatment. Only one patient (patient 006) received epinephrine during the up-dosing period for a Grade 2 home reaction that may not have required epinephrine. Attributing a cause for these reactions was not always straightforward, since many of the symptoms could have been caused by concomitant viral infections, which caused vomiting and some times wheezing, and which slowed the progress of dose escalation (Noro virus infection was particularly common in the community during the study). In addition, in some patients intolerance of the taste of peanut flour appeared to cause nausea and excessive salivation, since these symptoms improved when the peanut flour was swallowed in capsules rather than mixed in food. Nevertheless, these results demonstrate that when the subjects were on omalizumab, allergic reactions during the relatively rapid desensitization process were surprisingly mild.
During the maintenance phase (Weeks 20 to 52, Figure 1), after omalizumab was stopped and when patients took a daily dose of 4,000 mg peanut flour, six of the twelve patients (50%) had no reactions, and three others (25%) had only minor grade 1 or 2 reactions. However, two other patients (17%), required epinephrine treatments, receiving it at home. One patient (008) received epinephrine in the setting of a viral illness, menstruation or with NSAID use, and possible school related stress, even after she had tolerated multiple 4,000 mg doses of peanut and the second DBPCFC. These reactions all occurred more than two hours after the peanut dose. The second patient (007) received epinephrine on three occasions after passing the second DBPCFC for reactions associated with exercise. All of the episodes were associated with specific triggers (infection, exercise, menstruation or NSAID use), and were all easily controlled. We speculate that the frequency of these reactions might be reduced by a longer treatment period with omalizumab, particularly since the peanut-specific IgE levels, which were very high before treatment, were still high even at Week 52, when the median peanut-specific IgE was 70 kUA/L. This idea is also supported by the fact that the median initial peanut skin test wheal size (Tables 1 and 3) in the seven patients who had more frequent and severe reactions (median wheal size=19 mm) was significantly greater than that of the 6 patients who had no or grade 1 reactions (median wheal size=11 mm) (P=0.03, Wilcoxon rank sum test).
The persistence of high levels of peanut-specific IgE in our subjects on maintenance therapy suggests that peanut specific immunity was robust in our patients, and that long-term oral maintenance dosing may be required to drive down peanut-specific IgE levels to “non-allergic” levels. This idea might be consistent with the results of a recent study showing that only 28% of orally desensitized egg allergic patients developed “immunological tolerance”, as defined by the ability of egg desensitized patients to avoid egg for 2 months and still pass an oral egg challenge13, and suggests that the development of “immunological tolerance” after oral desensitization may require long periods of oral therapy. Currently, our recommendation to our study patients who tolerate doses of 10–20 peanuts is to remain on daily peanut dosing until peanut-specific IgE levels fall well below the initial levels, which may take many more months. We anticipate that tolerance of peanuts will improve over time on maintenance treatment, but we are being vigilant for complications, including eosinophilic esophagitis, which has been reported after oral desensitization38.
The major limitations of our study include the small sample size and the absence of a placebo group. However, our results, using omalizumab in oral peanut desensitization, are qualitatively distinct compared to previous studies of oral desensitization without omalizumab. As noted above, on the initial day of desensitization 100% of our subjects tolerated a cumulative dose of 992 mg peanut flour with minimal symptoms, whereas in several other studies without omalizumab, ≤26% of the subjects tolerated the equivalent of 200 mg of peanut flour16,34.
In conclusion, our study suggests that omalizumab may facilitate rapid oral desensitization in high-risk peanut allergic patients with high peanut-specific IgE. 100% of patients tolerated a cumulative dose of 992 mg of peanut flour on the first day of desensitization, and 92% were able to rapidly tolerate doses of 8,000 mg of peanut flour. Although we cannot yet recommend omalizumab to facilitate oral desensitization, our results provide strong evidence that omalizumab can effectively reduce allergic reactions and expedite successful and rapid oral peanut desensitization in patients with high peanut-specific IgE. Larger randomized placebo-controlled studies are currently being conducted to confirm a beneficial role of omalizumab in facilitating oral peanut desensitization.
Supplementary Material
Clinical Implications.
If confirmed with larger double-blind placebo-controlled studies, this approach using omalizumab to facilitate oral desensitization could change the clinical approach for large numbers of patients with clinicallysignificant peanut allergy.
Acknowledgments
We thank the Thrasher Research Foundation, the Clinical and Translational Science Center/Harvard Catalyst (NIH UL1 RR 025758), the Food Allergy Initiative, the Bunning Food Allergy Project, the Jasmine and Paul Mashikian Fund, and Genentech for support. Genentech was not involved in the design, implementation or conduct of this study. We also thank members of our Data and Safety Monitoring Board, Drs. Hans Oettgen, Francisco Bonilla and Doug McDonald for their unstinting help; Drs. Michael Young and Frank Twarog for referring their patients for this study; Dr. Michael Young for critically reviewing the manuscript; Drs. Leslie A. Kalish and Dionne Graham for help with the statistical analysis; Dr. Michael Pistiner and Tim Harrington for helping with the study; and our patients, who courageously participated in this study.
Funded by the Thrasher Research Foundation, the Clinical and Translational Science Center/Harvard Catalyst (NIH UL1 RR 025758), the Food Allergy Initiative, the Bunning Food Allergy Project, the Jasmine and Paul Mashikian Fund, and Genentech.
Abbreviations
- CI
confidence intervals
- CTSU
Clinical and Translational Research Unit
- DBPCFC
Double-blind, placebo-controlled food challenge
- SPT
skin prick test
Footnotes
Trial registration: ClinicalTrials.gov identifier: NCT01290913.
References
- 1.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116–25. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Lack G. Clinical practice. Food allergy. N Engl J Med. 2008;359:1252–60. doi: 10.1056/NEJMcp0800871. [DOI] [PubMed] [Google Scholar]
- 3.Burks AW. Peanut allergy. Lancet. 2008;371:1538–46. doi: 10.1016/S0140-6736(08)60659-5. [DOI] [PubMed] [Google Scholar]
- 4.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–85. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–6. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 7.Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy. 2010;65:933–45. doi: 10.1111/j.1398-9995.2010.02342.x. [DOI] [PubMed] [Google Scholar]
- 8.Fleischer DM, Conover-Walker MK, Christie L, Burks AW, Wood RA. The natural progression of peanut allergy: Resolution and the possibility of recurrence. J Allergy Clin Immunol. 2003;112:183–9. doi: 10.1067/mai.2003.1517. [DOI] [PubMed] [Google Scholar]
- 9.Meglio P, Bartone E, Plantamura M, Arabito E, Giampietro PG. A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy. 2004;59:980–7. doi: 10.1111/j.1398-9995.2004.00542.x. [DOI] [PubMed] [Google Scholar]
- 10.Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, et al. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J Allergy Clin Immunol. 2008;121:343–7. doi: 10.1016/j.jaci.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Staden U, Blumchen K, Blankenstein N, Dannenberg N, Ulbricht H, Dobberstein K, et al. Rush oral immunotherapy in children with persistent cow’s milk allergy. J Allergy Clin Immunol. 2008;122:418–9. doi: 10.1016/j.jaci.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. 2007;119:199–205. doi: 10.1016/j.jaci.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–43. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash S, Steele P, Kamilaris J, Pons L, Kulis M, Lee L, et al. Oral peanut immunotherapy for children with peanut allergy. J Allergy Clin Immunol. 2008;121:S147. [Google Scholar]
- 15.Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009;64:1218–20. doi: 10.1111/j.1398-9995.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009;124:286–91. 91 e1–6. doi: 10.1016/j.jaci.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enrique E, Pineda F, Malek T, Bartra J, Basagana M, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005;116:1073–9. doi: 10.1016/j.jaci.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–55. 55 e1–5. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvani M, Giorgio V, Miceli Sopo S. Specific oral tolerance induction for food. A systematic review. Eur Ann Allergy Clin Immunol. 2010;42:11–9. [PubMed] [Google Scholar]
- 20.Sampson H. Peanut oral immunotherapy: Is it ready for clinical practice? JACI:In Practice. 2013;1:15–21. doi: 10.1016/j.jaip.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91 e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Meglio P, Giampietro PG, Carello R, Gabriele I, Avitabile S, Galli E. Oral food desensitization in children with IgE-mediated hen’s egg allergy: a new protocol with raw hen’s egg. Pediatr Allergy Immunol. 2013;24:75–83. doi: 10.1111/j.1399-3038.2012.01341.x. [DOI] [PubMed] [Google Scholar]
- 23.Anagnostou K, Clark A, King Y, Islam S, Deighton J, Ewan P. Efficacy and safety of high-dose peanut oral immunotherapy with factors predicting outcome. Clin Exp Allergy. 2011;41:1273–81. doi: 10.1111/j.1365-2222.2011.03699.x. [DOI] [PubMed] [Google Scholar]
- 24.Fanta CH. Asthma. N Engl J Med. 2009;360:1002–14. doi: 10.1056/NEJMra0804579. [DOI] [PubMed] [Google Scholar]
- 25.Leung DY, Sampson HA, Yunginger JW, Burks AW, Jr, Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–93. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 26.Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, et al. A phase II, randomized, doubleblind, parallelgroup, placebocontrolled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127:1309–10 e1. doi: 10.1016/j.jaci.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 27.Nadeau K, Schneider L, Hoyte E, Borras I, Umetsu D. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol. 2011;127:1722–4. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadeau KC, Kohli A, Iyengar S, DeKruyff RH, Umetsu DT. Oral immunotherapy and anti-IgE antibody-adjunctive treatment for food allergy. Immunol Allergy Clin North Am. 2012;32:111–33. doi: 10.1016/j.iac.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Bedoret D, Singh AK, Shaw V, Hoyte EG, Hamilton R, Dekruyff RH, et al. Changes in antigen-specific T-cell number and function during oral desensitization in cow’s milk allergy enabled with omalizumab. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.dosing, Xolair. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000606/WC500057298.pdf.
- 31.MacGlashan DW, Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, et al. Down-regulation of Fc(epsilon) RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–45. [PubMed] [Google Scholar]
- 32.Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol. 1988;82:986–97. doi: 10.1016/0091-6749(88)90135-2. [DOI] [PubMed] [Google Scholar]
- 33.Nowak-Wegrzyn A, Fiocchi A. Is oral immunotherapy the cure for food allergies? Curr Opin Allergy Clin Immunol. 2010;10:214–9. doi: 10.1097/ACI.0b013e3283399404. [DOI] [PubMed] [Google Scholar]
- 34.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008;122:1154–60. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu GP, Weldon B, Neale-May S, Nadeau KC. The safety of peanut oral immunotherapy in peanut-allergic subjects in a single-center trial. Int Arch Allergy Immunol. 2012;159:179–82. doi: 10.1159/000336391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez-Garcia S, Rodriguez Del Rio P, Escudero C, Martinez-Gomez MJ, Ibanez MD. Possible eosinophilic esophagitis induced by milk oral immunotherapy. J Allergy Clin Immunol. 2012;129:1155–7. doi: 10.1016/j.jaci.2011.11.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


