Abstract
BACKGROUND
Ovarian failure is a common toxic effect of chemotherapy. Studies of the use of gonadotropin-releasing hormone (GnRH) agonists to protect ovarian function have shown mixed results and lack data on pregnancy outcomes.
METHODS
We randomly assigned 257 premenopausal women with operable hormone-receptor–negative breast cancer to receive standard chemotherapy with the GnRH agonist goserelin (goserelin group) or standard chemotherapy without goserelin (chemotherapy-alone group). The primary study end point was the rate of ovarian failure at 2 years, with ovarian failure defined as the absence of menses in the preceding 6 months and levels of follicle-stimulating hormone (FSH) in the postmenopausal range. Rates were compared with the use of conditional logistic regression. Secondary end points included pregnancy outcomes and disease-free and overall survival.
RESULTS
At baseline, 218 patients were eligible and could be evaluated. Among 135 with complete primary end-point data, the ovarian failure rate was 8% in the goserelin group and 22% in the chemotherapy-alone group (odds ratio, 0.30; 95% confidence interval, 0.09 to 0.97; two-sided P = 0.04). Owing to missing primary end-point data, sensitivity analyses were performed, and the results were consistent with the main findings. Missing data did not differ according to treatment group or according to the stratification factors of age and planned chemotherapy regimen. Among the 218 patients who could be evaluated, pregnancy occurred in more women in the goserelin group than in the chemotherapy-alone group (21% vs. 11%, P=0.03); women in the goserelin group also had improved disease-free survival (P = 0.04) and overall survival (P=0.05).
CONCLUSIONS
Although missing data weaken interpretation of the findings, administration of goserelin with chemotherapy appeared to protect against ovarian failure, reducing the risk of early menopause and improving prospects for fertility. (Funded by the National Cancer Institute and others; POEMS/S0230 ClinicalTrials.gov number, NCT00068601.)
Early ovarian failure is an important and potentially devastating long-term toxic effect of chemotherapy. Manifestations include menopausal symptoms, osteoporosis, and infertility. Concerns about fertility may influence treatment choices for young women with breast cancer1,2 despite the known survival benefit of adjuvant chemotherapy.
Trials of the coadministration of a gonadotropin-releasing hormone (GnRH) agonist with adjuvant chemotherapy for the purpose of protecting ovarian function have shown mixed results.3 A large randomized trial addressing this issue suggested that coadministration of a GnRH agonist with chemotherapy had an ovarian protective effect in a cohort of patients in which 86% had estrogen-receptor–positive breast cancer, with the return of menses within the first year used as the primary measure of ovarian function.4 The use of adjuvant endocrine therapy after chemotherapy complicates the assessment of longer-term ovarian function after administration of a GnRH agonist with chemotherapy. Furthermore, data on pregnancy outcomes after GnRH agonist treatment with chemotherapy are lacking. It has even been suggested that this approach may impair fertility.5
The Prevention of Early Menopause Study (POEMS)/S0230 was an international, phase 3, randomized study that was performed to evaluate whether administration of the GnRH agonist goserelin (Zoladex, AstraZeneca) with chemotherapy would reduce the rate of ovarian failure after adjuvant or neoadjuvant treatment of hormone-receptor–negative early breast cancer. The study was designed to compare the rate of ovarian failure at 2 years, the rate of ovarian dysfunction, and pregnancy outcomes between patients receiving chemotherapy with goserelin and those receiving chemotherapy without goserelin.
METHODS
STUDY OVERSIGHT
The protocol of the study was approved by the institutional review board at each participating site. All patients provided written informed consent for participation. The study was designed by the authors and monitored by an independent data and safety monitoring committee. The SWOG Cancer Research Group (SWOG) coordinated the study and was responsible for the design of the study and the collection, analysis, and reporting of the data. The authors vouch for the accuracy and completeness of the reported data and for the fidelity of the study to the protocol, which is available with the full text of this article at NEJM.org.
PATIENTS
Premenopausal women 18 to 49 years of age were eligible for enrollment if they had operable stage I to IIIA estrogen-receptor (ER)–negative and progesterone-receptor (PR)–negative breast cancer for which treatment with adjuvant or neoadjuvant cyclophosphamide-containing chemotherapy was planned. ER and PR negativity was defined according to the treating institution’s standard. Participants were enrolled from SWOG, the International Breast Cancer Study Group (IBCSG), the ECOG– ACRIN Cancer Research Group, and the Alliance for Clinical Trials in Oncology. Eligible participants had taken no estrogens, antiestrogens, selective estrogen-receptor modulators, aromatase inhibitors, or hormonal contraceptives within the month before enrollment. Exceptions were made for the use of hormonal contraception in women younger than 35 years of age that was discontinued before randomization and for hormonal treatment for up to 2 months for the purposes of in vitro fertilization and cryopreservation of embryos or oocytes before randomization. Interest in future fertility was not an eligibility requirement.
STUDY DESIGN
In this phase 3 trial, patients were randomly assigned, in a 1:1 ratio, to standard adjuvant or neoadjuvant chemotherapy with the GnRH agonist goserelin (goserelin group) or to chemotherapy without goserelin (chemotherapy-alone group). The choice of the standard cyclophosphamide-containing chemotherapy regimen was left to the discretion of the investigator. For patients randomly assigned to the goserelin group, goserelin at a dose of 3.6 mg was administered subcutaneously every 4 weeks beginning 1 week before the initial chemotherapy dose and was continued to within 2 weeks before or after the final chemotherapy dose. Randomization was stratified according to age (<40 years vs. 40 to 49 years) and chemotherapy regimen (3 to 4 cycles [about 3 months] vs. 6 to 8 cycles [about 6 months], and anthracycline-based vs. nonanthracycline-based). Use of trastuzumab was permitted in patients with human epidermal growth factor receptor 2 (HER2)–overexpressing tumors.
The primary objective was to compare the rate of ovarian failure between the two treatment groups. Ovarian failure was defined as amenorrhea for the preceding 6 months and follicle-stimulating hormone (FSH) levels in the post-menopausal range at 2 years. Patients who became pregnant were considered not to have had ovarian failure. Patients who underwent hysterectomy or bilateral oophorectomy were categorized as unable to be evaluated. Additional end points included pregnancy within 5 years, assessed annually, and ovarian dysfunction, defined as amenorrhea for the preceding 3 months and FSH, estradiol, or inhibin B levels in the postmenopausal range, assessed at both year 1 and year 2. Events in the analysis of overall survival included deaths due to any cause; events in the analysis of disease-free survival also included breast-cancer recurrence but not contralateral breast or nonbreast primary cancers. Only adverse events related to hormonal effects and serious adverse events that occurred during chemotherapy with or without goserelin were routinely assessed, with assessment according to the Common Terminology Criteria for Adverse Events, version 3.0.6
STATISTICAL ANALYSIS
The original target enrollment was 416 eligible patients. We estimated that with this sample size, the study, based on a two-group binomial design, would have more than 80% power to detect an absolute reduction of 15 percentage points in the rate of ovarian failure, assuming rates of ovarian failure in the chemotherapy-alone group in the range of 20 to 95% and an expected mortality by year 2 of 10%, at a one-sided significance level of 0.025. The study closed early owing to loss of funding for study-drug distribution. Post hoc power calculations that were based on actual enrollment indicated that the study had sufficient power (≥80%) to detect an absolute reduction of 20 percentage points in the rate of ovarian failure under the same design specifications.
The primary analysis was based on conditional logistic regression, with data from all eligible patients who could be evaluated and who had complete 2-year data, stratified according to age and type of chemotherapy regimen. An assessment window within 6 months before or after the 2-year time point was allowed. Owing to missing endpoint data, sensitivity analyses were performed to incorporate partial information. These included adding death and, separately, death plus hysterectomy or oophorectomy as treatment failures. In addition, given that amenorrhea and FSH levels are positively correlated and that these data were also available at year 1, we examined the risk of either amenorrhea or postmenopausal levels of FSH at year 2, as well as at year 1 or 2.
We analyzed patient characteristics according to randomization group. To assess whether missing data influenced the results for the primary analysis, we also evaluated the association between treatment and stratification variables according to status with respect to follow-up data (availability vs. nonavailability of data for the 2-year end point). We analyzed the number of patients reporting pregnancy and attempting pregnancy according to the randomization group over the course of 5 years. Finally, exploratory Kaplan–Meier curves for disease-free and overall survival were calculated and 4-year rates were estimated. Hazard ratios, 95% confidence intervals, and P values for differences in overall and disease-free survival were derived with the use of multivariable Cox regression, with adjustment for stratification factors and cancer stage. Pregnancy and survival rates were assessed in all patients who were eligible and could be evaluated.
According to the study-design specifications, a one-sided alpha level of 0.025 was used to indicate statistical significance for the primary end-point analysis of ovarian failure; for all other P values, a two-sided alpha level of 0.05 was used to indicate statistical significance. The cutoff date for all analyses was January 22, 2014.
RESULTS
PATIENTS
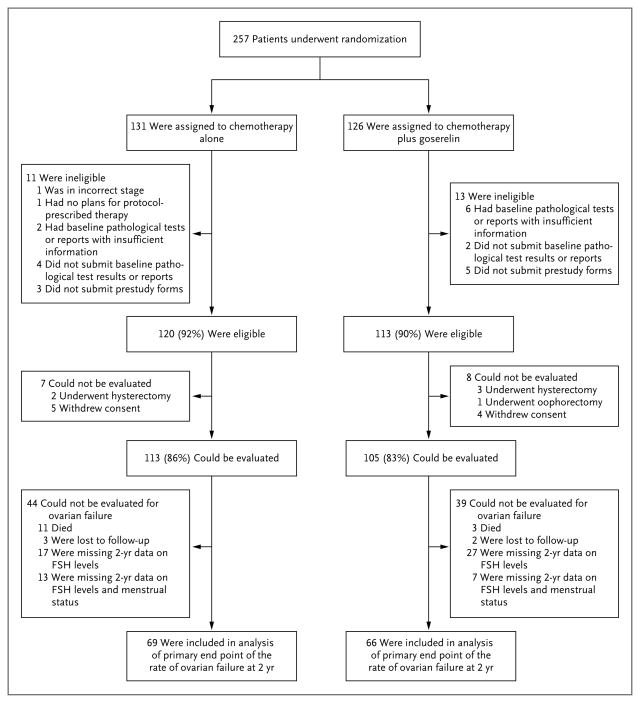
A total of 257 patients (14 from CALGB, 86 from ECOG, 104 from IBCSG, and 53 from SWOG) underwent randomization between February 2004 and May 2011. A total of 24 patients were ineligible, and 15 were considered unable to be evaluated for the study end points, leaving 218 patients who could be evaluated (113 in the chemotherapy-alone group and 105 in the goserelin group) (Fig. 1).
Figure 1.
Randomization, Eligibility, and Follow-up
FSH denotes follicle-stimulating hormone.
The median follow-up time among patients still alive at the time of the end-point analysis was 4.1 years. Patient characteristics according to study-group assignment are shown in Table 1. The median age of the patients was 38 years. A total of 91% of the patients received anthracycline-based therapy. The characteristics of the patients were well balanced between the two groups.
Table 1.
Baseline Characteristics of the Patients, According to Study Group.*
| Characteristic | All Eligible Patients | Patients with 2-Yr Data on Ovarian Failure | ||||
|---|---|---|---|---|---|---|
| Overall (N = 218) | Chemotherapy Alone (N = 113) | Chemotherapy plus Goserelin (N = 105) | Overall (N = 135) | Chemotherapy Alone (N = 69) | Chemotherapy plus Goserelin (N = 66) | |
| Age | ||||||
|
| ||||||
| Median (range) | 37.7 (25.1–49.9) | 38.7 (25.1–49.9) | 37.6 (26.1–48.6) | 36.9 (25.1–49.9) | 37.5 (25.1–49.9) | 36.1 (26.1–48.6) |
|
| ||||||
| <40 yr — no. (%) | 138 (63) | 70 (62) | 68 (65) | 94 (70) | 45 (65) | 49 (74) |
|
| ||||||
| ≥40 yr — no. (%) | 80 (37) | 43 (38) | 37 (35) | 41 (30) | 24 (35) | 17 (26) |
|
| ||||||
| Race or ethnic group — no./total no. (%)† | ||||||
|
| ||||||
| White | 122/136 (90) | 57/66 (86) | 65/70 (93) | 69/79 (87) | 33/39 (85) | 36/40 (90) |
|
| ||||||
| Black | 11/136 (8) | 6/66 (9) | 5/70 (7) | 7/79 (9) | 3/39 (8) | 4/40 (10) |
|
| ||||||
| Asian | 2/136 (1) | 2/66 (3) | 0 | 2/79 (3) | 2/39 (5) | 0 |
|
| ||||||
| Native American | 1/136 (1) | 1/66 (2) | 0 | 1/79 (1) | 1/39 (3) | 0 |
|
| ||||||
| Unknown | 82/218 (38) | 47/113 (42) | 35/105 (33) | 56/135 (41) | 30/69 (43) | 26/66 (39) |
|
| ||||||
| Hispanic or non-Hispanic ethnic group — no./total no. (%)† | ||||||
|
| ||||||
| Hispanic | 67/126 (53) | 26/60 (43) | 33/66 (50) | 39/71 (55) | 18/35 (51) | 14/36 (39) |
|
| ||||||
| Non-Hispanic | 59/126 (47) | 34/60 (57) | 33/66 (50) | 32/71 (45) | 17/35 (49) | 22/36 (61) |
|
| ||||||
| Unknown | 92/218 (42) | 53/113 (47) | 39/105 (37) | 64/135 (47) | 34/69 (49) | 30/66 (45) |
|
| ||||||
| Planned chemotherapy — no. (%) | ||||||
|
| ||||||
| 3–4 cycles of anthracycline-based therapy | 46 (21) | 22 (19) | 24 (23) | 27 (20) | 15 (22) | 12 (18) |
|
| ||||||
| 3–4 cycles of nonanthracycline-based therapy | 12 (6) | 7 (6) | 5 (5) | 8 (6) | 5 (7) | 3 (5) |
|
| ||||||
| 6–8 cycles of anthracycline-based therapy | 152 (70) | 80 (71) | 72 (69) | 96 (71) | 47 (68) | 49 (74) |
|
| ||||||
| 6–8 cycles of nonanthracycline-based therapy | 8 (4) | 4 (4) | 4 (4) | 4 (3) | 2 (3) | 2 (3) |
|
| ||||||
| Stage of cancer — no. (%) | ||||||
|
| ||||||
| I | 55 (25) | 32 (28) | 23 (22) | 34 (25) | 18 (26) | 16 (24) |
|
| ||||||
| II | 107 (49) | 52 (46) | 55 (52) | 70 (52) | 34 (49) | 36 (55) |
|
| ||||||
| IIIA | 54 (25) | 29 (26) | 25 (24) | 31 (23) | 17 (25) | 14 (21) |
|
| ||||||
| Unknown | 2 (1) | 0 | 2 (2) | 0 | 0 | 0 |
|
| ||||||
| HER2 status — no./total no. (%) | ||||||
|
| ||||||
| Positive | 32/215 (15) | 19/112 (17) | 13/103 (13) | 23/132 (17) | 11/68 (16) | 12/64 (19) |
|
| ||||||
| Negative | 183/215 (85) | 93/112 (83) | 90/103 (87) | 109/132 (83) | 57/68 (84) | 52/64 (81) |
|
| ||||||
| Unknown | 3/218 (1) | 1/113 (1) | 2/105 (2) | 3/135 (2) | 1/69 (1) | 2/66 (3) |
Among patients with 2-year end-point data, there were no significant differences between the groups in any of the characteristics listed in this table. Percentages may not sum to 100% for a given characteristic owing to rounding. HER2 denotes human epidermal growth factor receptor 2.
Data on race and ethnic group were self-reported or were reported by the investigator. Data on race and ethnic group were not collected at many of the sites outside the United States; for patients at those sites, data were recorded as unknown.
TOXIC EFFECTS
Two patients in the goserelin group could not be evaluated for adverse events because they received no intervention, and data on toxic effects were never collected for two patients in the chemotherapy-alone group. Of the 111 patients who could be evaluated for adverse events in the chemotherapy-alone group, 6 had grade 3 toxic effects; none of the patients in the group had grade 4 toxic effects. Of the 103 patients who could be evaluated for adverse events in the goserelin group, 1 had a grade 4 toxic effect (thromboembolism) and 6 had grade 3 toxic effects. Thus, 5% of the patients in the chemotherapy-alone group and 7% in the goserelin group had grade 3 or higher toxic effects (P = 0.89), and 24% and 48%, respectively, had grade 2 or higher toxic effects (P<0.001) (Table 2).
Table 2.
Grade 2 or Higher Toxic Effects.*
| Adverse Event | Chemotherapy Alone (N = 111) | Chemotherapy plus Goserelin (N = 103) | ||||
|---|---|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 | |
| Diarrhea | 2 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Fatigue | 1 | 0 | 0 | 2 | 0 | 0 |
|
| ||||||
| Hot flashes | 14 | 3 | 0 | 29 | 4 | 0 |
|
| ||||||
| Irregular menses | 2 | 0 | 0 | 5 | 2 | 0 |
|
| ||||||
| Decrease in libido | 6 | 0 | 0 | 9 | 0 | 0 |
|
| ||||||
| Agitation | 4 | 1 | 0 | 6 | 0 | 0 |
|
| ||||||
| Anxiety | 4 | 0 | 0 | 9 | 0 | 0 |
|
| ||||||
| Depression | 3 | 0 | 0 | 8 | 1 | 0 |
|
| ||||||
| Joint pain | 1 | 1 | 0 | 0 | 0 | 0 |
|
| ||||||
| Muscle pain | 2 | 0 | 0 | 1 | 0 | 0 |
|
| ||||||
| Headache | 1 | 1 | 0 | 12 | 0 | 0 |
|
| ||||||
| Sweating | 7 | 0 | 0 | 10 | 0 | 0 |
|
| ||||||
| Thromboembolism | 0 | 0 | 0 | 0 | 0 | 1 |
|
| ||||||
| Vaginal dryness | 9 | 0 | 0 | 12 | 0 | 0 |
Included are grade 2 or higher toxic effects that were reported in more than 1% of the patients in either study group. Patients may have had more than one toxic event for a given grade.
OVARIAN FAILURE
Data on both menstrual status and FSH levels at 2 years, which together composed the end point of ovarian failure, were available for 135 of the 218 patients who could be evaluated (62%). Among the 83 patients for whom data were unavailable, 14 (17%) died within the 2-year time window and 5 (6%) were lost to follow-up. The remaining 64 patients lacked data on FSH levels, with 20 of those also missing menstrual data (Fig. 1). There was no evidence that missing data changed the main findings of the study: 69 of 113 patients (61%) in the chemotherapy-alone group and 66 of 105 (63%) in the goserelin group had complete primary end-point data, and the association between treatment and stratification variables (age and chemotherapy category) did not differ significantly according to whether patients had missing data for the primary end point.
A total of 15 of 69 patients (22%) in the chemotherapy-alone group and 5 of 66 patients (8%) in the goserelin group had protocol-defined ovarian failure. In the protocol-specified stratified logistic-regression analysis, this difference was significant (odds ratio, 0.30; 95% confidence interval [CI], 0.09 to 0.97; one-sided P = 0.02, two-sided P = 0.04). The results were similar in the univariate regression analysis (odds ratio, 0.30; 95% CI, 0.10 to 0.87; one-sided P = 0.01, two-sided P = 0.03) and the multivariate regression analysis (odds ratio, 0.36; 95% CI, 0.11 to 1.14; one-sided P=0.04, two-sided P=0.08).
Secondary and sensitivity analyses related to the primary end point showed consistent results (additional details are provided in the Supplementary Appendix, available at NEJM.org). A benefit with goserelin therapy was observed when deaths were included as treatment failure (odds ratio, 0.25; 95% CI, 0.11 to 0.60; P = 0.002) and when deaths plus hysterectomy or oophorectomy were counted as treatment failure (odds ratio, 0.29; 95% CI, 0.16 to 0.75; P = 0.007). Similarly, a benefit with goserelin was observed when treatment failure was defined as amenorrhea or postmenopausal FSH levels at year 2 (odds ratio, 0.29; 95% CI, 0.12 to 0.70; P = 0.006) and when treatment failure was defined as amenorrhea or postmenopausal FSH levels at year 1 or 2 (with inclusion of year 1 data if year 2 data were missing) (odds ratio, 0.43; 95% CI, 0.22 to 0.85; P = 0.01).
OVARIAN DYSFUNCTION
Ovarian dysfunction was evaluated at years 1 and 2. Included in the analyses were patients with both menstrual-status data and at least two available laboratory values (i.e., two or more measurements of FSH, inhibin B, or estradiol levels). At year 1, data were available for 153 patients (70%). Ovarian dysfunction was present in 28 of 75 patients (37%) in the chemotherapy-alone group and in 18 of 78 patients (23%) in the goserelin group (odds ratio, 0.64; 95% CI, 0.30 to 1.37; P=0.25). At year 2, data were available for 130 patients (60%). Ovarian dysfunction was present in 22 of 67 patients (33%) in the chemotherapy-alone group and in 9 of 63 (14%) in the goserelin group (odds ratio, 0.35; 95% CI, 0.13 to 0.93; P = 0.03).
PREGNANCY OUTCOMES
Among the 218 patients who could be evaluated, 34 (16%) had at least one pregnancy: 12 of 113 (11%) in the chemotherapy-alone group and 22 of 105 (21%) in the goserelin group (odds ratio, 2.45; 95% CI, 1.09 to 5.51; P = 0.03). Women who became pregnant were younger than those who did not (median age, 32.9 years vs. 39.6 years; P<0.001) but were similar with respect to planned chemotherapy regimen. The analysis of the cumulative incidence of pregnancy at 5 years is shown in the Supplementary Appendix. A total of 18 patients in the chemotherapy-alone group (16%) and 25 in the goserelin group (24%) reported attempting pregnancy (odds ratio, 1.78; 95% CI, 0.85 to 3.72; P = 0.12). The number of reported miscarriages, elective terminations, and pregnancy complications were similar in the two groups. More patients in the goserelin group than in the chemotherapy-alone group successfully delivered 1 or more babies (P = 0.05). A total of 12 babies were born to women in the chemotherapy-alone group and 18 were born to women in the goserelin group. At the time of data submission, there were an additional 3 ongoing pregnancies reported in the chemotherapy-alone group and 5 ongoing pregnancies in the goserelin group (Table 3).
Table 3.
Pregnancy Outcomes.
| Outcome | Chemotherapy Alone (N = 113) | Chemotherapy plus Goserelin (N = 105) | Odds Ratio with Goserelin | P Value* |
|---|---|---|---|---|
| Attempted pregnancy — no. of patients (%) | 18 (16) | 25 (24) | 1.78 | 0.12 |
| Achieved pregnancy — no. of patients (%) | 12 (11) | 22 (21) | 2.45 | 0.03 |
| ≥1 delivery — no. of patients (%) | 8 (7) | 16 (15) | 2.51 | 0.05 |
| Delivery or ongoing pregnancy — no. of patients (%) | 10 (9) | 19 (18) | 2.45 | 0.04 |
| Babies born — no.† | 12 | 18 | ||
| Ongoing pregnancies at last report — no. | 3 | 5 | ||
| Adverse pregnancy event — no. of events | ||||
| Miscarriage | 5 | 4 | ||
| Elective termination | 3 | 2 | ||
| Delivery complication | 2 | 2 | ||
P values were adjusted for the stratification factors of age and type of planned chemotherapy. The cutoff date for data analysis was January 22, 2014; data up to that date are included.
This category may include more than one baby born to a woman.
DISEASE-FREE AND OVERALL SURVIVAL
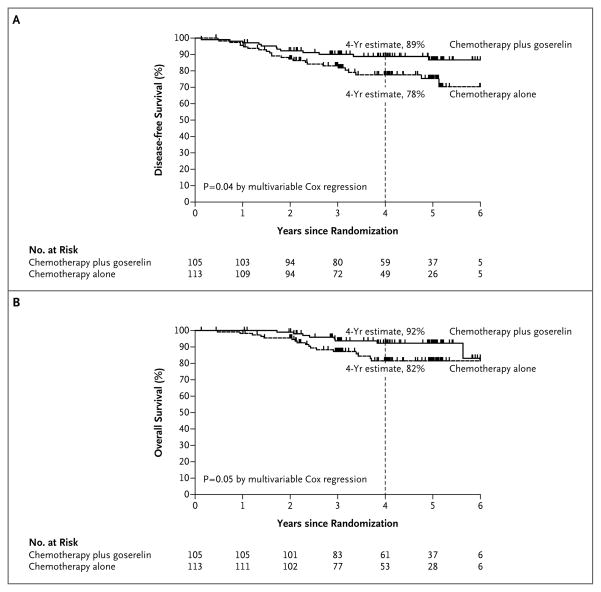
Among the 218 patients who could be evaluated, 24 in the chemotherapy-alone group and 12 in the goserelin group had a recurrence of disease or died. The 4-year Kaplan–Meier estimate of the rate of disease-free survival was 78% in the chemotherapy-alone group and 89% in the goserelin group (adjusted hazard ratio, 0.49; 95% CI, 0.24 to 0.97; P = 0.04) (Fig. 2A). A total of 17 patients in the chemotherapy-alone group and 8 in the goserelin group died. The 4-year Kaplan–Meier estimate of the rate of overall survival was 82% in the chemotherapy-alone group and 92% in the goserelin group (adjusted hazard ratio, 0.43; 95% CI, 0.18 to 1.00; P = 0.05) (Fig. 2B). Among all 257 patients who underwent randomization, the trend toward a higher rate of disease-free survival with goserelin was not significant (hazard ratio, 0.64; 95% CI, 0.35 to 1.17; P = 0.15), but the rate of overall survival was significantly higher in the goserelin group (hazard ratio, 0.45; 95% CI, 0.21 to 0.97; P = 0.04). There were three second primary cancer events in each study group: two contralateral breast cancers in each group, one melanoma in the goserelin group, and one anal cancer in the chemotherapy-alone group.
Figure 2. Disease-free and Overall Survival.
The 4-year estimates of disease-free survival (Panel A) and overall survival (Panel B) are Kaplan–Meier estimates. With respect to disease-free survival, there were 12 relapses or deaths in the chemotherapy-plus-goserelin group and 24 in the chemotherapy-alone group; with respect to overall survival, there were 8 deaths in the chemotherapy-plus-goserelin group and 17 in the chemotherapy-alone group.
DISCUSSION
The study findings confirm and extend the results of several previous randomized studies that suggested that administration of a GnRH agonist during the course of chemotherapy protects ovarian function.4,7,8 Other randomized studies that did not show ovarian protection with the use of GnRH agonists during chemotherapy were smaller and had relatively short follow-up times for the assessment of ovarian function.9–11 A recent meta-analysis of randomized trials of the use of GnRH analogues for protection of ovarian function during chemotherapy showed a 57% reduction in the risk of ovarian failure, a finding that is consistent with our results, although the definition of ovarian failure varied among the trials.12
Interpretation of the main findings is complicated by incomplete enrollment and missing data. We found no evidence of an imbalance in the primary end-point data according to study group, nor did we find evidence that the association between treatment assignment and stratification factors differed according to whether patients had primary end-point data. Therefore, although the missing data may affect the overall level of observed ovarian failure, there was no evidence that missing data influenced the relative comparison between randomized groups. Furthermore, the results of sensitivity analyses that incorporated partial information from patients with missing data were consistent with the main findings. Thus, the available data indicate a consistent benefit of goserelin in preserving ovarian function.
Current guidelines from the American Society of Clinical Oncology encourage early referral of female cancer patients who are interested in fertility preservation to reproductive specialists for consideration of embryo cryopreservation.13 Cost, timing issues, and the need for a partner, however, limit assisted reproduction options for many young women who are receiving chemotherapy. Coadministration of a GnRH agonist with chemotherapy may be a more accessible option for patients and can be used in conjunction with traditional fertility-preservation techniques. Side effects of GnRH agonists include vasomotor symptoms and loss of bone density; however, it is anticipated that long-term preservation of ovarian function may help avoid unwanted menopausal symptoms and loss of bone density even in women who are not interested in fertility preservation.
The improved rates of disease-free and overall survival in the goserelin group in this study were unexpected in this population of patients with ER-negative breast cancer. Luteinizing hormone–releasing hormone receptors are frequently present in triple-negative breast cancers, and preclinical studies have shown that the use of GnRH analogues is associated with growth inhibition, reduction in metastasis, and apoptotic cell death in xenograft models of triple-negative breast cancer.14–16 Disease risk factors were not stratified in the study, making it difficult to draw conclusions about any therapeutic effect of the GnRH agonist. However, adjustment for breast-cancer stage did not alter the disease-free or overall survival findings. The favorable disease-related outcomes confirm the safety of concurrent administration of a GnRH agonist with chemotherapy in patients with ER-negative breast cancer.
Since our study included only patients with ER-negative disease, it cannot address the safety of GnRH agonist therapy with chemotherapy in patients with ER-positive breast cancer. Concurrent use of endocrine therapy and chemotherapy fell out of favor after publication of the results of the SWOG-led INT-0100 randomized trial involving postmenopausal women with endocrine-responsive breast cancer, which suggested a disease-free survival advantage with sequential, as compared with concurrent, chemotherapy and tamoxifen.17,18 The mechanism of action of GnRH agonists, however, is different from that of tamoxifen. Multiple studies suggesting favorable effects of chemotherapy-induced amenorrhea on breast-cancer outcomes19–21 and the recently reported excellent survival results with triptorelin administered concurrently with chemotherapy in the Tamoxifen and Exemestane Trial22 indicate that ovarian suppression during chemotherapy is probably safe in women with hormone-sensitive breast cancer; however, caution is recommended in this population, for whom longer-term ovarian suppression may be desirable. Ovarian protection would also be anticipated with the use of GnRH analogues in young women with non-breast cancer who are receiving treatment with similar cyclophosphamide-based chemotherapy.
The results of a randomized study addressing the therapeutic role of GnRH agonists in ER-positive breast cancer have recently been reported23; however, any potential therapeutic role for GnRH agonists in hormone-receptor–negative breast cancer requires further investigation. Although missing data limit interpretation of the findings, the administration of a GnRH agonist with chemotherapy appears to protect against ovarian failure, reducing the risk of early menopause and improving prospects for fertility.
Supplementary Material
Acknowledgments
Supported by grants from National Cancer Institute at the National Institutes of Health (CA189974, CA180821, CA31946, CA075362, CA180820, CA27525, CA189808, CA180830, CA180801, CA189872, CA189822, CA189953, CA189858, CA180858, 189954, CA189957, CA189972) and by AstraZeneca, the Australia and New Zealand Breast Cancer Trials Group, and the Breast Cancer Institute of Australia. Dr. Phillips is an Australian National Breast Cancer Foundation Practitioner Fellow.
We thank the patients and investigators of the SWOG Cancer Research Group, the International Breast Cancer Study Group, ECOG–ACRIN Cancer Research Group, and the Alliance for Clinical Trials in Oncology.
APPENDIX
The authors’ affiliations are as follows: the Cleveland Clinic Foundation, Cleveland (H.C.F.M.); SWOG Cancer Research Group Statistical Center, Fred Hutchinson Cancer Research Center (J.M.U., W.E.B.), and Seattle Cancer Care Alliance and University of Washington (J.G.) — all in Seattle; Peter MacCallum Cancer Centre, University of Melbourne, Melbourne, VIC (K.-A.P., P.A.F.), Australia and New Zealand Breast Cancer Trials Group (ANZBCTG) (K.-A.P., P.A.F., J.F.F.), Calvary Mater Hospital, Newcastle, NSW (F.B., J.M.L., J.F.F.), and University of Sydney, Sydney (F.B.) — all in Australia; International Breast Cancer Study Group (IBCSG), Bern, Switzerland (K.-A.P., P.A.F.); National Institute of Oncology, Budapest, Hungary (E.H.); Auckland Regional Cancer and Blood Service, Auckland, New Zealand (D.P.); Fox Chase Cancer Center, Philadelphia (L.J.G.); Instituto de Enfermedades Neoplasicas (H.L.G.) and Oncosalud SAC (C.S.V.), Lima, Peru; Dana–Farber Cancer Institute (A.H.P., R.D.G.) and IBCSG Statistical Center (R.D.G.) — both in Boston; Wichita Community Clinical Oncology Program, Wichita (S.R.D.), and University of Kansas, Westwood (C.J.F.) — both in Kansas; University of Southern California Norris Cancer Center, Los Angeles (A.A.G.), the Angeles Clinic and Research Institute, Santa Monica (S.M.), and University of California at Irvine Chao Family Comprehensive Cancer Center, Orange (F.L.M) — all in California; National Cancer Institute, Division of Cancer Prevention, Bethesda, MD (L.M.); M.D. Anderson Cancer Center, Houston (G.N.H.); and Loyola University Medical Center, Cardinal Bernardin Cancer Center, Maywood, IL (K.S.A.).
Footnotes
The authors’ affiliations are listed in the Appendix.
The Prevention of Early Menopause Study (POEMS)/S0230 trial was performed in collaboration by investigators from the SWOG Cancer Research Group, the International Breast Cancer Study Group, the ECOG–ACRIN Cancer Research Group, and the Alliance for Clinical Trials in Oncology.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32:1151–6. doi: 10.1200/JCO.2013.52.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senkus E, Gomez H, Dirix L, et al. Attitudes of young patients with breast cancer toward fertility loss related to adjuvant systemic therapies. EORTC study 10002 BIG 3–98. Psychooncology. 2014;23:173–82. doi: 10.1002/pon.3384. [DOI] [PubMed] [Google Scholar]
- 3.Turner NH, Partridge A, Sanna G, Di Leo A, Biganzoli L. Utility of gonadotropin-releasing hormone agonists for fertility preservation in young breast cancer patients: the benefit remains uncertain. Ann Oncol. 2013;24:2224–35. doi: 10.1093/annonc/mdt196. [DOI] [PubMed] [Google Scholar]
- 4.Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306:269–76. doi: 10.1001/jama.2011.991. [DOI] [PubMed] [Google Scholar]
- 5.Oktay K, Sönmezer M, Oktem O, Fox K, Emons G, Bang H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12:1055–66. doi: 10.1634/theoncologist.12-9-1055. [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v3.0. ( http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf)
- 7.Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91:694–7. doi: 10.1016/j.fertnstert.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 8.Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat. 2009;117:561–7. doi: 10.1007/s10549-009-0313-5. [DOI] [PubMed] [Google Scholar]
- 9.Gerber B, von Minckwitz G, Stehle H, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011;29:2334–41. doi: 10.1200/JCO.2010.32.5704. [DOI] [PubMed] [Google Scholar]
- 10.Munster PN, Moore AP, Ismail-Khan R, et al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:533–8. doi: 10.1200/JCO.2011.34.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elgindy EA, El-Haieg DO, Khorshid OM, et al. Gonadatrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstet Gynecol. 2013;121:78–86. doi: 10.1097/aog.0b013e31827374e2. [DOI] [PubMed] [Google Scholar]
- 12.Del Mastro L, Ceppi M, Poggio F, et al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: systematic review and meta-analysis of randomized trials. Cancer Treat Rev. 2014;40:675–83. doi: 10.1016/j.ctrv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 14.Buchholz S, Seitz S, Schally AV, et al. Triple-negative breast cancers express receptors for luteinizing hormone-releasing hormone (LHRH) and respond to LHRH antagonist cetrorelix with growth inhibition. Int J Oncol. 2009;35:789–96. doi: 10.3892/ijo_00000391. [DOI] [PubMed] [Google Scholar]
- 15.Gründker C, Föst C, Fister S, Nolte N, Günthert AR, Emons G. Gonadotropin-releasing hormone type II antagonist induces apoptosis in MCF-7 and triple-negative MDA-MB-231 human breast cancer cells in vitro and in vivo. Breast Cancer Res. 2010;12:R49. doi: 10.1186/bcr2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schubert A, Hawighorst T, Emons G, Gründker C. Agonists and antagonists of GnRH-I and -II reduce metastasis formation by triple-negative human breast cancer cells in vivo. Breast Cancer Res Treat. 2011;130:783–90. doi: 10.1007/s10549-011-1358-9. [DOI] [PubMed] [Google Scholar]
- 17.Albain KS, Green SJ, Ravdin PM, et al. Adjuvant chemohormonal therapy for primary breast cancer should be sequential instead of concurrent: initial results from Intergroup trial 0100 (SWOG-8814); Presented in part at the ASCO annual meeting; Orlando, FL. May 18–21, 2002; abstract. [Google Scholar]
- 18.Albain KS, Barlow WE, Ravdin PM, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:2055–63. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagani O, O’Neill A, Castiglione M, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer. 1998;34:632–40. doi: 10.1016/s0959-8049(97)10036-3. [DOI] [PubMed] [Google Scholar]
- 20.Poikonen P, Saarto T, Elomaa I, Joensuu H, Blomqvist C. Prognostic effect of amenorrhoea and elevated serum gonadotropin levels induced by adjuvant chemotherapy in premenopausal node-positive breast cancer patients. Eur J Cancer. 2000;36:43–8. doi: 10.1016/s0959-8049(99)00225-7. [DOI] [PubMed] [Google Scholar]
- 21.Swain SM, Jeong JH, Geyer CE, Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–65. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–18. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in pre-menopausal breast cancer. N Engl J Med. 2015;372:436–46. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.