Abstract
Numerous in vitro and in vivo studies have shown that the endothelial cells of the microvasculature of the lung and kidney are damaged by exposure to ionizing radiation, and this sustained endothelial cell injury is involved in the early and late radiation effects observed in these tissues. It is well accepted that ionizing radiation causes the generation of reactive oxygen species during exposure that results in damage to DNA and cellular organelles. It is more controversial, however, whether additional biochemical events or long-lived radicals occur and persist postirradiation that amplify and initiate new forms of cellular damage. Two families of Eukarion (EUK) compounds have been synthesized that possess superoxide dismutase (SOD), catalase and peroxidase activities. The Mn porphyrins are available orally whereas the salen Mn complexes are administered by injection. In the present study we have examined the ability of these SOD/catalase mimetics to prevent apoptosis of endothelial cells when administered 1 h postirradiation (mitigation). A range of salen Mn complex (EUK-189 and EUK-207) and Mn porphyrins (EUK-418, -423, -425, -450, -451, -452, -453) were used to treat endothelial cells 1 h after the cells received 2–20 Gy ionizing radiation in vitro. Two lead compounds, EUK-207 at a dose of 30 μM and EUK-451 at a dose of 10 μM, exhibited low toxicity and mitigated radiation-induced apoptosis. Future animal studies will test whether these compounds protect when administered after radiation exposure as would be done after a radiological accident or a terrorism event.
INTRODUCTION
All forms of ionizing radiation trigger immediate biochemical events in living cells via photoelectric, Compton and Auger effects (1). Because tissues and cells are composed of ~80% water, much of the radiation damage from exposure to low-linear energy transfer radiation (X rays, γ rays and fast electrons) is due to radiolysis of water leading to the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). These radical species damage cellular components and can cause irreversible genetic defects leading to carcinogenesis, necrosis or apoptosis (2–4).
Apoptosis was defined by Kerr in 1972 (2) as having distinct morphological and biochemical attributes. Morphological changes include nuclear condensation, DNA cleavage into internucleosomal fragments, and the condensation of the DNA into apoptotic bodies without loss of plasma membrane integrity. Biochemical events include the expression of initiator (caspase 8, 9) and executioner (caspase 3, 7) caspases, cytochrome c release from mitochondria, appearance of phosphatidylserine on the external plasma membrane, and poly (ADP-ribose) polymerase (PARP) cleavage. In vivo, condensed apoptotic nuclei are recognized and apoptotic cells are removed by phagocytes, with minimal inflammation (4–6). In contrast, necrosis is initiated by cellular catastrophe after a toxic insult or irreversible physical damage to the cell. Morphological indications of necrosis are the appearance of cytoplasmic vacuoles and cellular leakage with a resulting inflammatory reaction in the vicinity of the dying cell. Cells that die from necrosis can exhibit hypertrophy and changes in nuclear morphology but not the organized chromatin condensation and DNA fragmentation characteristic of apoptosis (4, 5).
Agents that scavenge ROS, such as superoxide dismutase (SOD), glutathione peroxidase, catalase and some growth factors (e.g. fibroblast growth factor), can reduce or eliminate radiation-induced apoptosis and necrosis when administered prior to irradiation (7–11) or within 8 min after radiation exposure (12). If radioprotective agents selectively interfere with normal tissue damage while preserving malignant cell killing, these agents can increase effectiveness and dose tolerance in patients receiving radiation therapy.
The problems with most available radioprotectors and ROS scavengers are their toxicity, short half-life and low bioavailability. A family of small SOD/catalase mimetics [Eukarion (EUK) compounds], first described in 1993 (13), have been studied extensively with respect to their chemistry, their catalytic and cytoprotective properties, and their ability to protect normal tissue in vivo in models of diseases with an oxidative stress component. The injectable EUK compounds, the salen manganese (Mn) complexes, are synthetic low-molecular-weight compounds that catalytically mimic SOD, catalase and peroxidase, and they can neutralize ROS superoxide and hydrogen peroxide (14). These compounds also neutralize, via peroxidase-like mechanisms, reactive nitrogen species (RNS), including peroxynitrite (15). EUK compounds have demonstrated efficacy when given by injection or infusion in an oxidative stress injury rat stroke model (16), a rat seizure-induced hippocampal injury model (17), a mouse amyotrophic lateral sclerosis (ALS) model (18), a mouse Parkinson’s disease model (19, 20), a neurodegenerative mouse model in mice lacking mitochondrial SOD (SOD2−/−) (21), and a mouse model of human prion disease (22). These compounds also protected peripheral organs, including the lung (23), liver (24) and kidney (25), from ischemia-reperfusion, toxemia and other forms of oxidative stress. Recent studies demonstrated the efficacy of the injectable salen Mn complexes as radioprotectors when given within minutes of irradiation and every day thereafter in a hamster model of radiation-induced mucositis (Dr. Steven Sonis, personal communication). Another class of Mn-based SOD mimetics has shown radioprotection in another model of radiation-induced mucositis (26). A SOD mimetic, AEOL10113, given 15 min prior to 28 Gy and for 5 days after irradiation, reduced breathing frequency and fibrosis in rats in a shielded hemithoracic model of lung injury (27).
Fewer agents have been tested for their efficacy when administered postirradiation as mitigating agents, largely due to the dogma that events after radiation exposure were largely inconsequential to damage exhibited in tissues and cells. There is some evidence, however, that ROS and RNS do play a role in late radiation effects that manifest hours to days or even years after radiation exposure (28, 29); this could represent an additional opportunity to interfere with radiation-induced damage. A mitigating agent that spares cells or increases survival when administered an hour to days after the radiation exposure (12) could be an important countermeasure after nuclear plant accidents or radiological attacks.
Recent studies conducted in vitro and in vivo have demonstrated that several agents can increase survival of cells or animals when administered after exposure to lethal doses of radiation (30–32). For example, lethally irradiated embryonic cells treated up to 1 h postirradiation with a nitroxide, hemigramicidin-TEMPO, had significantly improved clonogenic survival (30). A fungus, Hirsutella sinensis (CorImmune), administered 2 h after lethal total-body irradiation (TBI) of BALB/c mice resulted in survival of more than half the mice, whereas all of the vehicle-treated mice died (31). After treatment of mice with 35 Gy, a subcutaneous injection of an adenovirus vector overexpressing manganese SOD significantly mitigated both acute and chronic radiation-induced skin damage (32). Another class of Mn-based SOD mimetic reduced micronucleus formation when given by intraperiteneal injection hours after irradiation in a rat model of lung pneumonitis but did not improve survival of rats (33). These and other such agents either have not been characterized or would be difficult to administer in a non-hospital setting, but these studies do provide proof of principle that postirradiation biochemical events are important to lethality and that opportunities exist to mitigate the effects of radiation exposures. In another study, an Mn porphyrin SOD mimic was administered subcutaneously 2 h after 28 Gy shielded hemithoracic irradiation in a rat lung model. The drug, which was given every day for 14 days, delayed fibrosis and improved breathing frequency (34). In contrast, the SOD mimetic AEOL10150 produced no benefit when administered 1 day before and for 1 week after hemithorax-shielded irradiation of the rat lung (28 Gy). However, rats that received the same SOD mimetic 1 day postirradiation and for 10 weeks after irradiation had significantly reduced fibrosis of the lungs and improved breathing function compared to either the irradiated or the radioprotected animals (35).
Other agents that are in clinical use for other applications and have a standard of practice for administration have been shown to have activity as mitigating agents. Prevastatin reduced intestinal complications in rats after radiation exposure (36), and captopril administered after TBI in rats reduced the severity of radiation-induced glomerulonephritis in a bone marrow transplantation model (37). It is not known how these drugs mitigate the damaging effects of radiation or how these drugs will affect the multiorgan responses of mammals to radiation exposures.
In the present study we examined the use of EUK compounds as mitigating agents to prevent radiation-induced apoptosis of endothelial cells, using doses that could be achieved in serum after either oral (EUK-400 compounds) or subcutaneous (both classes of compounds) administration.
MATERIALS AND METHODS
Reagents and Biochemicals
Fetal calf serum (FCS) was obtained from JRH Biosciences (Lenexa, KS). Glutamine and penicillin/streptomycin were from Irvine Scientific (Santa Ana, CA). Fungisone was purchased from Fisher Scientific (Pittsburgh, PA). The LDH assay kit and other chemicals were obtained from Sigma Chemicals (St. Louis, MO). Salen Mn EUK-189 and EUK-207 compounds were custom synthesized by Dalton Chemical Laboratories (Toronto), and EUK-400 series compounds were custom synthesized by Frontier Scientific (Logan, UT).
Cell Culture
Capillary endothelial cells were provided by Ms. Catherine Butterfield and the late Dr. Judah Folkman at Children’s Hospital, Boston, or were purchased from BioWittaker/Clonetics. These cells exhibit characteristics of normal nontransformed endothelial cells, including the ability to take up acetylated LDL (DiL-AcLDL) using the method of Voyta (38), von Willebrand-related antigen positivity after staining when using ab6994 from AbCAM, have a finite life span, and have the ability to form a cobblestone pattern at confluence. Stock cultures of endothelial cells obtained from Children’s Hospital were maintained in DMEM (Sigma, D6046) supplemented with 10% FCS (JRH Biosciences) and 3 ng/ml human recombinant bFGF (Sigma) as described previously (39, 40). Human lung microvascular endothelial cells from Clonetics were maintained according to the vendor’s instructions in EGM2MV with growth factor supplementation and 5% FCS. These cells tested positive for PECAM and negative for smooth muscle actin by the manufacturer. The cell lines exhibited identical kinetics and amount of apoptosis in response to 2–20 Gy ionizing radiation.
Radiation Treatments
For radiation experiments, endothelial cells were treated as reported previously (9, 39–41). Briefly, endothelial cells were plated into eight-chamber Labtek slides at 25,000 cells/well and allowed to attach for 18–24 h; then the medium was replaced with medium containing 10% FCS without growth factors. Endothelial cells were grown for 2 days and then either sham-exposed or X-irradiated with 2–50 Gy. At least 30 min and up to 1.5 h after radiation exposure, endothelial cells were treated with vehicle or with salen manganese (EUK-189, EUK-207) or porphyrin (EUK-418, -423, -425, -450, -451, -452, -453) EUK compounds. Six hours later, the time at which the first maximal peak of apoptosis occurs in response to radiation in these cells, endothelial cells were fixed, stained and analyzed as described previously (9, 40).
X-Ray Dosimetry
Dosimetry was performed prior to every experiment as described previously (9, 39, 40) using a calibrated Victoreen model 660-1 exposure meter with a model 660-4A probe (Inovision Radiation Measurements, Cleveland, OH) and Gemini 320 kV X-ray Generator (Picker Industrial Corp., Cleveland, OH). Because the Gemini 320 kV X-ray Generator (Picker Industrial Corp. Cleveland, OH, 250 kV potential and 12 mA current) produced exposure rates that exceeded the upper limit of the 660-1 meter, exposure rates were estimated by extrapolation of exposure rates measured at lower currents that were corrected for air pressure and temperature and converted to an absorbed dose rate using the conversion factor 9.196 × 10−3 Gy/R. The absorbed dose rate was approximately 1.2 Gy/min.
Quantification of Apoptosis using Nuclear Morphology
Cells were fixed by adding methanol to the medium (in a 1:1 volumetric equivalent). After 5 min incubation at room temperature, the medium/methanol was removed and replaced with 100% methanol. Slides with fixed cells were stored at 4°C without removing the methanol until staining with 4,6,diamidino-2-phenylindole dihydrochloride (DAPI). All subsequent staining procedures were performed in the dark. DAPI solution was prepared in methanol to a final concentration of 0.1 μg/ml. Fixed cells were washed once with DAPI solution, then incubated in DAPI solution at 37°C for 30 min. After staining, the solution was aspirated and the chambers air-dried for 5 min; then the Labtek slides were covered with cover slips using a solution of glycerol:PBS (2:1). DNA was visualized using a Nikon epifluorescence microscope equipped with a Nikon digital camera and a computer with image analysis capability. In microscope studies performed on coded slides in a blinded study, no fewer than five random fields per Labtek well were counted, scoring total nuclei (total cell number/set field area) and apoptotic and mitotic nuclei/cell number. A cell was considered to be apoptotic if at least three distinct clusters of fragmented DNA of different sizes were observed. For each condition, no less than 500 cells were counted in triplicate or quadruplicate chambers. Apoptosis was expressed as the percentage of the total cell number. The number of fields required per well to count 500 cells was also recorded.
Quantification of Apoptosis using Caspase Assays
The activities of executioner/effector caspases 3 and 7 were determined using luminescent Caspase-Glo 3/7 assays (Promega, Madison, WI) and measuring luminescence on a Synergy HT Multi-Detection Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT). For caspase assays, the endothelial cells were plated in full medium into 96-well plates at a seeding density of 25,000 cells/well. The cells were allowed to attach for 18–24 h; then fresh medium containing 10% FCS without growth factors was added to each well. Endothelial cells were grown for 2 days and then sham-exposed or irradiated. One hour after irradiation, the cells were treated with vehicle alone or various doses of EUK compounds. In a pilot study, plates were processed for caspase 3/7 determination 2, 3, 4 and 5 h after 10 Gy irradiation using different incubation times (1–3 h) and various luminescence sensitivities (25, 100, 150 and 175 wavelength units). We determined that caspase 3/7 detection was optimal 4 h postirradiation using a 1-h incubation time and measuring the luminescence sensitivity at 175 wavelength units.
Measurement of Necrosis using Lactate Dehydrogenase Quantitative Assays
Death of cells by necrosis was assessed by the release and detection of lactate dehydrogenase (LDH) into the medium after irradiation. LDH was measured using In Vitro Toxicology Assay Kit Lactate Dehydrogenase Based from Sigma. For LDH comparisons, medium from Labtek wells (130 μl) was taken 6 h after X irradiation. The samples were analyzed in triplicate for each experimental condition and compared to medium from sham-treated cultures (basal or control lysis) and cells were incubated in lysis buffer to lyse 100% of the cells. Experimental lysis was calculated according to the following formulas:
Treatment of Endothelial Cells with EUK Compounds
Within 1–2 h after exposure to X rays or sham treatment, endothelial cells received fresh medium with or without EUK compounds at concentrations ranging from 1 to 100 μM.
Statistical Analysis
All experiments were repeated at least three times under the same conditions, and the data were pooled. Statistical analyses were carried out using Student’s t test. A P value of less than 0.05 was accepted as statistically significant.
RESULTS
Chemical Properties and Structures of the EUK SOD Mimetics
We and others have shown that capillary endothelial cells respond to X-ray exposures by undergoing apoptosis with maximal peaks at 6–8 and 18–22 h postirradiation in vitro (6–45% of cells undergo apoptosis depending on the dose of radiation and cell growth conditions) (9, 11, 40) and, in many tissues, including the lungs and kidneys, in vivo (42–47). The endothelium is thought to be the focal point of critical cellular damage in the body after radiation exposure and thus is an excellent model to use to screen and identify radioprotectors and mitigating agents.
In the present study we evaluated the mitigating properties of two groups of novel EUK compounds: the salen Mn complexes EUK-189 and EUK-207 (Fig. 1A) and the Mn porphyrins, EUK-400 series compounds (Fig. 1B). These compounds have been well characterized chemically and have been shown to have SOD, catalase and peroxidase activities as well as cytoprotective activity in various other models for oxidative stress (48–50). These small synthetic SOD mimetics have several advantages over the native enzymes both for use in experimental systems and for rapid translation to clinical studies. They are likely to be poor immunogens, lacking species-specific domains, and they are readily absorbed into cells because they are lipophilic to varying degrees. We selected them for testing due to their catalytic activities and their stability in biological fluids (13, 14, 48–50). The original salen Mn complexes EUK-8, and EUK-134 and ELK-189, have cytoprotective activity in vitro and in vivo in a variety of experimental models (13, 20, 21, 33, 51). EUK-207 is one of the newer cyclized analogs, designed for greater stability, and also has demonstrated efficacy in vivo (52) and in culture systems (53). The EUK-400 series compounds have been synthesized more recently (48–50); they were developed as orally bioavailable agents with desirable features of the higher-molecular-weight Mn porphyrins with ROS and RNS scavenging and cytoprotective properties (54, 55).
FIG. 1.
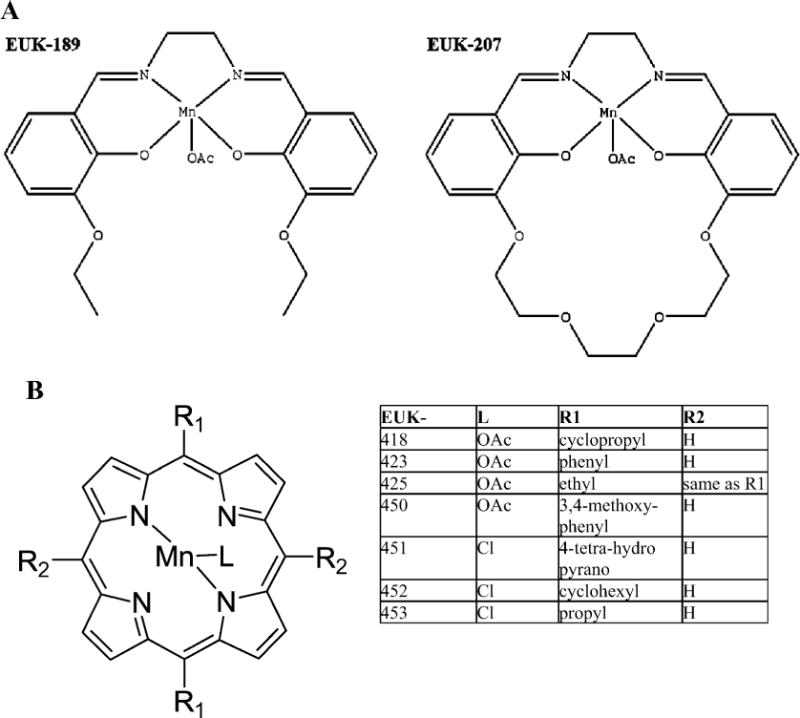
Panel A: The chemical structures of the salen Mn complexes EUK-189 and EUK-207. Panel B: The chemical structures of the Mn porphyrins compounds, the EUK-400 series.
Radiation-Induced Apoptosis of Capillary Endothelial Cells
We first determined the level of endothelial cell apoptosis at 6 h in response to X-ray doses ranging from 2 to 50 Gy using low-passage capillary endothelial cells (passages 5–8) (Fig. 2). A dose of 2 Gy caused a significant increase over basal apoptosis (0 Gy), and a dose response was apparent through a dose of 50 Gy.
FIG. 2.
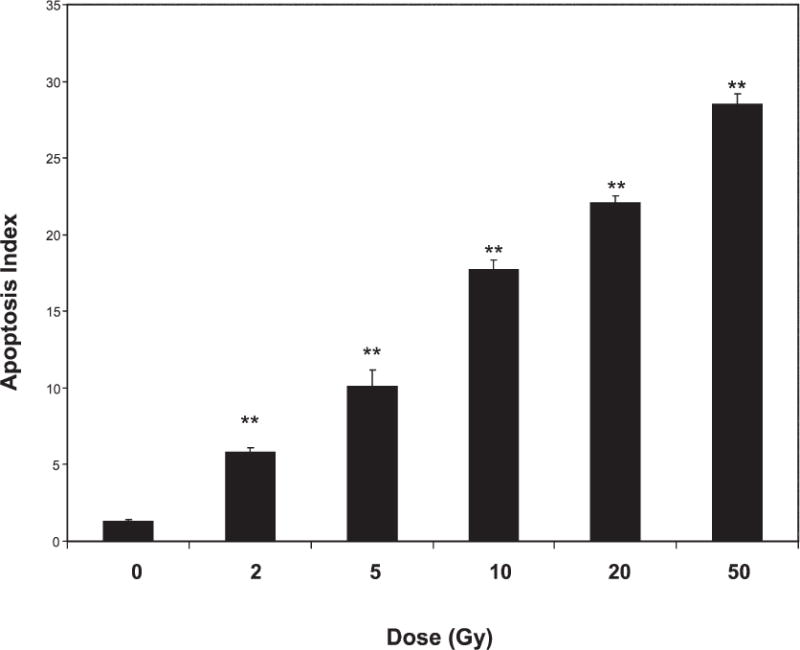
Apoptosis of endothelial cells after X irradiation. Cells (passages 5–8) were sham-treated (0 Gy) or exposed to various doses of X rays; 6 h later, the number of cells undergoing apoptosis was determined. Differences between sham-treated and irradiated cells were compared using Student’s t test (**P < 0.001). Shown are pooled data from treatments performed in duplicate per experiment in three separate experiments. Error bars are SE.
Screen for Toxicity and Effectiveness to Mitigate Radiation-Induced Apoptosis
The objective of the screen shown in Fig. 3 was to select lead compounds with the highest efficacy as mitigating agents and with the lowest intrinsic toxicity. We measured apoptosis in unirradiated cultures over a range of doses (Fig. 3). EUK-189 and EUK-207 did not increase apoptosis above baseline at doses up to and including 30 μM. EUK-451, -418 and -423 did not increase apoptosis above baseline at doses of 10 μM and lower but did increase apoptosis at doses of 30 μM and higher. EUK-425, -450, -453 and -452 produced a statistically significant increases in apoptosis of endothelial cells at doses of 10 μM and higher and were eliminated as candidates for use as mitigating agents.
FIG. 3.
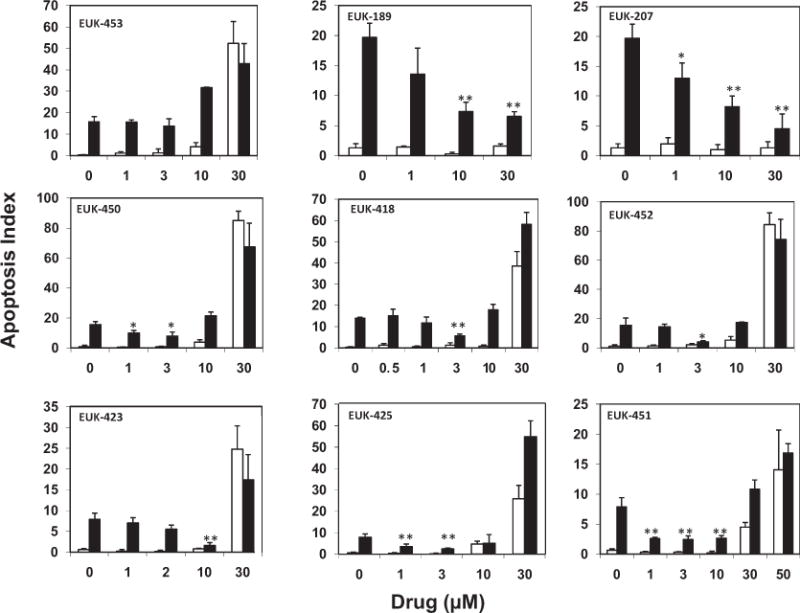
Apoptosis of endothelial cells after sham (open bars) or 20 Gy irradiation (black bars) with or without various doses of EUK compounds administered 1 h postirradiation. Apoptosis indices were determined using cells at passage numbers 5–8 (top row), 9–11 (middle row) or 12–15 (bottom row). Statistically significant differences are shown for matched irradiated groups with and without drug. (Student’s t test, *P < 0.05; **P < 0.001). Bars are pooled data from treatments performed in duplicate per experiment. Error bars are SE.
We also examined the ability of these compounds to mitigate the effects of ionizing radiation (Fig. 3) by measuring the apoptosis index of irradiated cultures for cells with or without EUK compounds added 1 h after X irradiation with 20 Gy. EUK-207 and -451 significantly reduced radiation-induced apoptosis, even when used at the lowest dose of 1 μM. EUK-425 and EUK-418 also reduced apoptosis at a dose of 3 μM, but during the course of these studies we noticed that the field number required to count 500 cells in each well had increased in both the irradiated and the sham cultures. Cell loss from the surface of the dishes during the first hour of incubation, when the drug is present, appeared to be indicative of necrosis in these endothelial cell cultures. To confirm this, we repeated the nonirradiated cell studies with a wider range of concentrations (1 to 100 μM) to determine the toxicity that resulted from necrosis instead of apoptosis using the LDH cytotoxicity assay (Fig. 4).
FIG. 4.
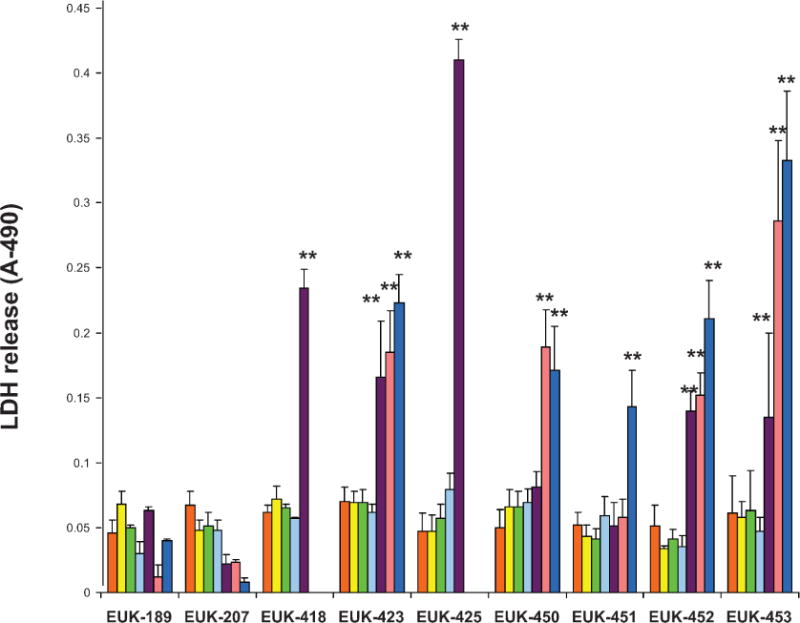
Quantification of LDH released from endothelial cells (passages 5–8) treated with various doses of EUK compounds: 1 μM (yellow), 3 μM (green), 10 μM (light blue), 30 μM (purple), 50 μM (pink) and 100 μM (dark blue). Differences were compared between groups without drug (orange bars) compared to drug treatment using Student’s t tests (*P < 0.05; **P < 0.001). Error bars are SE.
Drug Toxicity Leading to Necrosis Detected by Release of LDH
Several EUK compounds caused a statistically significant increase in LDH release from cells treated with 30 μM or more compared to that in the absence of the drug (Fig. 4). Of the EUK compounds that can be administered intravenously, neither EUK-189 or EUK-207 was toxic, and EUK-207 had lower than basal levels of LDH release at doses of 100 μM. Of the EUK compounds designed for oral administration, the 400 series compounds, EUK-451 showed no increase in LDH release until a dose of 100 μM was used.
Based on these and the previous results, we selected EUK-207 as the lead injectable EUK compound and EUK-451 as the lead orally available EUK compound. In the large screening experiments shown in Fig. 3, the endothelial cells exhibited a modest radioresistance at high passage numbers. Thus, in all subsequent studies, low-passages endothelial cells (passages 5–8) were used to maintain robust radiation-induced apoptosis.
Mitigation of Low- and High-Dose Radiation-Induced Apoptosis by EUK-451 and EUK-207 Measured by Detection of Apoptotic Nuclear Morphology
Using doses of EUK-207 and EUK-451 that were well tolerated by endothelial cells, we tested their ability to mitigate radiation-induced apoptosis. Low-passage endothelial cells exhibited a significantly increased the rate of apoptosis, detected using nuclear morphology, that increased with dose after exposure to 2–10 Gy (Fig. 5A). However, when EUK-451 (10 μM) or EUK-207 (30 μM) was added to the endothelial cells 1 h after irradiation, the radiation-induced apoptosis was eliminated after 2 Gy and reduced by approximately half after 5 or 10 Gy (Fig. 5A). There was no change in field number in any of these studies, and LDH release above the levels in sham cultures was not detected (data not shown). These results demonstrate that necrosis is not occurring in cultures of endothelial cells exposed to 2–10 Gy or to the doses of EUK compounds used here.
FIG. 5.
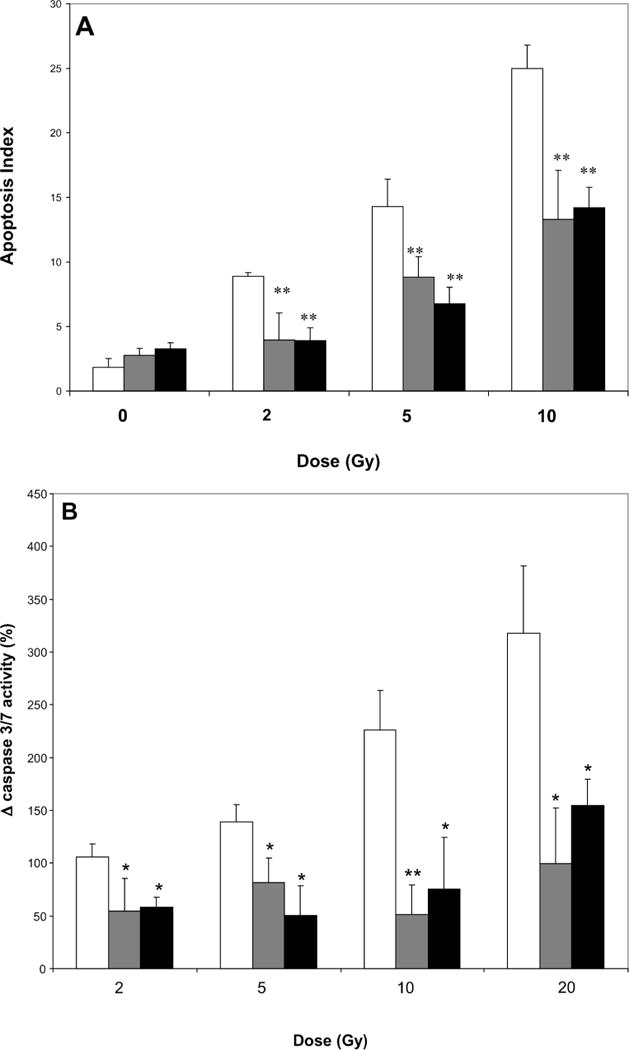
Panel A: Effects of EUK compounds in preventing apoptosis of endothelial cells (passages 5–8) when applied 1 h after cells were exposed to radiation as determined by enumerating apoptotic bodies. Gray bars: 10 μM EUK-451; black bars: 30 μM EUK-207; open bars: no drug; **P < 0.001. Panel B: Effects of EUK 451 (gray bars) and EUK 271 (black bars) when applied to endothelial cells (passages 5–8) 1 h after cells were exposed to radiation (no drug, open bars) as detected by quantification of caspase 3/7. Bars represent changes in expression of executioner caspases in irradiated cultures from levels in sham cultures. *P < 0.05; **P < 0.001. Error bars are SE.
Mitigation of Low- and High-Dose Radiation-Induced Caspase Production by EUK-451 and EUK-207 Measured by Detection of Caspase
Media and cell lysates from sham-treated and irradiated endothelial cells with and without EUK compounds administered 1 h after irradiation were collected and quantified using a chemiluminescence assay to measure caspase 3/7 production (Fig. 5B). Caspase 3 and 7 are both downstream effector caspases that act on protein substrates at the irreversible stage of apoptosis and are thus definitive indicators of apoptosis in the culture. We found a statistically significant increase in caspase 3/7 production that occurred in a dose–response fashion after exposure of endothelial cells to 2, 5, 10 and 20 Gy ionizing radiation. However, when EUK-451 (10 μM) or EUK-207 (30 μM) was added 1 h after irradiation, this radiation-induced production of caspase was reduced by half after doses of 2 and 5 Gy and was reduced to one-third after doses of 10 or 20 Gy (Fig. 5B). These results demonstrate the potent mitigating activity of EUK-451 and EUK-207 in preventing apoptosis of endothelial cells after irradiation.
DISCUSSION
Our previous studies showed that capillary endothelial cells react to ionizing radiation and menadione redox cycling-induced oxidative stress by undergoing apoptosis, with peaks of apoptotic bodies at 6–8 h and 18–22 h after oxidative stress treatments (9, 35, 36). The present study demonstrated that after irradiation with 2–50 Gy, endothelial cells exhibited a statistically significant dose-dependent increase in apoptosis at 6 h from 6% (2 Gy) to 28% (50 Gy). These results were confirmed by quantification of caspase 3/7 in parallel. Caspase 3/7 increased in a statistically significant dose-dependent manner after irradiation, with the peak activity occurring 4 h after irradiation. The kinetics of the caspase 3/7 rise preceded detectable internucleosomal DNA fragmentation because changes in nuclear morphology require cleavage of poly(ADP-ribose) polymerase (PARP) and DNA fragmentation, which are downstream events of caspase 3/7 effector functions (56).
It is well established that apoptosis after irradiation is due in part to the immediate production of reactive oxygen and nitrogen species (ROS/RNS) in irradiated cells and tissues (56). ROS/ RNS generation occurs within seconds of starting irradiation and persists for 2–5 min postirradiation (58). Thus early radiation responses such as immediate DNA damage occur during and within minutes after radiation exposure. DNA double-strand breaks have long been thought to be the most important form of cellular damage, with about 40 double-strand breaks per 1 Gy occurring in a cell during irradiation (59). What is more controversial is whether additional oxidative stress occurs after irradiation via secondary biochemical events that occur hours to days after irradiation and to what extent these ROS/RNS-generating reactions contribute to early and late forms of radiation damage to cells and to tissues.
One form of late radiation effects in vivo is the sterilization of cells that do not immediately die by apoptosis, which leaves these cells unable to repopulate during subsequent normal tissue turnover. This has been shown to occur in endothelium. Rats that received radiation to the brain showed a steady decline in the number of endothelial cells over a 65-week period unrelated to the initial apoptosis (60). The latent losses of endothelial cells via apoptosis correlated with normal endothelial cell repopulation in brains of nonirradiated rats and with dementia in irradiated rats.
There is also a class of late effects termed radiation-induced cellular dysfunction (or residual dysfunction) (60). Residual dysfunction refers to a dormant form of injury in irradiated cells that is unrelated to the cell’s inability to proliferate in which the cell appears morphologically normal but harbors a residual functional defect. A well-documented form of residual dysfunction after radiation exposure is the inability of the irradiated endothelium to regulate thrombogenic processes. Irradiated endothelial cells in many key dose-limiting tissues exhibit altered production of molecules involved in inflammation and coagulation months to years after radiation therapy (e.g. platelet activating factor, thrombomodulin, and von Willebrand factor) (61, 62). Whereas the underlying cause of acute gastrointestinal radiation syndrome is thought to be due to the immediate loss of endothelial cells via apoptosis, chronic radiation enteropathy is thought to be due to a completely different form of radiation-induced dysfunction when chronic changes in the remaining endothelial cell surface properties occur. This pro-inflammatory state leads to sclerosis and fibrosis of the capillaries, intestinal epithelial cell hypoxia and finally necrosis. Other forms of cellular dysfunction play a role in radiation-induced wound healing defects, lung pneumonitis, glomerulonephritis and oral mucositis. If these and other radiation effects are due only to events that occur during and minutes after radiation exposure, then early and late forms of damage would be inevitable and unavoidable, but if there are critical biochemical events that occur hours to days after exposure to ionizing radiation, then we have an opportunity for intervention and mitigation.
It has been estimated that 66% of damage caused by radiation is due to free radicals generated during radiation and that 33% of the damage results from persistent postirradiation oxidative stress (63). Given the known bioactivity of the SOD mimetics and the postirradiation administration of the drugs, we think it is highly unlikely that these compounds reduce direct radiation-induced DNA damage but that instead it is likely that they reduce the activity of long-lived radicals and scavenge new radicals generated by secondary biochemical events hours to days after irradiation. In recent years, several agents have shown promise as mitigating agents by preventing different forms of radiation damage, even when administered hours to days after exposure. For example, an agonist of the toll receptor, CBLB502, increased survival of primates but not mice when given 48 h after a lethal dose of ionizing radiation (64); a free radical scavenger, hesperidin, prevented liver necrosis in rats even though it was given days after γ irradiation (65); a SOD expression recombinant adenovirus, or a statin, injected under the irradiated skin of mice mitigated the appearance and severity of skin lesions (63, 66); and an angiotensin-converting enzyme inhibitor mitigated renal failure in a clinical trial of patients receiving total-body irradiation and then the ACE inhibitor in conjunction with bone marrow transplantation (37). These studies demonstrate the significant positive effects that compounds such as anti-inflammatory agents and free radical scavengers can have on experimental end points and clinical outcomes when administered after irradiation.
In the current study, we showed that several different forms of synthetic SOD/catalase mimetics are effective as mitigators of radiation-induced apoptosis of endothelial cells. In recent years, evidence has accumulated that after radiation exposure long-lived radicals persist (67–69). Watanabe et al. (68) showed that 20 min after X irradiation, short-lived radicals were no longer detected; however, a distinct class of radicals with much longer half-lives persisted and had transforming potential. Drugs that elevate intrinsic expression of SOD, altering the redox state of the cells 12–24 h after irradiation, have been shown to significantly reduce the malignant transformation and metastatic potential of irradiated tumor cells (69). One reason these long-lived radicals postirradiation were underappreciated is that they are not detected by standard methods such as the dichloroflourescern method (68). These findings support a role for long-lived radicals postirradiation in the death of normal cells and in malignant progression (66–69). Of the orally available 400 series EUK compounds, EUK-451 was the most active mitigator with the lowest cytotoxicity for endothelial cells; it also had the highest catalase activity of the EUK Mn porphyrins (50). The salen Mn complexes, EUK-189 and EUK-207, also mitigated radiation-induced endothelial cell apoptosis. The salen Mn complex with the highest potency and lowest intrinsic cytotoxicity, EUK-207, reduced radiation-induced apoptosis by 75% when administered 1 h or more after irradiation. Both agents have comparable catalytic activities, but the increased stability of EUK-207 relative to EUK-189 might contribute to its greater potency. Higher doses of the salen Mn complexes were required relative to the Mn porphyrins to achieve comparable radiation mitigation despite the fact that these salen Mn complexes have higher SOD/catalase/peroxidase activity. One explanation is that the different SOD mimetics may have a different site(s) of action. Recent studies have shown that the EUK compounds have a wide range of partition coefficients (50), which concentrates them in different subcellular compartments. In addition, the EUK-400 compounds, like other metalloporphyrins, are resistant to intracellular degradation. Another possibility is that the forms of long-lived radicals favor distinct scavenging properties. In our current study, more important than their potential differences in potency is the conclusion that two completely different chemical classes of Mn-ligand complexes, both with SOD/catalase activity, both show significant mitigating activity.
In summary, our work demonstrates that both classes of SOD/catalase/peroxidase mimetics, salen Mn complexes and Mn porphyrins, are mitigating agents that reduce or eliminate at least one form of radiation injury. We identified a potential lead analog in each class, EUK-207 and EUK-451, respectively. As the forms and longevity of radiation-induced redox reactions and long-lived radicals become clearer, it will be possible to determine experimentally precisely how these SOD mimetics mitigate radiation damage by their scavenging properties. After being tested in vivo, these agents could prove useful as a medical countermeasure in the event of accidental or malicious radiation exposures. Furthermore, this work and that of others would support the use of radioprotectors given before or during radiation therapy in combination with mitigating agents administered after radiotherapy. Such combined adjunct therapy may significantly alter the dose-limiting toxicity of tissues such as the intestine, kidney, skin and CNS, thereby reducing radiation damage to normal tissue while preserving the effectiveness of tumor treatment. Our future and ongoing studies will examine the efficacy of these two lead mitigating agents in preventing other forms of radiation-induced late damage and to mitigating neutron and γ-radiation-induced damage in vitro and in vivo.
Acknowledgments
This work was supported by the Center for Medical Countermeasures Against Radiological Terrorism (CMCRT) Pilot Grant Program at the Medical College of Wisconsin and awarded by the NIH National Institute of Allergy and Infectious Disease (NIAID) 1-U19-AI067734-01. We are grateful to Catherine Butterfield and the late Dr. Judah Folkman for originally providing us with the capillary endothelial cells. We acknowledge Bernard Meunier and Frederic Cosledan, who designed and synthesized EUK-418, -423 and -425, and Myra Robinson, who provided able technical assistance. We thank Dr. John Moulder, Dr. Jacqueline Williams and Dr. Michael Robbins for helpful suggestions during the progress of the work.
References
- 1.Nair CKK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res. 2001;42:21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- 2.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1990;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 4.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundquist T, Moravec R, Niles A, O’Brien M, Riss T. Timing your apoptosis assay. Cell Notes. 2006;16:18–21. [Google Scholar]
- 7.Samuni A, Mitchell JB, DeGraff W, Krishna CM, Samuni U, Russo A. Nitroxide SOD-mimics: modes of action. Free Radic Res Commun. 1991;12–13:187–194. doi: 10.3109/10715769109145785. [DOI] [PubMed] [Google Scholar]
- 8.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 9.Langley RE, Bump EA, Quartuccio SG, Medeiros D, Braunhut SJ. Radiation-induced apoptosis in microvascular endothelial cells. Br J Cancer. 1997;75:666–672. doi: 10.1038/bjc.1997.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuks Z, Alfieri A, Haimovitz-Friedman A, Seddon A, Cordon-Cardo C. Intravenous basic fibroblast growth factor protects the lung but not mediastinal organs against radiation-induced apoptosis in vivo. Cancer J Sci Am. 1995;1:62–72. [PubMed] [Google Scholar]
- 11.Fuks Z, Persaud RS, Alfieri A, McLoughlin M, Ehleiter D, Schwartz JL, Seddon AP, Cordon-Cardo C, Haimovitz-Friedman A. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res. 1994;54:2582–2590. [PubMed] [Google Scholar]
- 12.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI workshop, December 3–4, 2003. Radiat Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 13.Baudry M, Etienne S, Bruce A, Palucki M, Jacobsen E, Malfroy B. Salen-manganese complexes are superoxide dismutase-mimics. Biochem Biophys Res Commun. 1993;192:964–968. doi: 10.1006/bbrc.1993.1509. [DOI] [PubMed] [Google Scholar]
- 14.Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, Baker K, Lazarowych N, Malfroy B. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. J Med Chem. 2002;45:4549–4558. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 15.Sharpe M, Ollosson R, Stewart VC, Clark JB. Oxidation of nitric oxide by oxomanganese salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem J. 2002;366:97–107. doi: 10.1042/BJ20020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker K, Bucay-Marcus C, Huffman C, Kruk H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- 17.Rong Y, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc Natl Acad Sci USA. 1999;96:9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, Xu Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse myotrophic lateral sclerosis model. Neurosci Lett. 2001;304:157–160. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- 19.Doctrow SR, Huffman K, Marcus CB, Musleh W, Bruce A, Baudry M, Malfroy B. Salen-manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. In: Sies H, editor. Antioxidants in Disease Mechanisms and Therapeutic Strategies. Academic Press; New York: 1997. pp. 247–270. [DOI] [PubMed] [Google Scholar]
- 20.Peng J, Stevenson FF, Doctrow SR, Andersen JK. SOD/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantia nigra: implications for Parkinson’s disease. J Biol Chem. 2005;280:29194–29198. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- 21.Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Lifespan extension and rescue of neurodegeneration in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase/catalase mimetics. J Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brazier MW, Doctrow SR, Masters CL, Collins SJ. A manganese-superoxide dismutase/catalase mimetic extends survival in a mouse model of human prion disease. Free Radic Biol Med. 2008;45:184–192. doi: 10.1016/j.freeradbiomed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez PK, Zhuang J, Doctrow SR, Malfroy B, Benson PF, Menconi MJ, Fink MP. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J Pharmacol Exp Ther. 1995;275:798–806. [PubMed] [Google Scholar]
- 24.Zhang HJ, Doctrow SR, Xu L, Oberley LW, Beecher B, Morrison J, Oberley TD, Kregel KC. Redox modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress. FASEB J. 2004;18:1547–1549. doi: 10.1096/fj.04-1629fje. [DOI] [PubMed] [Google Scholar]
- 25.Gianello P, Saliez A, Bufkens X, Pettinger R, Misseleyn D, Hori S, Malfroy B. EUK-134, a synthetic superoxide dismutase and catalase mimetic, protects rat kidneys from ischemia-reperfusion-induced damage. Transplantation. 1996;62:1664–1666. doi: 10.1097/00007890-199612150-00022. [DOI] [PubMed] [Google Scholar]
- 26.Murphy CK, Fey EG, Watkins BA, Wong V, Rothstein D, Sonis ST. Efficacy of superoxide dismutase mimetic M40403 in attenuating radiation-induced oral mucositis in hamsters. Clin Cancer Res. 2008;14:4292–4297. doi: 10.1158/1078-0432.CCR-07-4669. [DOI] [PubMed] [Google Scholar]
- 27.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TW, Fridovich I, Dewhirst MW, Anscher MS. A small molecular weight catalytic metallopophyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33:857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 28.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 29.Riley PA. Free radicals in biology: oxidative stress and the effect of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 30.Jiang J, Belikova NA, Hoye AT, Zhao Q, Epperly MW, Greenberger JS, Wipf P, Kagan VE. A mitochondria-targeted nitroxide/hemigramicidin S conjugate protects mouse embryonic cells against gamma irradiation. Int J Radiat Oncol Biol Phys. 2008;70:816–825. doi: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xun C, Shen N, Li B, Zhang Y, Wang F, Yang Y, Shi X, Schafermyer K, Brown SA, Thompson JS. Radiation mitigation effect of cultured mushroom fungus Hirsutella Sinensis (CorImmune) isolated from a Chinese/Tibetan herbal preparation – Cordyceps Sinensis. Int J Radiat Biol. 2008;84:139–149. doi: 10.1080/09553000701797070. [DOI] [PubMed] [Google Scholar]
- 32.Yan S, Brown SL, Kolozsvary A, Freytag SO, Lu M, Kim JH. Mitigation of radiation-induced skin injury by AAV2-mediated MnSOD gene therapy. J Gene Med. 2008;10:1012–1008. doi: 10.1002/jgm.1226. [DOI] [PubMed] [Google Scholar]
- 33.Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006;79:231–238. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Batinic-Haberle I, Vukaskovic Z. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic Biol Med. 2008;44:982–989. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabbini ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, Vujaskovic Z. Long-term administration of a small molecular weight calalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haydont V, Gilliot O, Rivera S, Bourgier C, François A, Aigueperse J, Bourhis J, Vozenin-Brotons MC. Successful mitigation of delayed intestinal radiation injury using pravastatin is not associated with acute injury improvement or tumor protection. Int J Radiat Oncol Biol Phys. 2007;68:1471–1482. doi: 10.1016/j.ijrobp.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 37.Moulder JE, Fish BL, Cohen EP. ACE inhibitors and all receptor antagonists in the treatment and prevention of bone marrow transplant nephropathy. Curr Pharm Design. 2003;9:737–749. doi: 10.2174/1381612033455422. [DOI] [PubMed] [Google Scholar]
- 38.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984;99:2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren MC, Bump EA, Medeiros D, Braunhut SJ. Oxidative stress-induced apoptosis of endothelial cells. Free Radic Biol Med. 2000;29:537–547. doi: 10.1016/s0891-5849(00)00353-1. [DOI] [PubMed] [Google Scholar]
- 40.Vorotnikova E, Tries M, Braunhut SJ. Retinoids and TIMP1 prevent radiation-induced apoptosis of capillary endothelial cells. Radiat Res. 2004;161:174–184. doi: 10.1667/rr3107. [DOI] [PubMed] [Google Scholar]
- 41.Braunhut SJ, Medeiros D, Lai L, Bump EA. Tempol prevents impairment of the endothelial cell wound healing response caused by ionizing radiation. Br J Cancer Suppl. 1996;27:157–160. [PMC free article] [PubMed] [Google Scholar]
- 42.Law MP. Radiation-induced vascular injury and its relation to late effects in normal tissues. Adv Radiat Biol. 1981;9:37–73. [Google Scholar]
- 43.Adamson IYR, Bowden DH. Endothelial injury and repair in radiation induced pulmonary fibrosis. Am J Pathol. 1983;112:224–230. [PMC free article] [PubMed] [Google Scholar]
- 44.Fajardo LF. The endothelial cell as a unique target of radiation: an overview. In: Rubin DB, editor. The Radiation Biology of Vascular Endothelium. CRC Press; Boca Raton, FL: 1997. pp. 1–12. [Google Scholar]
- 45.Milliat F, François A, Tamarat R, Benderitter M. Role of endothelium in radiation-induced normal tissue damages. Ann Cardiol Angieol (Paris) 2008;57:139–148. doi: 10.1016/j.ancard.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Glatstein E, Fajardo LF, Brown JM. Radiation injury in the mouse kidney. Int J Radiat Oncol Biol Physiol. 1977;2:933–943. doi: 10.1016/0360-3016(77)90191-2. [DOI] [PubMed] [Google Scholar]
- 47.Fajardo LF, Brown JM, Glatstein E. Glomerular and juxtaglomerular lesions in radiation nephropathy. Radiat Res. 1976;68:177–183. [PubMed] [Google Scholar]
- 48.Meunier B, Cosledan F. Orally bioavailable low molecular weight metalloporphyrins as antioxidants. 20060241095. USPTO Application no. 2006
- 49.Rosenthal RA, Fisette LW, Huffman KD, Damphousse CA, Callaway WB, Malfroy B, Doctrow SR. Orally active Mn porphyrins are SOD/catalase mimetics which protect cells from apoptosis induced by oxidative stress. Free Radic Biol Med. 2007;43:188–189. [Google Scholar]
- 50.Rosenthal RA, Huffman KD, Fisette LW, Damphousse CA, Callaway WB, Malfroy B, Doctrow SR. Orally available Mn porphyrins with superoxide dismutase and catalase activities. J Biol Inorg Chem. 2009;14:979–991. doi: 10.1007/s00775-009-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pong K, Doctrow SR, Huffman KD, Adinolfi CA, Baudri M. Attenuation of staurosporine-induced apoptosis, oxidative stress and mitochondrial dysfunction by synthetic superoxide dismutase and catalase mimetics in cultured cortical neurons. Exp Neurol. 2001;171:84–97. doi: 10.1006/exnr.2001.7747. [DOI] [PubMed] [Google Scholar]
- 52.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci USA. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou M, Baudry M. EUK-207, a superoxide dismutase/catalase mimetic, is neuroprotective against oxygen/glucose deprivation-induced neuronal death in cultured hippocampal slices. Brain Res. 2009;1247:28–37. doi: 10.1016/j.brainres.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falkner KM, Liochev SI, Fridovich I. Stable Mn (III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J Biol Chem. 1994;269:23741–23476. [PubMed] [Google Scholar]
- 55.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 56.Decker P, Muller S. Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr Pharm Biotechnol. 2002;3:275–283. doi: 10.2174/1389201023378265. [DOI] [PubMed] [Google Scholar]
- 57.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 58.Leach JK, Van Tuyle G, Lin PS, Schmidt-Ullrich RK, Mikkelsen RB. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001;61:3894–3901. [PubMed] [Google Scholar]
- 59.Ward JF. Nature of lesions formed by ionizing radiation. In: Nickoloff JA, Hoekstra MF, editors. DNA Damage and Repair: DNA Repair in Higher Eukaryotes. Humana Press; Totowa, NJ: 1998. pp. 65–84. [Google Scholar]
- 60.Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, Wheeler KT. Endothelial cell loss precedes and parallels the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J Neurol Sci. 2007;257:67–71. doi: 10.1016/j.jns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 61.Boerma M, Kruse JJ, van Loenen M, Klein HR, Bart CI, Zurcher C, Wondergem J. Increased deposition of von Willebrand factor in the rat heart after local ionizing irradiation. Strahlenther Onkol. 2004;180:109–116. doi: 10.1007/s00066-004-1138-0. [DOI] [PubMed] [Google Scholar]
- 62.McManus LM, Ostrom KK, Lear C, Luce EB, Gander DL, Pinckard RN, Redding SW. Radiation-induced increased platelet-activating factor activity in mixed saliva. Lab Invest. 1993;68:118–124. [PubMed] [Google Scholar]
- 63.Yan S, Brown SL, Kolozsvary A, Freytag SO, Lu M, Kim JH. Mitigation of radiation-induced skin injury by AAV2-mediated MnSOD gene therapy. J Gene Med. 2008;10:1012–1018. doi: 10.1002/jgm.1226. [DOI] [PubMed] [Google Scholar]
- 64.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Gudkov AV. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pradeep K, Park SH, Ko KC. Hesperidin, a flavanoglycone protects against gamma-irradiation induced hepatocellular damage and oxidative stress in Sprague-Dawley rats. Eur J Pharmacol. 2008;587:273–280. doi: 10.1016/j.ejphar.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 66.Holler V, Buard V, Gaugler MH, Guipaud O, Baudelin C, Sache A, Perez MD, Squiban C, Tamarat R, Benderitter M. Pravastatin limits radiation-induced vascular dysfunction in the skin. J Invest Dermatol. 2009;129:1280–1291. doi: 10.1038/jid.2008.360. [DOI] [PubMed] [Google Scholar]
- 67.Murley JS, Kataoka Y, Weydert C, Oberley L, Grdina DJ. Delayed cytoprotection after enhancement of SOD2 (MnSOD) gene expression in SA-NH mouse sarcoma cells exposed to WR-1065, the active metabolite of amifostine. Radiat Res. 2002;158:101–109. doi: 10.1667/0033-7587(2002)158[0101:dcaeos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 68.Koyama S, Kodama S, Suzuki K, Matsumoto T, Miyazaki T, Watanabe M. Radiation-induced long-lived radicals which cause mutation and transformation. Mutat Res. 1998;421:45–54. doi: 10.1016/s0027-5107(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 69.Hafer K, Konishi T, Schiestl R. Radiation-induced long-lived extracellular radicals do not contribute to measurement of intracellular reactive oxygen species using the dichlorofluorescein method. Radiat Res. 2008;169:469–473. doi: 10.1667/RR1211.1. [DOI] [PubMed] [Google Scholar]


