Abstract
Background
Chronic kidney disease (CKD) is found at epidemic levels in certain populations of the Pacific Coast in northwestern Nicaragua especially in younger men. There are knowledge gaps concerning CKD’s prevalence in regions at higher altitudes.
Methods
A cross-sectional study of adults between the ages of 20 and 60 years in 1 coffee-growing village in Nicaragua located at 1,000 m above sea level (MASL) altitude was performed. Predictors included participant sex, age, occupation, conventional CKD risk factors and other factors associated with CKD suggested by previous surveys in Central America. Outcomes included serum creatinine (SCr) values >1.2 mg/dL for men and >0.9 mg/dL for women, estimated glomerular filtration rate (GFR) <60 ml/min per 1.73 m2, dipstick proteinuria stratified as microalbuminuria (30–300 mg/dL) and macroalbuminuria (>300 mg/dL), hypertension and body mass index.
Results
Of 324 eligible participants, 293 were interviewed (90.4%), and 267 of those received the physical exam (82.4% overall). Of the sample, 45% were men. Prevalence rate of estimated GFR <60 ml/min per 1.73 m2 was 0 for men (0%) and 2 for women (1.4%). The prevalence of at least microalbuminuria was significantly higher among men compared with women (27.5% vs. 21.4%, respectively; p=0.02).
Conclusions
The CKD prevalence in this village is comparable to a previously studied Nicaraguan coffee-farming region and much lower than previously screened portions of northwestern Nicaragua. There is heterogeneity in CKD prevalence across Nicaragua. At this time, screenings should target individuals living in previously identified, higher risk regions. More work is needed to understand determinants of CKD in this resource-poor nation.
Keywords: Altitude, Chronic kidney disease, Nicaragua, Pesticides, Risk factors, Rural
Introduction
Rates of chronic kidney disease (CKD) are rising alarmingly in the developing world (1, 2). Many cases are linked to known CKD risk factors (3–9) but this is not a universal pattern. Some regions have seen a mixture of established and as-yet-unknown risk factor correlations for developing CKD (10–15). One such perplexing region is found in Central America (16) and includes northwestern Nicaragua (17). There the high rates of disease have been linked to known risk factors, agricultural work and locally produced alcohol (13, 17–19). Younger men are disproportionately affected (18). A series of surveys performed in 2007 in 5 different communities in Nicaragua demonstrated that rates of CKD affliction were not uniform throughout the region (17). The highest rates of disease were seen in the subsistence farming/mining village and the banana/sugarcane growing village. The lowest rates were seen in 2 villages with service industry and coffee-growing economies (17). O’Donnell also found high rates of CKD among rural, agricultural populations. In both, an inverse correlation between village altitude and CKD prevalence was observed (17, 18). Of all the villages surveyed, the fewest participants were recruited from the elevated, coffee-growing village (92 total). This small sample meant the low rates reported required further follow-up, especially because coffee growing is at least as widespread in Nicaragua as mining or banana and sugarcane farming. If the original data were to prove inaccurate, many cases of CKD in the Nicaraguan population might be missed. Nicaragua would struggle to address this serious problem, as more developed Latin American nations fight to contain the costs related to caring for patients with CKD and its sequelae (20). If this data were accurate, it would argue for a more localized epidemic with a separate etiology.
The aim of this study was to estimate the prevalence of CKD in an elevated coffee-growing community and continue to map Nicaragua’s CKD epidemic. Furthermore, we planned to assess the extent to which environmental exposures and usual CKD risk factors were associated with decrements in estimated glomerular filtration rate (eGFR) and proteinuria.
Subjects and Methods
This cross-sectional survey established the prevalence of decreased kidney function in adults aged 20–60 years in 1 coffee-growing village in north-central (Matagalpa) Nicaragua. The village was selected because of its economy and isolation from northwest Nicaragua and high altitude compared with previously screened villages (1,000 m above sea level [MASL]). The study sample included 324 eligible participants, of whom 293 were interviewed (90.4%) and 267 of those received the physical exam and provided blood samples (82.4%) (Fig. 1). Data was collected from June to July 2009. All participants gave informed consent, and the study was performed in adherence with the Declaration of Helsinki. Patient demographics and clinical characteristics were collected using an interviewer administered questionnaire. This study was performed with the approval of both the UNAN-Leon Bioethics Committee and the University of Pittsburgh Institutional Review Board.
Fig. 1.
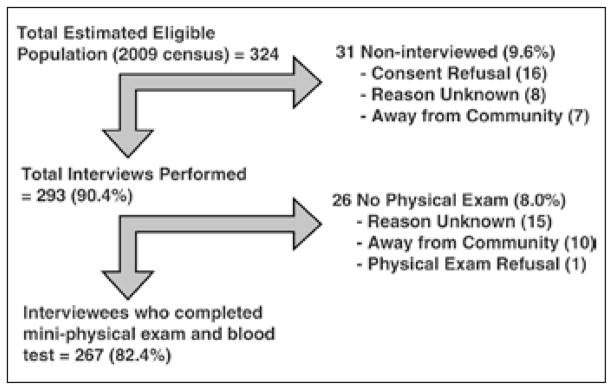
Study recruitment.
Participants then went to a central location to receive a kidney health checkup: first, height and weight were measured using a calibrated clinical scale; second, blood pressure was measured after 5 minutes of seated rest, using a previously calibrated sphygmomanometer; finally, blood and urine samples were obtained. Participants gave a random, late-catch urine sample (approx. 50 mL) in a sterile collector and a nonfasting capillary blood fingerstick. These measures were generally collected on the weekends or in the evenings to control for dehydration.
Creatinine levels were measured with capillary fingersticks using the Nova Biomedical (Waltham, MA) StatSensor device (sensitivity 59%, specificity 88%) (21). Each urine sample was examined for glucosuria and proteinuria. The urine was analyzed using Bayer’s Multistix Urine Test Strips (sensitivity 97%, specificity 62%) (22). Each sample was read 1 minute later and graded by the color of the test strip by 1 student and independently confirmed by another student. Glucosuria was defined as a value of ≥ +. Microalbuminuria (30–300 mg/dL) was defined as a returned value of + or ++. Macroalbuminuria (>300 mg/dL) was defined as returned value +++ or ++++.
Serum creatinine (SCr) levels >1.2 mg/dL for men and >0.9 mg/dL for women were used to identify CKD. Glomerular filtration rate (GFR) <60 ml/min per 1.73 m2 was estimated based on the Modification of Diet in Renal Disease (MDRD) Study equation (23).
Hypertension was defined as systolic blood pressure >140 mm Hg, diastolic pressure >90 mm Hg or self-reported medical history. Diabetes mellitus was defined as glucosuria (glucose excretion >100 mg/dL) or self-reported medical history. Obesity was defined as body mass index >30 (calculated as kg/m2). Use of nephrotoxic drugs, including nonsteroidal antiinflammatory drugs, was defined as consuming at least 1 dose for 7 consecutive days at any point in the person’s life.
Occupation was classified into primary, secondary and economically inactive populations. The primary sector included categories of agriculture and animal husbandry. The secondary sector included categories of artisan manufacturing (food) and construction. Economically inactive included housewives, students and the unemployed.
Data was described with frequencies and analyzed with chi-squared test, Mann-Whitney test, Fisher’s exact test or t-test as appropriate. Binary logistic regression was performed using presence of proteinuria as an outcome. The logistic regression models included age, sex, diabetes, BMI, hypertension and work-related pesticide use. All statistics were performed using SPSS 19.0 for Windows.
Results
The mean age ± SD of the sample population was 34 ± 10.4 years (range 20–60 years). The population was 45% male, 7.5% hypertensive, 1.7% diabetic and 14% obese. There were no significant differences between men and women with regard to age, blood pressure or diabetes. Men and women worked different jobs, men smoked and drank alcohol more and women were noticeably more obese. Women also reported more family history of kidney disease (Tab. I). The prevalence of CKD and proteinuria by sex is shown in Table II. Only 2 individuals in the sample (0.7% of the population), both women, had stage 3 CKD at the time of measurement. The overall prevalence of CKD was not statistically different between the sexes. Men had a higher overall rate of proteinuria, though this difference was not statistically significant. Nine percent (6/65) of those with proteinuria had concomitant urinary leukocytes. Microalbuminuria was predominant in both sexes. There were differences in distribution of microalbuminuria and macroalbuminuria between the sexes (p=0.02). Men who had stage 1 or 2 CKD tended to have proteinuria (30/33, 90.9%); 28 of those 30 (93.3%) had stage 1 CKD.
Table I.
POPULATION CHARACTERISTICS
| Total n=267 (100%) | Men n=120 (47.6%) | Women n=147 (52.4%) | p Value | |
|---|---|---|---|---|
| Age mean ± SD, years | 34.15 ± 10.4 | 34.03 ± 10.4 | 34.24 ± 10.5 | 0.83 |
| Age range, no. (%) | ||||
| - 20–29 | 106 (39.7) | 51 (42.5) | 55 (37.4) | 0.80 |
| - 30–39 | 79 (29.6) | 35 (29.2) | 44 (29.9) | |
| - 40–49 | 55 (20.6) | 22 (18.3) | 33 (22.4) | |
| - 50–60 | 27 (10.1) | 12 (10.0) | 15 (10.2) | |
| Blood pressure, no. (%) | ||||
| - Normotensive | 224 (83.9) | 98 (81.7) | 126 (85.7) | 0.66 |
| - Pre-HTN | 23 (8.6) | 12 (10.0) | 11 (7.5) | |
| - HTN | 20 (7.5) | 10 (8.3) | 10 (6.8) | |
| Diabetes, no. (%) | 9 (3.4) | 2 (1.7) | 7 (4.8) | 0.19 |
| BMI,* no. (%) | ||||
| - Normal | 122 (46.0) | 71 (59.2) | 51 (35.2) | <0.01 |
| - Overweight | 105 (39.6) | 45 (37.5) | 60 (41.4) | |
| - Obese (all classes) | 38 (14.3) | 4 (3.3) | 34 (23.4) | |
| Family Hx (1st deg), no. (%) | ||||
| - Kidney disease | 109 (41.4) | 39 (32.8) | 70 (48.6) | 0.01 |
| - Diabetes mellitus | 41 (15.4) | 18 (15.0) | 23 (15.6) | 0.88 |
| - Hypertension | 119 (44.6) | 52 (43.3) | 67 (45.6) | 0.71 |
| Personal habits, no. (%) | ||||
| - Smoking currently | 56 (21.0) | 50 (41.7) | 6 (4.1) | <0.01 |
| - Past smoking | 45 (16.9) | 33 (27.5) | 12 (8.2) | <0.01 |
| - Current alcohol consumption | 51 (19.1) | 45 (37.5) | 6 (4.1) | <0.01 |
| - Past alcohol consumption | 64 (24.0) | 54 (45.0) | 10 (6.8) | <0.01 |
| Work experience, no. (%) | ||||
| - Primarily work in agriculture | 117 (43.8) | 111 (92.5) | 6 (4.1) | <0.01 |
| - Work preparing/applying pesticides | 140 (52.4) | 118 (98.3) | 22 (15.05) | <0.01 |
| History of nephrotoxic drug use, no. (%) | 188 (70.4) | 73 (60.8) | 115 (78.2) | <0.01 |
BMI = body mass index; HTN = hypertensive; Hx = history.
Did not include 2 underweight women (BMI <18.5).
Table II.
PREVALENCE OF CKD/PROTEINURIA BY SEX
| Total n=267 (100%) | Men n=120 (47.6%) | Women n=147 (52.4%) | p Value | |
|---|---|---|---|---|
| CKD stage (GFR/classification), no. (%) | ||||
| - Stage 0 | 201 (75.3) | 87 (72.5) | 114 (77.6) | <0.01 |
| - Stage 1 (≥90 with evidence of kidney damage) | 47 (17.6) | 30 (25.0) | 17 (11.6) | |
| - Stage 2 (60–89) | 17 (6.4) | 3 (2.5) | 14 (9.5) | |
| - Stage 3 (30–60) | 2 (0.7) | 0 (0.0) | 2 (1.4) | |
| All CKD stages, no. (%) | 66 (24.7) | 33 (27.5) | 33 (22.5) | 0.34 |
| Proteinuria classification, no. (%) | ||||
| - No proteinuria | 202 (75.7) | 87 (72.5) | 115 (78.2) | 0.02 |
| - Microalbuminuria, + or ++ | 59 (22.1) | 27 (22.5) | 32 (21.8) | |
| - Macroalbuminuria, +++ or ++++ | 6 (2.2) | 6 (5.0) | 0 | |
| Presence of any proteinuria, no. (%) | 65 (24.3) | 33 (27.5) | 32 (21.8) | 0.28 |
No stage 4 or 5 CKD was found in this study population.
CKD = chronic kidney disease; GFR = glomerular filtration rate.
There was a correlation between CKD prevalence and age grouping, with rates increasing with age (p=0.034). The same pattern of increasing prevalence with age was also seen for diabetes mellitus (p=0.016) (Tab. III). The relationships between presence of proteinuria and selected CKD risk factors are shown in Table IV. Proteinuria prevalence was higher in younger participants in both univariate and adjusted analyses. Simultaneous analysis of sex and work with pesticide could not be performed due to collinearity.
Table III.
CKD STAGE, DIABETES AND AGE GROUP
| 20–29 years n=106 (39.7%) | 30–39 years n=80 (30.0%) | 40–49 years n=52 (19.5%) | 50–60 years n=27 (10.1%) | p Value | |
|---|---|---|---|---|---|
| CKD classification, no. (%) | |||||
| - No CKD | 81 (76.4) | 57 (71.3) | 44 (84.6) | 19 (70.4) | 0.03 |
| - CKD stage 1 | 20 (18.9) | 19 (23.7) | 6 (11.5) | 2 (7.4) | |
| - CKD stage 2 | 5 (4.7) | 4 (5.0) | 1 (1.9) | 5 (18.5) | |
| - CKD stage 3 | 0 | 0 | 1 (1.9) | 1 (3.7) | |
| Diabetes, no. | |||||
| - None | 105 | 78 | 50 | 25 | 0.02 |
| - Diagnosed / glucosuria | 1 | 1 | 5 | 2 | |
There was no significant clinical link between CKD stage and diabetes mellitus (p=0.155). CKD = chronic kidney disease.
Table IV.
| Univariate analysis odds ratio | Adjusted odds ratio* | Multivariate analysis odds ratio† | |
|---|---|---|---|
| Table IVa | |||
| Age group | |||
| - 20–39 years | 2.34 (1.17–4.67) | 2.32 (1.16–4.62) | 2.43 (1.20–4.94) |
| - 40–60 years (Control) | |||
| Sex | |||
| - Men | 1.36 (0.78–2.39) | 1.33 (0.75–2.34) | 1.55 (0.85–2.85) |
| - Women (Control) | |||
| Table IVb | |||
| Age group | |||
| - 20–39 | 2.34 (1.17–4.67) | 2.34 (1.18–4.68) | 2.44 (1.20–4.94) |
| - 40–60 (Control) | |||
| Work with pesticides | |||
| - Yes | 0.99 (0.57–1.73) | 0.97 (0.55–1.71) | 1.09 (0.60–1.98) |
| - No (Control) | |||
Discussion
This study demonstrates that GFR rates and CKD prevalence in this high-altitude, coffee-farming village are comparable to or lower than the previously studied coffee farming region of Nicaragua and the rates reported in the 1988–1994 or 1999–2004 Third National Health and Nutrition Examination Survey (NHANES) (24) (Fig. 2). The overall percentage of individuals in the town with GFR’s <60 ml/min per 1.73 m2 was 2/267 (0.75%), the lowest prevalence rate documented in Nicaragua to date. This is likely somewhat explained by a hypertension prevalence of 7.5%. However, this village was also the most elevated thus far screened in Nicaragua (1,000 MASL). These observations concur with the previously observed inverse relationship between altitude and CKD prevalence in Nicaragua (17, 18), and this trend agrees with reports in the literature on the beneficial effects of higher altitudes on the kidney (25–27). However, these studies noticed the greatest effect at altitudes much higher than our coffee village – generally >6,000 feet (1,828 m). Only Winkelmayer et al found a positive benefit at an altitude of 1,000 m, with 7% less all-cause mortality among dialysis patients (25).
Fig. 2.
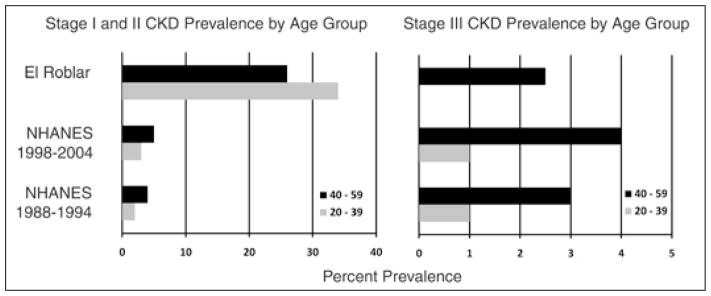
Chronic kidney disease (CKD) stage 1 and 2 prevalence (left) and CKD stage 3 prevalence (right) by age group, compared with Third National Health and Nutrition Examination Survey (NHANES) (24).
In both Torres et al and O’Donnell et al (17, 18), many cases of clinically significant CKD did not present with any level of proteinuria, suggesting tubulointerstitial disease potentially tied to environmental or occupational exposure(s). In this village, 51/66 (77.3%) of CKD cases presented with ≥1+ proteinuria, much more consistent with the picture caused by glomerulonephritides. This pattern is similar to CKD’s presentation in the developed world, where CKD is linked to known comorbidities, including increasing age which was significantly linked to CKD in this population. These observations support the hypothesis that varying CKD prevalence rates seen in different regions of Nicaragua are undergirded by different causative forces.
Due to prior links of CKD to agricultural work, justifiable concerns about a link between high prevalence rates of CKD and pesticide use (28) have been voiced. The men in the coffee-growing village in this study reported high levels of working with pesticides (92.5%). While this study can neither prove nor disprove these conjectures, pesticide exposure of the kind experienced in this coffee-growing village (both indirect and direct) is apparently not singularly sufficient to cause any increased prevalence of CKD.
While the rates of other known comorbidities such as hypertension (7.8%), diabetes (3.4%) and obesity (14.3%) are probably sufficient to explain the 66 cases of all-stage CKD in this population, they probably are not sufficient to explain the higher prevalence of any form of proteinuria among study participants (24.3%). Further, despite low overall CKD prevalence, the prevalence of proteinuria was higher in younger study participants, peaking in those 30–39 years of age (Fig. 3). This divergence of GFR reduction and proteinuria and higher rates of proteinuria among younger study participants are the opposite of what would be expected. One possible explanation is Nicaragua’s recent history. In 1960, life expectancy in Nicaragua was 47 years; in 2008, it was 73.1 years (29). Among older study participants (those 40–60 years old), only the healthiest (and luckiest) minority were still alive. Younger study participants grew up after war had ended, and Nicaragua began the epidemiologic transition associated with modernization. Both urban and rural Central American diets have recently come to be based more on fried foods, meats and processed foods (30). This observation is borne out by the fact that in this population, chronic disease risk factors increased only slightly with older age, and only diabetes did so in a significant manner (Fig. 3). In our study population, those younger than 40 years grew up exposed to many of the risk factors that predispose developed world populations to CKD – a CKD that often presents with proteinuria as we observed here – while older study participants did not. We suspect that as this population ages this divergence between reduced GFR and proteinuria will diminish. However, even as this divergence becomes less pronounced, the etiologic underpinning of CKD in this region will be different from that of the cases previously observed by Torres et al and O’Donnell et al (17, 18).
Fig. 3.
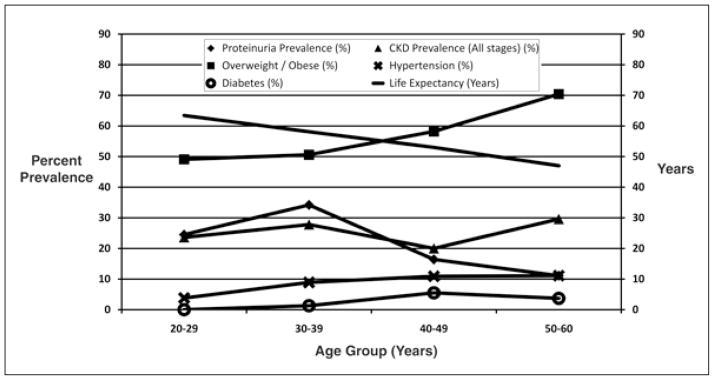
Chronic disease prevalence by age group and life expectancy (29).
Nicaragua is a resource-poor nation that must initiate screening to prevent further CKD cases or risk overwhelming its health care infrastructure. It appears that at least 2 mechanisms of disease development exist in Nicaragua – an as-yet-undescribed type of employment or environmental exposure (17, 18) and known risk factors as seen in this coffee-growing village at higher altitude. In light of limited resources, public health officials may have a greater impact by focusing on rural residents living at <500 MASL in northwestern Nicaragua. There, adult men of all ages should be screened because of their increased risk both overall and at younger ages (17, 18). At this time there is not sufficient evidence to justify screening Nicaraguans living outside the northwest or at higher altitudes (>500 MASL).
Acknowledgments
We would like to thank Li Wang (MS, University of Pittsburgh) and Dr. Claire Bunker (PhD, University of Pittsburgh) for their assistance with statistical calculations.
Financial support: NIH T35 Grant #2 T35 DK065521- 06, UPSOM Medical Alumni Association Scholarship, University of Pittsburgh Nationality Rooms Scholarships, NCRR Grant # 5UL1 RR024153-05.
Footnotes
Conflict of interest statement: Mark Unruh MD, MSc has consulted for Baxter Healthcare Medical Advisory Board Sigma-Tau, UCB and Abbot in the past three years. None for all other authors.
References
- 1.Barsoum RS. Chronic kidney disease in the developing world. N Engl J Med. 2006;354(10):997–999. doi: 10.1056/NEJMp058318. [DOI] [PubMed] [Google Scholar]
- 2.Hossain MP, Goyder EC, Rigby JE, El Nahas M. CKD and poverty: a growing global challenge. Am J Kidney Dis. 2009;53(1):166–174. doi: 10.1053/j.ajkd.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Cusumano A, Garcia-Garcia G, Di Gioia C, et al. End-stage renal disease and its treatment in Latin America in the twenty-first century. Ren Fail. 2006;28(8):631–637. doi: 10.1080/08860220600925693. [DOI] [PubMed] [Google Scholar]
- 4.Cusumano A, Garcia Garcia G, Gonzalez-Bedat C. The Latin American Dialysis and Transplant Registry: report 2006. Ethn Dis. 2009;19(Suppl 1):S1–3.6. [PubMed] [Google Scholar]
- 5.Arogundade FA, Barsoum RS. CKD prevention in Sub-Saharan Africa: a call for governmental, nongovernmental, and community support. Am J Kidney Dis. 2008;51(3):515–523. doi: 10.1053/j.ajkd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Correa-Rotter R, Cusumano AM. Present, prevention, and management of chronic kidney disease in Latin America. Blood Purif. 2008;26(1):90–94. doi: 10.1159/000110572. [DOI] [PubMed] [Google Scholar]
- 7.Ayodele OE, Alebiosu CO. Burden of chronic kidney disease: an international perspective. Adv Chronic Kidney Dis. 2010;17(3):215–224. doi: 10.1053/j.ackd.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Alebiosu CO, Ayodele OE. The global burden of chronic kidney disease and the way forward. Ethn Dis. 2005;15(3):418–423. [PubMed] [Google Scholar]
- 9.McClellan WM. Epidemiology and risk factors for chronic kidney disease. Med Clin North Am. 2005;89(3):419–445. doi: 10.1016/j.mcna.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Sumaili EK, Cohen EP, Zinga CV, Krzesinski JM, Pakasa NM, Nseka NM. High prevalence of undiagnosed chronic kidney disease among at-risk population in Kinshasa, the Democratic Republic of Congo. BMC Nephrol. 2009;10(1):18. doi: 10.1186/1471-2369-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumaili EK, Krzesinski JM, Cohen EP, Nseka NM. Epidemiology of chronic kidney disease in the Democratic Republic of Congo: review of cross-sectional studies from Kinshasa, the capital. Nephrol Ther. 2010;6(4):232–239. doi: 10.1016/j.nephro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Amato D, Alvarez-Aguilar C, Castañeda-Limones R, et al. Prevalence of chronic kidney disease in an urban Mexican population. Kidney Int Suppl. 2005;97(97):S11–S17. doi: 10.1111/j.1523-1755.2005.09702.x. [DOI] [PubMed] [Google Scholar]
- 13.Trabanino RG, Aguilar R, Silva CR, Mercado MO, Merino RL. Nefropatía terminal en pacientes de un hospital de referencia en El Salvador. Rev Panam Salud Publica. 2002;12(3):202–206. doi: 10.1590/s1020-49892002000900009. [DOI] [PubMed] [Google Scholar]
- 14.Harman C. Is poverty a risk factor for CKD? Nat Rev Nephrol. 2009;5(5):241. doi: 10.1038/nrneph.2009.66. [DOI] [PubMed] [Google Scholar]
- 15.Lou-Meda R. ESRD in Guatemala and a model for preventive strategies: outlook of the Guatemalan Foundation for Children with Kidney Diseases. Ren Fail. 2006;28(8):689–691. doi: 10.1080/08860220600938258. [DOI] [PubMed] [Google Scholar]
- 16.Gracia-Trabanino R, Domínguez J, Jansà JM, Oliver A. Proteinuria and chronic renal failure in the coast of El Salvador: detection with low cost methods and associated factors. Nefrologia. 2005;25(1):31–38. [PubMed] [Google Scholar]
- 17.Torres C, Aragón A, González M, et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55(3):485–496. doi: 10.1053/j.ajkd.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell JK, Tobey M, Weiner DE, et al. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011;26(9):2798–805. doi: 10.1093/ndt/gfq385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanoff SL, Callejas L, Alonso CD, et al. Positive association of renal insufficiency with agriculture employment and unregulated alcohol consumption in Nicaragua. Ren Fail. 2010;32(7):766–777. doi: 10.3109/0886022X.2010.494333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ardiles LG, Poblete H, Ortiz M, et al. The health system in Chile: the nephrologist perspective. J Nephrol. 2011;24(2):149–154. doi: 10.5301/jn.2011.6371. [DOI] [PubMed] [Google Scholar]
- 21.Korpi-Steiner NL, Williamson EE, Karon BS. Comparison of three whole blood creatinine methods for estimation of glomerular filtration rate before radiographic contrast administration. Am J Clin Pathol. 2009;132(6):920–926. doi: 10.1309/AJCPTE5FEY0VCGOZ. [DOI] [PubMed] [Google Scholar]
- 22.Collier G, Greenan MC, Brady JJ, Murray B, Cunningham SK. A study of the relationship between albuminuria, proteinuria and urinary reagent strips. Ann Clin Biochem. 2009;46 (Pt 3):247–249. doi: 10.1258/acb.2009.008189. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Coresh J, Greene T, et al. Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 25.Winkelmayer WC, Liu J, Brookhart MA. Altitude and all-cause mortality in incident dialysis patients. JAMA. 2009;301(5):508–512. doi: 10.1001/jama.2009.84. [DOI] [PubMed] [Google Scholar]
- 26.Yang CC, Ma MC, Chien CT, Wu MS, Sun WK, Chen CF. Hypoxic preconditioning attenuates lipopolysaccharide-induced oxidative stress in rat kidneys. J Physiol. 2007;582 (Pt 1):407–419. doi: 10.1113/jphysiol.2006.122747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brookhart MA, Schneeweiss S, Avorn J, et al. The effect of altitude on dosing and response to erythropoietin in ESRD. J Am Soc Nephrol. 2008;19(7):1389–1395. doi: 10.1681/ASN.2007111181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS. Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis. 2010;17(3):254–264. doi: 10.1053/j.ackd.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 29.World Bank. [Accessed March 26, 2011];World development indicators: the Google public data page. Available at: http://www.google.com/publicdata?ds=wb-wdi&met=sp_dyn_le00_in&idim=country:NIC&dl=en&hl=en&q=life+expectancy+nicaragua.
- 30.Thow AM, Hawkes C. The implications of trade liberalization for diet and health: a case study from Central America. Global Health. 2009;5(1):5. doi: 10.1186/1744-8603-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


