Patients with the most common aggressive AIDS-related non-Hodgkin lymphomas can expect improved outcomes approaching those of immunocompetent patients in the contemporary era. When treated with appropriate antilymphoma therapy, these improvements apply to every subgroup of patients, including those with low CD4 counts and high-risk disease.
Keywords: lymphoma, AIDS, HIV, diffuse large B-cell lymphoma, Burkitt lymphoma, IPI
Abstract
Background
We undertook the present analysis to examine the shifting influence of prognostic factors in HIV-positive patients diagnosed with aggressive non-Hodgkin lymphoma (NHL) over the last two decades.
Patients and methods
We carried out a pooled analysis from an existing database of patients with AIDS-related lymphoma. Individual patient data had been obtained prior from prospective phase II or III clinical trials carried out between 1990 until 2010 in North America and Europe that studied chemo(immuno)therapy in HIV-positive patients diagnosed with AIDS-related lymphomas. Studies had been identified by a systematic review. We analyzed patient-level data for 1546 patients with AIDS-related lymphomas using logistic regression and Cox proportional hazard models to identify the association of patient-, lymphoma-, and HIV-specific variables with the outcomes complete response (CR), progression-free survival, and overall survival (OS) in different eras: pre-cART (1989–1995), early cART (1996–2000), recent cART (2001–2004), and contemporary cART era (2005–2010).
Results
Outcomes for patients with AIDS-related diffuse large B-cell lymphoma and Burkitt lymphoma improved significantly over time, irrespective of baseline CD4 count or age-adjusted International Prognostic Index (IPI) risk category. Two-year OS was best in the contemporary era: 67% and 75% compared with 24% and 37% in the pre-cART era (P < 0.001). While the age-adjusted IPI was a significant predictor of outcome in all time periods, the influence of other factors waxed and waned. Individual HIV-related factors such as low CD4 counts (<50/mm3) and prior history of AIDS were no longer associated with poor outcomes in the contemporary era.
Conclusions
Our results demonstrate a significant improvement of CR rate and survival for all patients with AIDS-related lymphomas. Effective HIV-directed therapies reduce the impact of HIV-related prognostic factors on outcomes and allow curative antilymphoma therapy for the majority of patients with aggressive NHL.
introduction
The introduction of combination antiretroviral therapy (cART) in 1996 drastically altered the natural history of HIV infection and with it the management and prognosis of most patients with AIDS-related lymphoma (ARL) [1–4]. Currently, five classes of antiretroviral agents are available allowing excellent viral suppression in adherent patients [5]. In addition, improved supportive care of HIV-associated morbidity has moved the management of HIV infection into the realm of a chronic disease [6]. Selected patients with ARL can now tolerate appropriately aggressive therapy and achieve outcomes similar to immunocompetent patients with de novo non-Hodgkin lymphoma (NHL) [7–13].
In the pre-cART era, ARL outcomes were universally poor without regard to histology. Poor prognosis was associated with various patient-specific [age, i.v. drug use, performance status (PS)], HIV-specific (history of prior AIDS-defining illnesses, low CD4 count), and lymphoma-specific [stage, lactate dehydrogenase (LDH) level, extranodal disease] factors [14, 15]. Management of ARL has since evolved through different treatment eras, including evaluation of risk-adapted therapy, modifications of CHOP-based (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy, the use of infusional and more dose-intense regimens, the introduction and increasing utilization of rituximab in combination with chemotherapy, and improved supportive care. Outcomes improved in the early cART era, but not uniformly, notably patients with Burkitt or Burkitt-like lymphoma (BL/BLL) histology continued to have a poor prognosis [16]. At the same time, the prognostic significance of tumor-related factors [non-diffuse large B-cell lymphoma (DLBCL) histology and International Prognostic Index (IPI)] was found to be exceedingly important [17].
In order to explore changes in prognostic factors over time on a background of the evolving management of ARL, we analyzed clinical data from over 1500 patients diagnosed with ARL within the last two decades. We previously reported how treatment-factors affected outcome in this group of patients, and our findings supported the use of rituximab, infusional regimens, and concurrent use of cART [18]. Here, we report additional analysis describing the impact of patient-, HIV-, and lymphoma-specific factors. Our objective was to determine how prognostic factors in ARL have evolved over time and to quantify their influence in the contemporary era of effective antiretroviral and lymphoma-directed therapy.
methods
systematic review and data collection
We carried out a systematic review of the literature to identify prospective phase II and III clinical trials conducted in North America and Europe evaluating treatment of HIV-positive adults with newly diagnosed NHL. Details of the systematic review process, data collection, and abstraction have been previously described and can be found in supplementary Figure S1, available at Annals of Oncology online [18]. Briefly, 42 eligible studies were identified and data were available for 19 of the 42 studies. We pooled patient-level data from each study into one dataset, checked for inconsistencies, and reanalyzed centrally. The following variables were utilized in this analysis: date of enrollment, age at enrollment, sex, histological NHL subtype (DLBCL, BL/BLL, or ‘other’ lymphomas), stage, LDH (normal versus elevated), number of involved extranodal sites, baseline ECOG PS, baseline CD4 count (< or ≥50 cells/mm3), history of AIDS-defining events before lymphoma (h/o AIDS; absent versus present), type of chemotherapy, use of rituximab, and concurrent use of cART with chemotherapy. The study was approved by the institutional review board of Albert Einstein College of Medicine.
statistical analysis
Treatment eras were defined by the following enrollment periods each spanning ∼5 years: pre-cART (1989–1995; n = 388), early cART (1996–2000; n = 694), recent cART (2001–2004; n = 282), and contemporary cART era (2005–2010; n = 182). Patient characteristics were compared between pre-cART and cART eras as well as within the cART eras using Pearson χ2 test or Fisher's exact test for categorical variables and ANOVA or Kruskal–Wallis test for continuous variables.
The outcomes examined were complete response (CR) as defined by the original study protocol, progression-free survival (PFS), and overall survival (OS), defined as the time from enrollment to death from any cause (OS) or relapse or death from any cause (PFS). We calculated the age-adjusted IPI (aaIPI) based on the available information on stage, LDH, and PS. Associations of the covariates with the outcomes CR, PFS, and OS were first determined by univariate analysis and then multivariate analysis using logistic regression models for CR and Cox proportional hazard models for PFS and OS, adjusted for the other covariates, including enrollment period, type of chemotherapy, use of rituximab, and concurrent use of cART. We used separate Cox proportional hazard models adjusted for the same covariates to identify significant factors in each treatment era. We then tested for difference in association with OS over enrollment periods by combining all enrollments in one Cox model stratifying on enrollment period while including interaction between enrollment and prognostic factor of interest. OS was estimated and plotted according to the Kaplan–Meier method and stratified according to treatment era and a log-rank test was used to compare survival curves across treatment era. P values <0.05 were considered statistically significant. We used SAS software, version 9.2 (SAS Institute, Inc., SAS Campus Drive, Cary, NC) for statistical analysis.
results
trials and patient characteristics
Our analysis included patient-level data from 1546 patients enrolled on 19 trials. Thirty-one patients (2.0%) had incomplete follow-up data and were excluded from the survival analysis. Characteristics of the included and excluded trials have been published previously [18]. Details can be found in supplementary Appendix (Table S1, available at Annals of Oncology online). Patient, lymphoma, HIV, and treatment characteristics for patients included in the pooled analysis are shown in Table 1. Seventy-five percent (N = 1158) of patients were enrolled since the advent of cART, with the largest cohort enrolled during the early cART era (1996–2000; N = 694). Most were male (84%) and had DLBCL (69%). There was significant heterogeneity in characteristics across the treatment eras. In the later cART eras, patients were older and were more likely to have BL/BLL, intermediate-high or high-risk aaIPI, a prior history of AIDS, and a lower CD4 count at baseline, although the proportion of patients with CD4 <50 cells/mm3 was stable over time (12%–17%).
Table 1.
Patient, lymphoma, HIV, and treatment characteristics as per treatment era
| cART eras |
||||||||
|---|---|---|---|---|---|---|---|---|
| All patients (N = 1546) | Pre-cART (1989–1995) (N = 388) | cART (1996–2010) (N = 1158) | P* | Early (1996–2000) (N = 694) | Recent (2001–2004) (N = 282) | Contemporary (2005–2010) (N = 182) | P** | |
| Age in years, median (range) | 40 (18–76) | 38 (19–67) | 41 (18–76) | <0.001 | 39 (18–73) | 41 (22–76) | 44 (20–74) | <0.001 |
| Gender, n (%) | ||||||||
| Male | 1228 (84) | 343 (89) | 885 (82) | 0.001 | 513 (83) | 216 (77) | 156 (86) | <0.001 |
| Histology, n (%) | ||||||||
| DLBCL | 1059 (69) | 282 (73) | 777 (67) | 477 (69) | 220 (78) | 80 (44) | ||
| BL/BLL | 399 (26) | 97 (25) | 302 (26) | 0.003 | 146 (21) | 55 (20) | 101 (55) | <0.001 |
| Othera | 88 (5) | 9 (2) | 79 (7) | 71 (10) | 7 (2) | 1 (1) | ||
| Age-adjusted IPI, n (%) | ||||||||
| 0/1 | 535 (41) | 147 (42) | 388 (41) | 0.63 | 259 (44) | 78 (34) | 51 (36) | 0.03 |
| 2/3 | 769 (59) | 202 (58) | 567 (59) | 325 (56) | 150 (66) | 92 (64) | ||
| HIV characteristics | ||||||||
| CD4 count, median cells/mm3 (IQR) | 248 (551) | 425 (1480) | 229 (412) | <0.001 | 258 (670) | 184 (242) | 217 (267) | <0.001 |
| Concurrent cART, n (%) | 779 (52) | 0 | 779 (70) | <0.001 | 453 (67) | 201 (72) | 125 (80) | <0.001 |
| CD4 <50 cells/mm3, n (%) | 207 (14) | 46 (12) | 161 (15) | 0.18 | 89 (14) | 46 (17) | 26 (15) | 0.34 |
| Prior history of AIDS, n (%) | 480 (38) | 118 (32) | 362 (40) | 0.013 | 215 (41) | 79 (34) | 68 (46) | 0.041 |
| Treatment characteristics, n (%) | ||||||||
| Rituximab | 542 (35) | 0 | 542 (47) | <0.001 | 140 (20) | 233 (83) | 169 (93) | <0.001 |
| Dose-intense regimensb | 313 (20) | 107 (28) | 206 (18) | <0.001 | 95 (13) | 24 (9) | 87 (48) | <0.001 |
| CHOP | 632 (41) | 122 (31) | 510 (44) | 346 (50) | 129 (46) | 35 (19) | ||
| Infusional regimensc | 357 (23) | 24 (6) | 333 (28) | 144 (20) | 129 (46) | 60 (33) | ||
| Less-dose-intense regimensd | 244 (16) | 145 (35) | 109 (9) | 109 (16) | 0 | 0 | ||
*P values compare pre- and cART era.
P values compare within cART eras.
a‘Other histologies’ include unknown (N = 4), non-classifiable (N = 56), other-not otherwise specified (N = 23), anaplastic large-cell lymphoma (N = 4), and lymphoplasmacytoid lymphoma (N = 1).
bGMALL, LMB86, LAL3/97, ACVBP and LNHIV regimens—details as in supplementary Table S1, available at Annals of Oncology online.
cEPOCH (etoposide, prednisone, vincristine, doxorubicin, and cyclophosphamide) and CDE (cyclophosphamide, doxorubicin, etoposide).
dLow-dose or dose-modified CHOP, VS (vincristine and steroids), oral ‘Remick’ regimen (oral lomustine, etoposide, cyclophosphamide, procarbazine).
cART, combination antiretroviral therapy; DLBCL, diffuse large B-cell lymphoma; BL, Burkitt lymphoma; BLL, Burkitt-like lymphoma; IPI, International Prognostic Index; IQR, interquartile range; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; VS, vincristine and steroid.
There was also significant heterogeneity in HIV and lymphoma-directed treatment strategies across the enrollment periods. Concurrent cART usage increased over time reaching 80% in the contemporary era. CHOP was the most common chemotherapy regimen overall, with infusional (EPOCH: etoposide, prednisone, vincristine, doxorubicin, and cyclophosphamide, CDE: cyclophosphamide, doxorubicin, and etoposide) and intensive regimens investigated in more recent years. Rituximab use was sparse during the early cART era (20%) with increased utilization in the recent cART (83%) and contemporary (93%) eras.
patient- and lymphoma-specific factors (histology and aaIPI) and outcomes
Multivariate analysis (adjusted for age, sex, histology, aaIPI, CD4 count, prior history of AIDS, use of cART, enrollment period, chemotherapy regimen and use of rituximab) correlating prognostic factors with treatment outcomes (CR, PFS, and OS) for all 1546 patients is shown in Table 2. Neither age nor sex was associated with any outcome. BL/BLL histology compared with DLBCL was associated in both uni- and multivariate analysis with a reduced CR rate [OR 0.71; 95% confidence interval (CI) 0.48–1.07; P = 0.10], shortened PFS [hazard ratio (HR) 1.36; 95% CI 1.06–1.75; P = 0.02] and OS (HR 1.18; 95% CI 0.94–1.47; P = 0.16), although this was only statistically significant for PFS. In addition, in multivariate analysis, histology other than DLBCL or BL/BLL was associated with lack of CR (OR 0.39; 95% CI 0.15–0.99; P = 0.047) and worse PFS (HR 1.61; 95% CI 0.93–2.62) and OS (HR 1.17; 95% CI 0.71–1.83), although the association was not statistically significant for survival outcomes. In contrast, in both univariate and multivariate models, each 1-point increase in aaIPI (range 0–3) was strongly associated with inferior clinical outcomes (P < 0.001 for all measures): CR (OR 0.48; 95% CI 0.40–0.58), PFS (HR 1.58; 95% CI 1.40–1.80), and OS (HR 1.52; 95% CI 1.37–1.69).
Table 2.
Associations of patient, lymphoma, and HIV factors with treatment outcomes for all 1546 patients
| Univariate |
Multivariatea |
|||||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI; P) | Hazard ratio (95% CI; P) |
Odds ratio (95% CI; P) | Hazard ratio (95% CI; P) |
|||
| CR | PFS | OS | CR | PFS | OS | |
| Patient factors | ||||||
| Ageb | 1.01 (1.00–1.02; 0.23) | 0.99 (0.98–1.00; 0.099) | 1.00 (0.99–1.01; 0.88) | 0.99 (0.97–1.01; 0.17) | 1.00 (0.99–1.01; 0.90) | 1.01 (1.00–1.02; 0.08) |
| Sexc | 1.01 (0.75–1.36; 0.93) | 0.88 (0.70–1.09; 0.25) | 0.89 (0.74–1.07; 0.21) | 0.80 (0.52–1.24; 0.32) | 1.07 (0.81–1.40; 0.63) | 1.08 (0.85–1.36; 0.53) |
| Lymphoma factors | ||||||
| Histology | ||||||
| DLBCL | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| BL/BLL | 1.04 (0.80–1.34; 0.076) | 1.22 (1.02–1.45; 0.03) | 0.93 (0.80–1.09; 0.39) | 0.71 (0.48–1.07; 0.10) | 1.36 (1.06–1.74; 0.02) | 1.18 (0.94–1.47; 0.16) |
| Otherd | 0.84 (0.53–1.35; 0.49) | 1.38 (.098–1.88; 0.053) | 1.21 (0.89–1.61; 0.20) | 0.39 (0.15–0.99; 0.047) | 1.61 (0.93–2.62; 0.07) | 1.17 (0.71–1.83; 0.52) |
| aaIPIe | 0.57 (0.50–0.66; <0.001) | 1.35 (1.22–1.49; <0.001) | 1.39 (1.28–1.51; <0.001) | 0.48 (0.40–0.58; <0.001) | 1.58 (1.40–1.80; <0.001) | 1.52 (1.37–1.69; <0.001) |
| HIV factors | ||||||
| CD4 count <50 cells/mm3 | 0.50 (0.37–0.69; <0.001) | 1.37 (1.08–1.70; 0.006) | 1.85 (1.54–2.22; <0.001) | 0.42; (0.26–0.67; <0.001) | 0.76 (0.56–1.05; 0.09) | 1.78; (1.38–2.27; <0.001) |
| Prior history of AIDS | 0.54 (0.42–0.68; <0.001) | 1.05 (0.88–1.26; 0.57) | 1.28 (1.10–1.49; 0.001) | 0.66 (0.45–0.96; 0.03) | 1.16 (0.91–1.48; 0.23) | 1.25 (1.01–1.53; 0.04) |
aAll estimates in the multivariate analysis were adjusted for rituximab use, treatment, concurrent use of cART, age, sex, histological subtype, age-adjusted international prognostic index, CD4 count at baseline, prior history of AIDS, and enrollment period.
bAssociations are determined per each additional year.
cReference variable is male.
d‘Other’ histologies include unknown (N = 4), non-classifiable (N = 56), other-not otherwise specified (N = 23), anaplastic large-cell lymphoma (N = 4), and lymphoplasmacytoid lymphoma (N = 1).
eAssociations are determined per 1 point increase in score (range 0–3).
CR, complete response; PFS, progression-free survival; OS, overall survival; DLBCL diffuse large B-cell lymphoma; BL, Burkitt lymphoma; BLL, Burkitt-like lymphoma; aaIPI, age-adjusted International Prognostic Index.
HIV-specific factors (prior AIDS and CD4 <50) and outcomes
Factors correlating with advanced HIV infection were also associated with significantly inferior clinical outcomes. In univariate analysis, an AIDS-defining illness before lymphoma was associated with lack of CR and worse OS, but not with PFS. These associations remained significant in multivariate analysis (OR for CR: 0.66; 95% CI 0.45–0.96; P = 0.031, and HR for OS: 1.25, 95% CI 1.01–1.53; P = 0.036). Similarly, patients with baseline CD4 <50 cells/mm3 had inferior CR rates (OR 0.42; 95% CI 0.26–0.67; P < 0.001) and OS (HR for CD4 <50: 1.78; 95% CI 1.38–2.27; P < 0.001) in both univariate and multivariate analysis. In univariate analysis, CD4 <50 cells/mm3 was also associated with inferior PFS (HR 1.37, 95% CI 1.08–1.70; P = 0.006) but not significantly after multivariate adjustment (P = 0.09).
treatment eras and outcomes
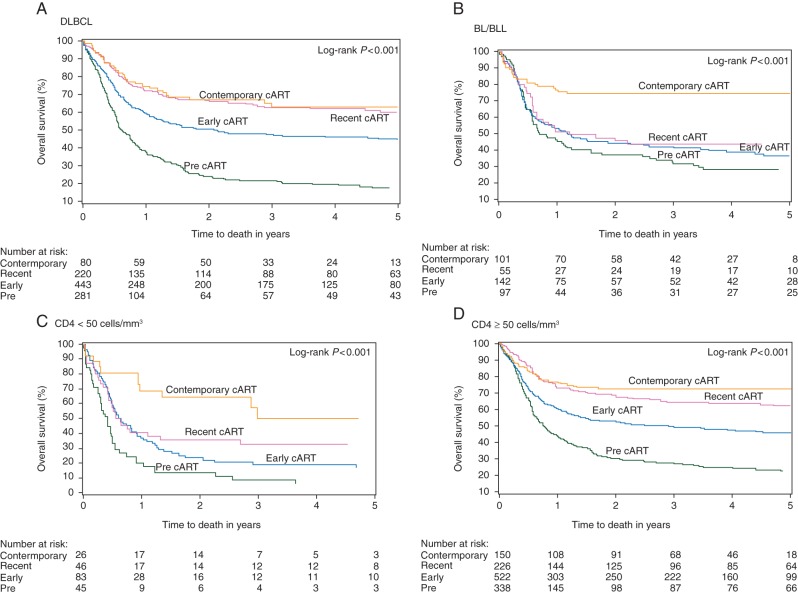
We then compared treatment outcomes both between pre-cART and cART era as well as within the cART era (early, recent, contemporary). Treatment outcomes (CR, PFS, and OS) significantly improved both for patients with DLBCL and BL/BLL throughout successive treatment eras since the introduction of cART (Table 3 and supplementary Table S2, available at Annals of Oncology online; Figure 1A and B). Compared with the pre-cART era patients with DBCL in the cART era had improved CR (47%–61%), 2-year PFS (43%–65%), and 2-year OS (24%–57%), with the most substantive gains in the contemporary era (CR 65%, 2-year PFS 76%, 2-year OS 67%). The corresponding gain in CR rates for patients with BL/BLL in the cART era was less pronounced (56% pre-cART to 59% cART) but reached 78% in the contemporary era. Patients with BL/BLL also had improvements in 2-year PFS (46%–56%) and 2-year OS (37%–55%). Whereas substantial survival gains were first observed for patients with DLBCL in the recent era, they were delayed for patients with BL/BLL until the contemporary era. Treatment outcomes also improved for patients with CD4 counts <50 (except PFS) and ≥50 cells/mm3 in the cART era (Figure 1C and D) and for patients with all aaIPI risk groups (except for CR in patients with low-risk aaIPI; see supplementary Table S2 and Figure S2, available at Annals of Oncology online). Improvement in survival was most substantial for patients with CD4 <50 cells/mm3 enrolled in the contemporary era when the 2-year OS rate reached 65% from only 16% in the pre-cART era. The best outcomes were seen among patients with low (aaIPI = 0) aaIPI in the contemporary era when 2-year OS was 100%. Although CR rates also improved for high-risk (aaIPI = 3) patients from pre-cART into the contemporary era (25%–51%), survival remained poor (median OS 1.01 years; 2-year OS 49%). See supplementary Table S2, available at Annals of Oncology online, for results on CR rate and PFS within the cART eras and supplementary Figure S2, available at Annals of Oncology online, which provides KM curves according to aaIPI risk groups.
Table 3.
Two-year overall survival according to enrollment period
| 2-year overall survival (%, 95% CI) |
|||||
|---|---|---|---|---|---|
| Pre-cART (1990–1995) (N = 388) | cART (1996–2010) (N = 1158) | Early (1996–2000) (N = 694) | Recent (2001–2004) (N = 282) | Contemporary (2005–2010) (N = 182) | |
| Histology | |||||
| DLBCL | 24 (19–30) | 57 (53–61) | 51 (46–55) | 67 (60–73) | 67 (56–76) |
| BL/BLL | 37 (28–47) | 55 (49–60) | 45 (36–53) | 46 (32–58) | 75 (65–82) |
| CD4 count | |||||
| <50 cells/mm3 | 13 (5–25) | 34 (27–42) | 24 (15–34) | 36 (22–50) | 65 (43–80) |
| ≥50 cells/mm3 | 30 (25–35) | 60 (56–63) | 53 (48–57) | 68 (61–74) | 73 (65–79) |
| aaIPI | |||||
| Low | 57 (41–70) | 74 (64–82) | 65 (51–76) | 86 (55–96) | 100 (100–100) |
| Intermediate | 27 (22–33) | 58 (54–62) | 51 (46–56) | 68 (60–75) | 77 (66–85) |
| High | 15 (8–25) | 40 (33–48) | 29 (20–39) | 51 (37–63) | 49 (34–63) |
cART, combination antiretroviral therapy; DLBCL, diffuse large B-cell lymphoma; BL, Burkitt lymphoma; BLL, Burkitt-like lymphoma; aaIPI, age-adjusted International Prognostic Index.
Figure 1.
Kaplan–Meier plots comparing OS for patients enrolled on trials in different treatment eras. Patients with AIDS-related lymphomas who had (A) DLCBL, (B) BL/BLL, (C) CD4 <50 cells/mm3 or (D) CD4 ≥50 cells/mm3 achieved significantly longer overall survival when treated in later treatment eras.
prognostic factors and treatment era
The significant prognostic factors identified in Table 2 (histological subtype, aaIPI, prior history of AIDS and CD4 <50) pertain to the entire cohort of 1546 patients analyzed ‘en masse’ throughout the study period. Given substantial improvements in outcomes observed over time, we then sought to determine whether the relevance of prognostic factors was influenced by enrollment period even within the cART era. Table 4 provides the HRs for OS for the relevant prognostic factors stratified by enrollment in the pre-cART (1989–1995), early cART (1996–2000), recent cART (2001–2004), and contemporary eras (2005–2010), showing how significant risk factors for death differed across time periods studied. Although not associated with OS in the whole-group analysis, BL/BLL histology when compared with DLBCL correlated with improved OS in the pre-cART era (HR 0.71; 95% CI 0.53–0.95; P = 0.02), and worse OS only in the recent cART era (HR 2.11; 95% CI 1.22–3.67; P = 0.008). Also not previously identified as a significant prognostic factor, increasing age was associated with worse OS in the pre-cART (HR 1.02; 95% CI 1.01–1.04; P < 0.01) and again in the contemporary era (HR 1.05; 95% CI 1.00–1.10; P = 0.035).
Table 4.
Factors associated with overall survival according to enrollment period
| HR for overall survivala (95% CI; P) |
||||
|---|---|---|---|---|
| Pre-cART (1989–1995) (N = 388) | Early cART (1996–2000) (N = 694) | Recent cART (2001–2004) (N = 282) | Contemporary (2005–2010) (N = 182) | |
| Patient factors | ||||
| Ageb | 1.02 (1.01–1.04; <0.01) | 0.99 (0.98–1.01; 0.34) | 0.99 (0.95–1.02; 0.37) | 1.05 (1.00–1.10; 0.035) |
| Sexc | 1.20 (0.82–1.76; 0.34) | 0.85 (0.61–1.18; 0.33) | 1.29 (0.75–2.20; 0.36) | 1.54 (0.67–3.55; 0.31) |
| Lymphoma factors | ||||
| Histology | ||||
| DLBCL | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| BL/BLL | 0.71 (0.53–0.95; 0.02) | 0.95 (0.71–1.29; 0.75) | 2.11 (1.22–3.67; 0.008) | 0.59 (0.31–1.14; 0.11) |
| Other | 0.74 (0.30–1.83; 0.52) | 1.54 (0.91–2.61; 0.10) | 3.36 (0.76–14.77; 0.11) | ND |
| aaIPId | 1.54 (1.34–1.78; <0.0001) | 1.49 (1.28–1.74; <0.0001) | 1.52 (1.11–2.07; 0.0089) | 2.34 (1.54–3.56; 0.0001) |
| HIV factors | ||||
| CD4 <50 cells/mm3 | 1.33 (0.89–2.00; 0.17) | 1.79 (1.23–2.56; 0.002) | 2.77 (1.49–5.00; 0.001) | 1.53 (0.71–3.33; 0.27) |
| Prior history of AIDS | 1.25 (0.94–1.66; 0.12) | 1.31 (1.00–1.70; 0.047) | 1.59 (0.96–2.63; 0.07) | 0.74 (0.38–1.42; 0.36) |
ND indicates not determined as too few patients.
aAll estimates in the multivariate analysis were adjusted for rituximab use, treatment, concurrent use of cART, age, sex, histological subtype, age-adjusted international prognostic index, CD4 count at baseline, prior history of AIDS, and enrollment period.
bReference variable is male.
cHR are determined per each additional year.
dHR are determined per 1 point increase in score (range 0–3).
The prognostic significance of HIV-specific factors was limited to the early and recent cART eras. Thus, baseline CD4 <50 cells/mm3 was associated with worse OS during early and recent cART but was not significant in neither pre-cART nor contemporary era. Similarly, a prior history of AIDS increased the risk of death only in the early cART era and with borderline significance during recent cART. In contrast, the aaIPI was significantly associated with inferior survival in each treatment era, with the greatest magnitude in the contemporary era (HR 2.34; 95% CI 1.54–3.56; P < 0.0001). There was no significant interaction between each prognostic factor and enrollment.
discussion
This pooled analysis of patient-level data from 1546 patients with ARL examined outcomes and prognostic factors over 20 years that span pre-cART and cART eras and evolving HIV- and lymphoma-directed therapies. We found significant and continually improving outcomes for patients with ARL through the cART eras, including those with DLBCL and BL/BLL subtypes, irrespective of CD4 count or aaIPI. As previously described by others [14, 17], the significance of important prognostic factors changed over time with the IPI being the only consistent prognostic factor in all time periods.
We also noted significant shifts in patient characteristics of our cohort over time, the most striking of which is an inversion of the histological distribution of lymphomas enrolled into trials: while DLBCL was the most common diagnosis in the pre-cART era (73%), more patients in our cohort were diagnosed with BL/BLL in the contemporary era (55%). While this redistribution could represent a selection bias in our cohort, the observation is supported by other studies. In the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) study, which examined a large general population cohort of HIV-infected patients in the cART era stratified by the same three time periods, a proportional increase in BL was found [19]. Similarly, the HIV/AIDS Cancer Match Study described a decreased incidence of HIV-associated DLBCL and PCNSL but not BL [20].
In general, our findings confirm earlier analyses showing improved outcomes for patients with DLBCL in the cART era and further support extension of these gains for patients with BL/BLL, but only in the contemporary era. Earlier studies described improved survival in the cART era for patients with DLBCL but not for BL/BLL [16, 21]. Such initial failure for BL outcomes to keep pace with DLBCL after cART can likely be attributed to the ineffectiveness of early treatment strategies, especially the use of less intensive CHOP-based regimens [22]. Our findings support more recent evidence showing feasibility and improved outcomes for BL treated with dose-intensive strategies or infusional regimens [10, 11, 23, 24]. In contrast, the CNICS study found ARL mortality to be static across the cART era, and to be inferior to what is expected for HIV-uninfected patients [19]. Whereas our study included only patients enrolled on clinical trials, the poorer outcomes in the CNICS cohort are likely multifactorial: selection bias in our own cohort; the lag time between clinical trial findings and changes to standard of care; and the impact on survival of access to care and various other unaccounted social factors [25]. Nevertheless, our results likely reflect increased utilization of intensive and/or infusional regimens, and of rituximab, which reached 93% in the contemporary era, as well as improved HIV control and supportive care. Interpretation of the results for patients with histologies other than DLBCL or BL/BLL in our analysis is limited by the lack of available information on the exact histological subtypes.
In keeping with improved outcomes and the evolving treatment paradigm of ARL, we found a modification of significant prognostic factors over time. As outcomes improved, tumor-related factors became increasingly important. In our study, the aaIPI is the most important and consistent prognostic factor in patients with ARL with increasing value in the contemporary era. While the validity of the IPI in risk-stratification of patients with ARL has been consistently demonstrated [14, 17, 26–28], there have been variable findings on the relevance of HIV-specific factors, with some demonstrating less importance over time [14, 27] and others failing to demonstrate any prognostic relevance at all [17]. We found that while HIV-specific factors (prior AIDS and CD4 <50 cells/mm3) were associated with poor prognosis in the whole-group analysis, their relevance waxed and waned. HIV-related factors appeared to have less impact on survival in the pre-cART era, when outcomes were universally poor and determined by the inability to deliver effective lymphoma care. The same was observed again in the contemporary era; the most likely explanation for this finding is the high efficiency of contemporary HIV-directed care that enables excellent HIV control for most patients and results in the relative loss of prognostic importance of a single-individual HIV-related factor. Similarly, the relative shift of prognostic influence of histology on outcome in ARL is most likely a reflection of the shifts in lymphoma-directed care over the different eras.
Limitations of our analysis may affect the generalizability of some findings. Selection bias is inherent in clinical trial enrollment and thus the analyzed patient population may not reflect the general population of HIV-infected patients with NHL. Also, unexpectedly high CD4 counts observed in the pre-cART era may further reflect selection bias during that era. Additional bias may arise from our failure to obtain data from all eligible trials, although the majority of these were conducted in the pre-cART era and were of small sample size. While we used patient-level data and were able to control for many confounders, we relied on investigator reports of classifications of response, relapse, and histology. This particularly limits the utility of the outcomes CR and PFS, but should not affect the estimates for OS as this is a variable independent of investigator bias. In addition, missing data may have led to imprecise estimates, and although we adjusted for known variables, other unknown factors over the 20-year time frame may confound results.
On the other hand, several factors strengthen the robustness of this analysis. Previous reports on outcomes and prognostic factors in the cART era were limited by sample size, retrospective analysis, and shorter timeframes. This analysis is the largest and most comprehensive to date, including the majority of patients enrolled on clinical trials of ARL in the cART era at major centers in the United States and Europe, and the first such analysis to include patients treated in the contemporary era. We were able to adjust estimates for all known prognostic factors and important confounding variables, especially treatment, the use of rituximab and enrollment period. The large sample size also allowed subgroup analysis to reveal subtle trends in outcomes and prognostic factors over time.
In conclusion, outcomes have improved dramatically for all patients with ARL, despite a lag in benefit for patients with BL/BLL histology. The importance of HIV-related factors has waned over time and for the two most common subtypes (DLBCL and BL), histology may no longer be an important prognostic factor with current histology-directed treatment approaches. Thus, the aaIPI prevailed as the only consistent prognostic factor. As evidenced by the survival rates, we observed in the contemporary era, cure for HIV-positive patients with NHL can be achieved similarly to immunocompetent patients when adequate antiviral and histology-directed antilymphoma therapy is being utilized. Given this, patients with lymphoma should no longer be excluded from participation in lymphoma clinical trials solely based on their HIV status.
funding
This work was supported in parts by the AIDS Malignancy Consortium (AMC; grant UO1CA232947), the Paul Calabresi Career Development Award for Clinical Oncology (K12CA132783-03 Grant), the ASCO Cancer Foundation 2010 Young Investigator Award, grant RD12/0036/0029 from RTICC, Instituto de Salud Carlos III, Spain, and by the CTSA Grants UL1 RR025750 and KL2 RR025749 and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Noy A. Update in HIV lymphoma. Curr Opin Oncol 2006; 18: 449–455. [DOI] [PubMed] [Google Scholar]
- 2.Sparano JA. HIV-associated lymphoma: the evidence for treating aggressively but with caution. Curr Opin Oncol 2007; 19: 458–463. [DOI] [PubMed] [Google Scholar]
- 3.Levine AM. HIV-associated lymphoma. Blood 2010; 115: 2986–2987. [DOI] [PubMed] [Google Scholar]
- 4.Levine AM. Management of AIDS-related lymphoma. Curr Opin Oncol 2008; 20: 522–528. [DOI] [PubMed] [Google Scholar]
- 5.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA 2012; 308: 387–402. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan LD. HIV-associated lymphoma. Best Pract Res Clin Haematol 2012; 25: 101–117. [DOI] [PubMed] [Google Scholar]
- 8.Oriol A, Ribera J, Esteve J, et al. Lack of influence of human immunodeficiency virus infection status in the response to therapy and survival of adult patients with mature B-cell lymphoma or leukemia. Results of the PETHEMA-LAL3/97 study. Haematologica 2003; 88: 445–453. [PubMed] [Google Scholar]
- 9.Oriol A, Ribera JM, Bergua J, et al. High-dose chemotherapy and immunotherapy in adult Burkitt lymphoma: comparison of results in human immunodeficiency virus-infected and noninfected patients. Cancer 2008; 113: 117–125. [DOI] [PubMed] [Google Scholar]
- 10.Ferreri AJM, Bruno Ventre M, Donadoni G, et al. Safety and activity of a new intensive short-term chemoimmunotherapy in HIV-positive patients with Burkitt lymphoma. Br J Haematol 2012; 159: 252–255. [DOI] [PubMed] [Google Scholar]
- 11.Montoto S, Wilson J, Shaw K, et al. Excellent immunological recovery following CODOX-M/IVAC, an effective intensive chemotherapy for HIV-associated Burkitt's lymphoma. AIDS 2010; 24: 851–856. [DOI] [PubMed] [Google Scholar]
- 12.Coutinho R, Pria AD, Gandhi S, et al. HIV status does not impair the outcome of patients diagnosed with diffuse large B-cell lymphoma treated with R-CHOP in the cART era. AIDS 2014; 28: 689–697. [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein PG, Aboulafia DM, Zloza A. Malignancies in HIV/AIDS: from epidemiology to therapeutic challenges. AIDS 2014; 28: 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim ST, Karim R, Tulpule A, et al. Prognostic factors in HIV-related diffuse large-cell lymphoma: before versus after highly active antiretroviral therapy. J Clin Oncol 2005; 23: 8477–8482. [DOI] [PubMed] [Google Scholar]
- 15.Straus DJ, Huang J, Testa MA, et al. Prognostic factors in the treatment of human immunodeficiency virus-associated non-Hodgkin's lymphoma: analysis of AIDS Clinical Trials Group protocol 142—low-dose versus standard-dose m-BACOD plus granulocyte-macrophage colony-stimulating factor. National Institute of Allergy and Infectious Diseases. J Clin Oncol 1998; 16: 3601–3606. [DOI] [PubMed] [Google Scholar]
- 16.Lim ST, Karim R, Nathwani BN, et al. AIDS-related Burkitt's lymphoma versus diffuse large-cell lymphoma in the pre-highly active antiretroviral therapy (HAART) and HAART eras: significant differences in survival with standard chemotherapy. J Clin Oncol 2005; 23: 4430–4438. [DOI] [PubMed] [Google Scholar]
- 17.Miralles P, Berenguer J, Ribera JM, et al. Prognosis of AIDS-related systemic non-Hodgkin lymphoma treated with chemotherapy and highly active antiretroviral therapy depends exclusively on tumor-related factors. J Acquir Immune Defic Syndr 2007; 44: 167–173. [DOI] [PubMed] [Google Scholar]
- 18.Barta SK, Xue X, Wang D, et al. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood 2013; 122: 3251–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopal S, Patel MR, Yanik EL, et al. Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J Natl Cancer Inst 2013; 105: 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiels MS, Engels EA, Linet MS, et al. The epidemic of non-Hodgkin lymphoma in the United States: disentangling the effect of HIV, 1992–2009. Cancer Epidemiol Biomarkers Prev 2013; 22: 1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spina M, Simonelli C, Talamini R, Tirelli U. Patients with HIV with Burkitt's lymphoma have a worse outcome than those with diffuse large-cell lymphoma also in the highly active antiretroviral therapy era. J Clin Oncol 2005; 23: 8132–8133; author reply 8133–. [DOI] [PubMed] [Google Scholar]
- 22.Dunleavy K, Wilson WH. How I treat HIV-associated lymphoma. Blood 2012; 119: 3245–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noy A, Kaplan L, Lee JY. A modified dose intensive R- CODOX-M/IVAC for HIV-associated Burkitt and Atypical Burkitt Lymphoma (BL) demonstrates high cure rates and low toxicity: prospective multicenter phase II trial of the AIDS Malignancy Consortium (AMC 048). Blood 2013; 122: 639 (abstract). [Google Scholar]
- 24.Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt's lymphoma. N Engl J Med 2013; 369: 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunleavy K, Wilson WH. Implications of the shifting pathobiology of AIDS-related lymphoma. J Natl Cancer Inst 2013; 105: 1170–1171. [DOI] [PubMed] [Google Scholar]
- 26.Navarro JT, Ribera JM, Oriol A, et al. International prognostic index is the best prognostic factor for survival in patients with AIDS-related non-Hodgkin's lymphoma treated with CHOP. A multivariate study of 46 patients. Haematologica 1998; 83: 508–513. [PubMed] [Google Scholar]
- 27.Mounier N, Spina M, Gabarre J, et al. AIDS-related non-Hodgkin lymphoma: final analysis of 485 patients treated with risk-adapted intensive chemotherapy [see comment]. Blood 2006; 107: 3832–3840. [DOI] [PubMed] [Google Scholar]
- 28.Bower M, Gazzard B, Mandalia S, et al. A prognostic index for systemic AIDS-related non-Hodgkin lymphoma treated in the era of highly active antiretroviral therapy. Ann Intern Med 2005; 143: 265–273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.