Abstract
Streptococcus agalactiae (group B streptococci (GBS)) is an important infections agent in newborns associated with maternal vaginal colonization. Intrapartum antibiotic prophylaxis in GBS-colonized pregnant women has led to a significant reduction in the incidence of early neonatal infection in various geographic regions. However, this strategy may lead to resistance selecting among GBS, indicating the need for new alternatives to prevent bacterial transmission and even to treat GBS infections. This study reported for the first time the effect of eugenol on GBS isolated from colonized women, alone and in combination with silver nanoparticles produced by Fusarium oxysporum (AgNPbio). Eugenol showed a bactericidal effect against planktonic cells of all GBS strains, and this effect appeared to be time-dependent as judged by the time-kill curves and viability analysis. Combination of eugenol with AgNPbio resulted in a strong synergistic activity, significantly reducing the minimum inhibitory concentration values of both compounds. Scanning and transmission electron microscopy revealed fragmented cells and changes in bacterial morphology after incubation with eugenol. In addition, eugenol inhibited the viability of sessile cells during biofilm formation and in mature biofilms. These results indicate the potential of eugenol as an alternative for controlling GBS infections.
1. Introduction
Streptococcus agalactiae (Group B Streptococcus (GBS)) is an important cause of invasive diseases, mainly in newborns, pregnant women, and elderly individuals [1, 2]. Neonatal early onset diseases, which are characterized by sepsis, pneumonia, or meningitis, are strongly associated with maternal vaginal colonization and may occur vertically by aspiration of infected amniotic fluid or during passage through the birth canal [3]. It is estimated that 10–37% of pregnant women are colonized with GBS, and in the absence of any intervention, 30–70% of newborns become colonized, of which about 1–3% develop invasive diseases with high mortality rates [3, 4].
The active prevention strategy for GBS neonatal infections based on bacterial screening and intrapartum antibiotic prophylaxis (IAP) has led to a significant reduction in the incidence of early neonatal infection in various regions of the world. Current IAP consists of intravenous administration of beta-lactams (penicillin or ampicillin) to GBS-colonized pregnant woman for 4 h before delivery. Erythromycin, clindamycin, and vancomycin are the agents recommended in cases of colonization during pregnancy with beta-lactam-resistant GBS or beta-lactam allergy [4].
In general, GBS isolates have been shown to be sensitive to beta-lactams [5–7]. However, GBS isolates with reduced susceptibility and even resistance to beta-lactam agents have been reported in recent years [8, 9]. Moreover, resistance to erythromycin and clindamycin among GBS isolates has emerged in different regions of the world, with rates ranging from 14.5% to 70% and 8.2% to 70% for erythromycin and clindamycin, respectively [10–12].
Besides the emergence of GBS resistant to current antibiotics, the development of vaccines against GBS is still in progress, indicating that new safe and effective alternatives should be developed to prevent the transmission of this bacterium. Several authors have described the antibacterial activity of natural products against GBS [13–15], indicating their potential as alternatives for IAP, reducing the risk of development of resistance to commercially available antibiotics.
Eugenol, a major constituent of essential oils extracted from various plants, has been widely studied due to its medicinal properties, such as the following: antibacterial [16–18], antifungal [19], antiviral [20], antileishmanial [21], antioxidant [22], anticarcinogenic [23], and analgesic and anti-inflammatory [24]. Similarly, silver nanoparticles have been extensively investigated because of their antimicrobial properties alone [25, 26] or in combination with other compounds [27–29].
In this study, we evaluated the antibacterial effect of the phenylpropanoid eugenol on planktonic cells and biofilm of GBS isolated from colonized women, including those exhibiting resistance to erythromycin and clindamycin and its synergistic interaction with silver nanoparticles produced by Fusarium oxysporum (AgNPbio).
2. Material and Methods
2.1. Bacteria and Growth Conditions
Six nonduplicates strains of Streptococcus agalactiae (GBS) recovered from vaginal-rectal swabs of colonized women were taken from the bacterial collection of the Laboratory of Clinical Microbiology of University Hospital of Londrina, Paraná, Brazil. Bacterial strains were included according to phenotypic and genotypic characteristics [11] (Table 1). Bacteria were kept at −20°C in tryptic soy broth (TSB, Oxoid) containing 20% glycerol and 5% sheep blood. The reference strain S. agalactiae ATCC 13813 (kindly donated by FIOCRUZ, Rio de Janeiro, Brazil) was also included in this study. Bacteria were grown in TSB, pH 6.5, at 37°C for 24 h before the assays. The study protocol was approved by the Ethics Committee of the Universidade Estadual de Londrina (document 186/09-CEP/UEL). Written informed consent was obtained from the patients for the publication of this report and any accompanying images.
Table 1.
Phenotypic and genotypic characteristics and antibacterial susceptibility profile of eugenol for planktonic and sessile cells of Streptococcus agalactiae.
|
GBS strains |
MLVA genotypesa | Capsular typesa | E (µg/mL) | DA (µg/mL) | Antimicrobial resistance genesa | Eugenol (%) | ||
|---|---|---|---|---|---|---|---|---|
| MICb | MICb/MBCc | SMIC100 d | SMIC50 e | |||||
| 50 | 8 | Ia | 0.25 | 0.25 | — | 0.5 | 0.25 | 0.09 |
| 72 | 5 | III | 0.125 | 0.25 | — | 0.125 | 0.25 | 0.06 |
| 80 | 6 | V | 0.25 | 0.125 | — | 0.25 | 0.125 | 0.05 |
| 89 | 13 | Ia | 0.25 | 0.125 | — | 0.25 | 0.25 | 0.09 |
| 115 | 7 | V | >1024 | 1024 | ermB | 0.125 | 0.5 | 0.02 |
| 121 | 8 | Ia | 16 | 0.125 | mefA/E | 0.125 | 0.5 | 0.25 |
| ATCC 13813 | — | — | 0.125 | 0.125 | — | 0.25 | 0.06 | 0.04 |
aThe genetic diversity, the capsular type, and the resistance genes were previously determined by Otaguiri et al. [11]. bMinimum inhibitory concentration (MIC) of the compound which resulted in total inhibition of visible planktonic cell growth defined according to CLSI (2012) [31] guidelines by broth microdilution assays. cMinimum bactericidal concentration (MBC) of the eugenol. dSessile MIC (SMIC) of the eugenol which resulted in total reduction in metabolic activity of sessile cells during biofilm formation, using the XTT-reduction assay, after 24 h. eSMIC of the eugenol which resulted in 50% of reduction in metabolic activity of sessile cells from mature biofilm (24 h), using the XTT-reduction assay. MLVA: multiple locus variable number of tandem repeat analysis; E: erythromycin; DA: clindamycin.
2.2. Eugenol and Biologically Synthesized Silver Nanoparticles
A stock solution of 10% eugenol (SSWhite, Brazil) was prepared in TSB, pH 6.5, containing 10% (v/v) dimethyl sulfoxide (DMSO; Sigma Chemical Co., USA). The DMSO final concentration in the assays did not exceed 1.0%. Biological silver nanoparticles (AgNPbio) were obtained after AgNO3 reduction by F. oxysporum as previously described [30]. Briefly, F. oxysporum was cultured in broth medium containing 0.5% yeast extract at 28°C for 6 days. The fungal biomass was filtered, added (approximately 10 g) to 100 mL of distilled water, and incubated at 28°C for 72 h. A fungal-free solution was obtained by filtration and mixed with 1.0 mM AgNO3 and the mixture was kept at 28°C for 28 h. AgNPbio were purified and characterized by scanning electron microscopy and energy-dispersive spectroscopy.
2.3. Antibacterial Activity of Eugenol against Planktonic Cells
The minimal inhibitory concentration (MIC) of eugenol was determined by broth microdilution method according to the Clinical and Laboratory Standards Institute [31], with modifications. Twofold serial dilutions of eugenol (1–0.015%) in TSB were prepared in sterile polystyrene 96-well plates (Techno Plastic Products, Switzerland). Wells contained medium alone or medium plus 1% DMSO and untreated planktonic cells, and medium alone served as growth and sterility controls. After 24 h incubation, optical density was measured at 600 nm with a microtiter plate reader (Synergy HT, Biotek) and MIC was determined at total inhibition of growth after 24 h incubation compared to untreated planktonic cells [32]. MICs for erythromycin and clindamycin were also determined as described above, and Streptococcus pneumoniae ATCC 49619 was used as the quality control. To determine the minimal bactericidal concentration (MBC), the content from the wells (10 μL) showing no growth was transferred to plates with tryptic soy agar (TSA, Oxoid) containing 5% sheep blood and incubated at 37°C for 24 h. MBC was defined as 100% decrease in colony forming units (CFU) compared to untreated bacteria. All assays were carried out in triplicate on two different occasions.
2.4. Time-Kill Curves
For time-kill curve analysis, planktonic cells of GBS 89, GBS 121, and S. agalactiae ATCC 13813 (1–5 × 105 CFU/mL) were incubated in TSB containing MIC levels of eugenol. At determined time points (0, 1, 2, 4, 6, 8, 10, and 24 h), aliquots were aseptically transferred to TSA plus 5% sheep blood plates and the CFU counts were determined after incubation at 37°C for 24 h. The bacterial viability of eugenol-treated GBS was also evaluated, using LIVE/DEAD BacLight staining kit (Molecular Probes, Invitrogen) according to the manufactures' recommendations. This assay is based on the detection of two nucleic acid fluorescent stains. Green-fluorescent SYTO 9 labels live and dead bacteria, whereas the red fluorescent propidium iodide selectively labels bacteria with permeable (damaged) membranes. Bacterial cells were placed on a glass coverslip and analyzed by fluorescence microscopy (LEICA DM2000). All assays were carried out in triplicate on three different occasions.
2.5. Antibacterial Activity of Eugenol against Biofilms
Biofilms of GBS strains and S. agalactiae ATCC 13813 were formed on polystyrene surface of flat-bottomed 96-well microtiter plates (Techno Plastic Products, Switzerland) in a static model at 37°C according to Borges et al. [33]. Briefly, bacterial cells were grown in TSB at 37°C for 18 h, harvested by centrifugation, and washed with sterile 0.15 M phosphate-buffered saline (PBS), pH 7.2, and the cell density was adjusted to 1.0 × 108 CFU/mL in the same medium. Twofold serial dilutions of eugenol (2–0.03%) were prepared in TSB. For analysis of eugenol effect during biofilm formation, a 20 μL aliquot of each cell suspension was transferred to each well containing 180 μL of TSB with different concentrations of eugenol, and the plates were incubated for 24 h. For analysis of eugenol effect on mature biofilm, 20 μL of each cell suspension was added to each well containing 180 μL of TSB. After 24 h of biofilm formation, the medium was aspirated off and each well was washed with sterile PBS. A 200 μL aliquot of TSB containing different concentrations of eugenol was added and the plates were incubated for another 24 h. Controls included eugenol-free wells and biofilm-free wells. Sessile (biofilm) minimum inhibitory concentrations were determined at 50 and 100% inhibition (SMIC) compared to eugenol-free control wells using the 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT)-reduction assay [34]. A 200 μL aliquot of XTT-menadione [0.5 mg/mL XTT, 1 mM menadione (Sigma Chemical Co., USA)] was added to each well, and the plates were incubated in the dark at 37°C for 90 min, after which the optical density was measured at 490 nm with a microtiter plate reader (Synergy HT, Biotek). Experiments were carried out in quintuplicate on two different occasions.
2.6. Electron Microscopy Analysis
Planktonic cells treated with MIC levels of eugenol for 5 h were fixed for 2 h at room temperature with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2. For scanning electron microscopy (SEM), postfixed cells were dehydrated with a series of ethanol washes (15, 30, 50, 70, 80, 90, 95, and 100%), critical-point dried in CO2, coated with gold, and examined with a SHIMADZU SS-550 scanning electron microscope. For transmission electron microscopy (TEM), postfixation was carried out in 1% osmium tetroxide in cacodylate buffer containing 0.8% potassium ferrocyanide and 5 mM CaCl2 at room temperature for 1 h. The cells were dehydrated in acetone and embedded in Epon resin. Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Zeiss EM900 electron microscope.
2.7. Analysis of the Combinations of Eugenol and AgNPbio
The effect of eugenol combined with AgNPbio on planktonic cells of GBS was assessed by checkerboard method in 96-well microtiter plates, as previously described [35]. Twofold serial dilutions of the compounds were prepared in TSB, and the final concentrations of eugenol and AgNPbio ranged from 1 to 0.015% and 500 to 0.49 μM, respectively. Bacteria (1.0 × 105 cells) were grown with eugenol or AgNPbio individually and in combinations at 37°C for 24 h. The interactions of the two compounds were analyzed by the fractional inhibitory concentration index (FICI), which was defined as the sum of FICeugenol and FICAgNPbio. FIC of the material is the concentration that kills when being used in combination with another divided by the concentration that has same effect when used individually [35].
2.8. Statistical Analysis
The results were evaluated by one-way ANOVA using the GRAPHPAD PRISM version 5.0 (GRAPHPAD Software, San Diego, CA). P < 0.05 was considered significant.
3. Results
3.1. Antibacterial Activity of Eugenol against Planktonic Cells
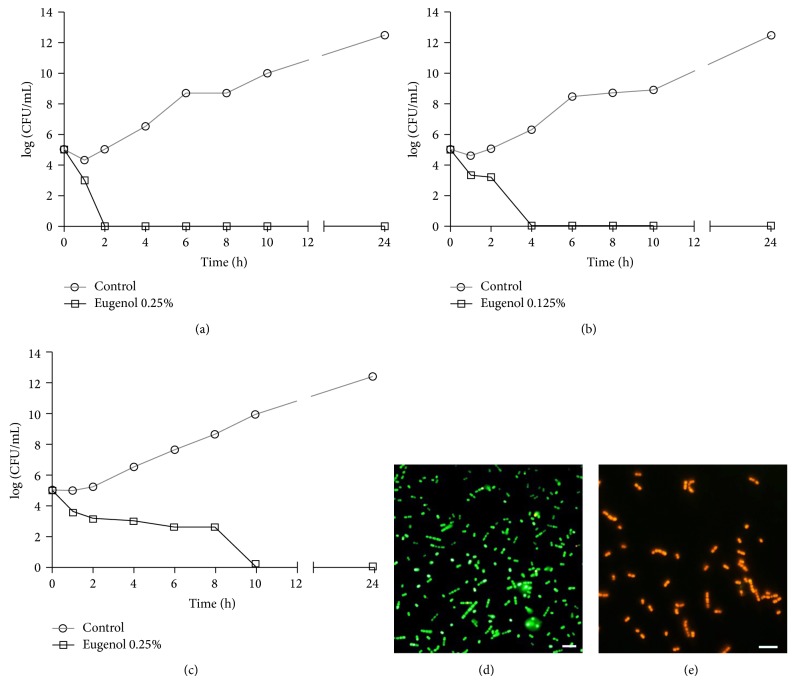
Eugenol inhibited the planktonic growth of all GBS strains, including those resistant to erythromycin and/or clindamycin. MIC and MBC values were exactly at the same concentrations for all GBS strains analyzed in this study. The mean MIC/MBC value was 0.23 ± 0.13% ranging from 0.125 to 0.5% (Table 1). To evaluate the killing kinetics of eugenol against GBS, survival of planktonic cells from GBS 89, GBS 121, and reference strain was assessed during 24 h in the presence of the phenylpropanoid at MIC. As shown in Figure 1, a time-dependent bactericidal effect was observed for all strains. The presence of eugenol at MIC reduced the planktonic cell population approximately by 1 to 2 log10 CFU/mL (P < 0.05) after one hour of incubation. No colony forming units (Figures 1(a), 1(b), and 1(c)) or cellular viability (Figures 1(d) and 1(e)) was detected after 2, 4, and 10 h of incubation in the presence of eugenol for GBS 89, GBS 121, or reference strain. At the specified time, all eugenol-treated GBSs were red-fluorescent, reflecting dead bacteria with damaged membranes, in contrast to green-fluorescent untreated cells.
Figure 1.
Effect of eugenol on growth (a–c) and viability (d-e) of Streptococcus agalactiae. Time-kill curves of GBS 89 (a), GBS 121 (b), and S. agalactiae ATCC 13813 (c) strains: bacteria were incubated with eugenol at MIC for 24 h at 37°C and the CFU counts were determined at specified time points. Viability of the cells was determined with live-dead staining and GBSs with intact membranes were green-fluorescent (d), whereas eugenol-treated GBSs (at MIC) with damaged membranes were red-fluorescent (e). Representative images are shown. Bar = 5 μm.
The combination of eugenol with AgNPbio significantly reduced (P < 0.05) the MIC value of both compounds, and the calculated FICI indicated a synergistic effect between them against all GBS strains (Table 2). AgNPbio alone at 125 μM inhibited the growth of all GBS strains. When the two compounds were combined, the MIC values of eugenol and AgNPbio decreased 4- to 8-fold and 4- to 256-fold, respectively.
Table 2.
In vitro synergistic effect of eugenol and biological silver nanoparticles on Streptococcus agalactiae growth.
| GBS strains | MICa | FICId | Effect | ||
|---|---|---|---|---|---|
| Eugenolb (%) | AgNPbioc (µM) | Eugenol/AgNPbio | |||
| 50 | 0.5 | 125 | 0.06/15.62 | 0.25 | Synergism |
| 72 | 0.125 | 125 | 0.03/31.25 | 0.5 | Synergism |
| 80 | 0.25 | 125 | 0.06/0.49 | 0.25 | Synergism |
| 89 | 0.25 | 125 | 0.03/7.8 | 0.19 | Synergism |
| 115 | 0.25 | 125 | 0.03/31.25 | 0.38 | Synergism |
| 121 | 0.125 | 125 | 0.03/3.9 | 0.281 | Synergism |
| ATCC 13813 | 0.25 | 125 | 0.03/15.62 | 0.25 | Synergism |
aMIC: minimum inhibitory concentration. bMIC of eugenol used alone. cMIC of silver nanoparticle used alone. dFICI: fractional inhibitory concentrations index were calculated according to Yadav et al. [35] and classified as follows: synergistic if FIC ≤0.5, additive if FIC >0.5 and ≤1.0, indifferent if FIC >1.0 and ≤2.0, and antagonistic if FIC >2.0.
3.2. Morphological and Ultrastructural Alterations in Eugenol-Treated Planktonic Cells
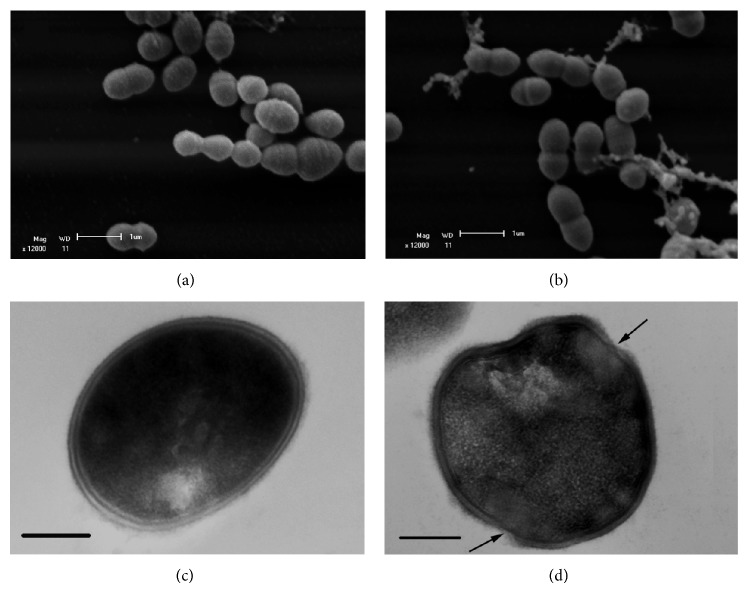
SEM images showed untreated control cells with typical morphology arranged in an organized manner (Figure 2(a)). In contrast, the incubation of GBS planktonic cells with an inhibitory concentration of eugenol for 5 h led to cell lysis resulting in leakage of cytoplasmic contents (Figure 2(b)). Likewise, TEM imaging of control cells showed an intact cell wall with regular electron density (Figure 2(c)) and treated cells displaying various alterations such as changes in bacterium morphology; disruption of cell wall (arrow), corroborating the SEM results; and decrease in electron density (Figure 2(d)).
Figure 2.
Scanning electron microscopy (a and b) and transmission electron microscopy images (c and d) of the effect of eugenol on Streptococcus agalactiae ATCC 13813. Untreated cells (a and c) and treated cells with eugenol 0.25% for 5 h (b and d). Bar: (a and b) = 1 μm, (c and d) = 200 nm.
3.3. Antibacterial Activity of Eugenol in Biofilms
Eugenol inhibited biofilm formation of GBS, and no metabolic activity was detected at concentrations ranging from 0.06 to 0.5%, where these values were considered, SMIC100 (Table 1). In addition, eugenol decreased significantly the viability of mature (24 h) biofilm at concentrations ranging from 0.03 to 2%, with SMIC50 ranging from 0.02 to 0.25% (Table 1). However, except for the reference strain, no total inhibition was observed at the highest concentration tested in this study. At 2% eugenol, reduction in metabolic activity ranged from 60 to 90% for the other GBS strains, where GBS 121 biofilm was the least susceptible.
4. Discussion
Eugenol is a remarkably versatile molecule, which can be incorporated as a functional compound in various products applied not only in the pharmaceutical industry but also in the agricultural, food, and cosmetic industries as well [36].
In this study, the bactericidal activity of eugenol against human isolates of S. agalactiae, including those exhibiting different mechanisms of resistance to erythromycin and/or clindamycin, is reported for the first time. The antibacterial activity of eugenol against planktonic cells of different species of the genus Streptococcus has been previously reported [35, 37–39]. Baskaran et al. [37] reported the bactericidal activity of eugenol against planktonic cells of five agents of bovine mastitis, including S. agalactiae, whose MIC and MBC were 0.4 and 0.8%, respectively.
The bactericidal effect of eugenol on S. agalactiae seems to be dependent on changes in the cell envelope, as judged by alterations in the morphology and ultrastructure observed in treated cells. In fact, other authors reported that eugenol induces cell lysis through protein and lipid leakage, leading to extrusion of cytoplasmic content in various Gram-negative and Gram-positive bacteria, including Streptococcus pyogenes [17, 35, 38]. In addition, eugenol is capable of inhibiting the membrane-bound ATPase activity of Escherichia coli and Listeria monocytogenes [40].
In this study, silver nanoparticles synthesized by an ecofriendly method using the filamentous fungus F. oxysporum showed inhibitory activity against planktonic cells of all GBS strains. Moreover, the synergistic antibacterial interaction of AgNPbio with eugenol against GBS is reported for the first time. Metallic nanoparticles have been widely studied because of their broad spectrum antimicrobial effect, even at low concentrations [25–28]. In addition, the combination of these nanoparticles with several compounds has shown potent antimicrobial activity in different microbial species, including those displaying resistance to conventional antibiotics [26–29]. Accordingly, additive or synergistic effect of essential oil component cinnamaldehyde with chemically synthesized silver nanoparticles against Gram-positive and Gram-negative bacteria has been reported elsewhere [29].
Current antibacterial agents have limited efficacy on biofilms. Sessile (adhered) bacteria in these communities have different physiological characteristics compared to free-floating planktonic cells. Clinically, these features can result in protection against the host immune system and less susceptibility to antimicrobial agents, contributing to persistent infections and difficult treatment [41]. Biofilms are also established during host colonization, enabling the bacteria to withstand removal by mechanical processes [42]. Except for S. agalactiae, the antibiofilm activity of eugenol for various bacterial species has been reported elsewhere [35, 43]. In this study, eugenol also exhibited an antibacterial activity against biofilms of S. agalactiae, showing the ability to inhibit its formation as well as the viability of mature biofilm, under in vitro conditions. Similarly, Yadav et al. [35] reported the inhibitory effect of eugenol against biofilms of S. pneumoniae. Furthermore, Adil et al. [44] described that Streptococcus mutans incubated in the presence of subinhibitory concentrations of eugenol showed decreased expression of genes related to biofilm formation.
A limitation of this study, which may reduce the generalization of the results, is the number of GBS strains. Despite this limitation, the results presented here showed the antibacterial activity of eugenol against S. agalactiae, alone or in combination with AgNPbio, opening a promising strategy for application in IAP for women colonized with this bacterium. In this sense, a variety of evidence supports the application of eugenol in human healthcare. The addition of eugenol and other essential oils in animal feed reduced the Clostridium perfringens load in the gut of broiler chickens. Although this bacterium can be a harmless intestinal inhabitant of chickens, it is the leading agent of necrotic enteritis in these hosts [45]. Topical application of eugenol on teeth reduced the incidence and severity of carious lesions caused by S. mutans in rats [39]. Preliminary in vivo studies have shown the safety and efficacy of topical use of eugenol in combination with thymol in the treatment of bacterial vaginosis and vaginal candidiasis [46]. The incorporation of eugenol in polymeric material has been shown to reduce the biofilm formation on surfaces [43].
5. Conclusion
The results obtained in this study demonstrated the bactericidal activity of eugenol and its synergistic effect with AgNPbio against planktonic cells of S. agalactiae. Furthermore, this compound inhibited biofilm formation and viability of mature biofilm formed on polystyrene.
Acknowledgments
This study was supported by grants from Programa de Pesquisa para o SUS: Gestão Compartilhada em Saúde (PPSUS, Edição 2011)/Fundação Araucária/SESA-PR/MS/CNPq; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Grant no. 402728/2013-0); and Programa de Pós-Graduação em Microbiologia da Universidade Estadual de Londrina. R. P. Biasi-Garbin was funded by a student scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). This work is part of the M.S. dissertation of R. P. Biasi-Garbin. The authors thank Dr. A. Leyva for English editing of the paper and Complexo de Centrais de Apoio à Pesquisa (COMCAP) of Universidade Estadual de Maringá for supporting the electron microscopy analysis.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clinical Microbiology Reviews. 1998;11(3):497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skoff T. H., Farley M. M., Petit S., et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clinical Infectious Diseases. 2009;49(1):85–92. doi: 10.1086/599369. [DOI] [PubMed] [Google Scholar]
- 3.Melin P. Neonatal group B streptococcal disease: from pathogenesis to preventive strategies. Clinical Microbiology and Infection. 2011;17(9):1294–1303. doi: 10.1111/j.1469-0691.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 4.Verani J. R., McGee L., Schrag S. J. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. Morbidity and Mortality Weekly Report. 2010;59(RR-10):1–36. [PubMed] [Google Scholar]
- 5.Palmeiro J. K., Dalla-Costa L. M., Fracalanzza S. E., et al. Phenotypic and genotypic characterization of group B streptococcal isolates in southern Brazil. Journal of Clinical Microbiology. 2010;48(12):4397–4403. doi: 10.1128/JCM.00419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garland S. M., Cottrill E., Markowski L., et al. Antimicrobial resistance in group B streptococcus: the Australian experience. Journal of Medical Microbiology. 2011;60, part 2:230–235. doi: 10.1099/jmm.0.022616-0. [DOI] [PubMed] [Google Scholar]
- 7.Turner C., Turner P., Po L., et al. Group B streptococcal carriage, serotype distribution and antibiotic susceptibilities in pregnant women at the time of delivery in a refugee population on the Thai-Myanmar border. BMC Infectious Diseases. 2012;12, article 34 doi: 10.1186/1471-2334-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longtin J., Vermeiren C., Shahinas D., et al. Novel mutations in a patient isolate of Streptococcus agalactiae with reduced penicillin susceptibility emerging after long-term oral suppressive therapy. Antimicrobial Agents and Chemotherapy. 2011;55(6):2983–2985. doi: 10.1128/aac.01243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagano N., Nagano Y., Toyama M., et al. Nosocomial spread of multidrug-resistant group B streptococci with reduced penicillin susceptibility belonging to clonal complex 1. Journal of Antimicrobial Chemotherapy. 2012;67(4):849–856. doi: 10.1093/jac/dkr546.dkr546 [DOI] [PubMed] [Google Scholar]
- 10.Lambiase A., Agangi A., Del Pezzo M., et al. In vitro resistance to macrolides and clindamycin by group B streptococcus isolated from pregnant and nonpregnant women. Infectious Diseases in Obstetrics and Gynecology. 2012;2012:5. doi: 10.1155/2012/913603.913603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otaguiri E. S., Morguette A. E. B., Tavares E. R., et al. Commensal Streptococcus agalactiae isolated from patients seen at University Hospital of Londrina, Paraná, Brazil: capsular types, genotyping, antimicrobial susceptibility and virulence determinants. BMC Microbiology. 2013;13(1, article 297) doi: 10.1186/1471-2180-13-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fröhlicher S., Reichen G., Müller M., et al. Serotype distribution and antimicrobial susceptibility of group B streptococci in pregnant women: results from a Swiss tertiary centre. Swiss Medical Weekly. 2014;144 doi: 10.4414/smw.2014.13935.w13935 [DOI] [PubMed] [Google Scholar]
- 13.Alves M. J., Ferreira I. C. F. R., Martins A., Pintado M. Antimicrobial activity of wild mushroom extracts against clinical isolates resistant to different antibiotics. Journal of Applied Microbiology. 2012;113(2):466–475. doi: 10.1111/j.1365-2672.2012.05347.x. [DOI] [PubMed] [Google Scholar]
- 14.Elaissi A., Rouis Z., Salem N. A. B., et al. Chemical composition of 8 eucalyptus species' essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complementary and Alternative Medicine. 2012;12, article 81 doi: 10.1186/1472-6882-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruíz F. O., Gerbaldo G., García M. J., Giordano W., Pascual L., Barberis I. L. Synergistic effect between two bacteriocin-like inhibitory substances produced by lactobacilli strains with inhibitory activity for Streptococcus agalactiae . Current Microbiology. 2012;64(4):349–356. doi: 10.1007/s00284-011-0077-0. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura C. V., Ueda-Nakamura T., Bando E., Negrão Melo A. F., Garcia Cortez D. A., Dias Filho Filho B. P. Antibacterial activity of Ocimum gratissimum L. essential oil. Memorias do Instituto Oswaldo Cruz. 1999;94(5):675–678. doi: 10.1590/s0074-02761999000500022. [DOI] [PubMed] [Google Scholar]
- 17.Devi K. P., Nisha S. A., Sakthivel R., Pandian S. K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. Journal of Ethnopharmacology. 2010;130(1):107–115. doi: 10.1016/j.jep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Devi K. P., Sakthivel R., Nisha S. A., Suganthy N., Pandian S. K. Eugenol alters the integrity of cell membrane and acts against the nosocomial pathogen Proteus mirabilis . Archives of Pharmacal Research. 2013;36(3):282–292. doi: 10.1007/s12272-013-0028-3. [DOI] [PubMed] [Google Scholar]
- 19.de Paula S. B., Bartelli T. F., Di Raimo V., et al. Effect of eugenol on cell surface hydrophobicity, adhesion, and biofilm of Candida tropicalis and Candida dubliniensis isolated from oral cavity of HIV-infected patients. Evidence-Based Complementary and Alternative Medicine. 2014;2014:8. doi: 10.1155/2014/505204.505204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai J.-P., Zhao X.-F., Zeng J., et al. Drug screening for autophagy inhibitors based on the dissociation of Beclin1-Bcl2 complex using BiFC technique and mechanism of eugenol on anti-influenza A virus activity. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061026.e61026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueda-Nakamura T., Mendonça-Filho R. R., Morgado-Díaz J. A., et al. Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum . Parasitology International. 2006;55(2):99–105. doi: 10.1016/j.parint.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Gülçin I. Antioxidant activity of eugenol: a structure-activity relationship study. Journal of Medicinal Food. 2011;14(9):975–985. doi: 10.1089/jmf.2010.0197. [DOI] [PubMed] [Google Scholar]
- 23.Jaganathan S. K., Supriyanto E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules. 2012;17(6):6290–6304. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniel A. N., Sartoretto S. M., Schmidt G., Caparroz-Assef S. M., Bersani-Amado C. A., Cuman R. K. N. Anti-inflammatory and antinociceptive activities of eugenol essential oil in experimental animal models. Revista Brasileira de Farmacognosia. 2009;19(1):212–217. doi: 10.1590/s0102-695x2009000200006. [DOI] [Google Scholar]
- 25.Durán N., Marcato P. D., de Conti R., Alves O. L., Costa F. T. M., Brocchi M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. Journal of the Brazilian Chemical Society. 2010;21(6):949–959. doi: 10.1590/s0103-50532010000600002. [DOI] [Google Scholar]
- 26.Herman A., Herman A. P. Nanoparticles as antimicrobial agents: their toxicity and mechanisms of action. Journal of Nanoscience and Nanotechnology. 2014;14(1):946–957. doi: 10.1166/jnn.2014.9054. [DOI] [PubMed] [Google Scholar]
- 27.Allahverdiyev A. M., Kon K. V., Abamor E. S., Bagirova M., Rafailovich M. Coping with antibiotic resistance: combining nanoparticles with antibiotics and other antimicrobial agents. Expert Review of Anti-Infective Therapy. 2011;9(11):1035–1052. doi: 10.1586/eri.11.121. [DOI] [PubMed] [Google Scholar]
- 28.Cardozo V. F., Oliveira A. G., Nishio E. K., et al. Antibacterial activity of extracellular compounds produced by a Pseudomonas strain against methicillin-resistant Staphylococcus aureus (MRSA) strains. Annals of Clinical Microbiology and Antimicrobials. 2013;12, article 12 doi: 10.1186/1476-0711-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh I. N., Patil S. D., Sharma T. K., Srivastava S. K., Pathania R., Navani N. K. Synergistic action of cinnamaldehyde with silver nanoparticles against spore-forming bacteria: a case for judicious use of silver nanoparticles for antibacterial applications. International Journal of Nanomedicine. 2013;8:4721–4731. doi: 10.2147/ijn.s49649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durán N., Marcato P. D., Alves O. L., de Souza G. I. H., Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. Journal of Nanobiotechnology. 2005;3, article 8 doi: 10.1186/1477-3155-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clinical and Laboratory Standards Institute (CLSI) Document. M100-S22. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2012. Clinical and Laboratory Standards Institute: performance standards for antimicrobial susceptibility testing; twentieth informational supplement. [Google Scholar]
- 32.Kwieciński J., Eick S., Wójcik K. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. International Journal of Antimicrobial Agents. 2009;33(4):343–347. doi: 10.1016/j.ijantimicag.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Borges S., Silva J., Teixeira P. Survival and biofilm formation by Group B streptococci in simulated vaginal fluid at different pHs. Antonie van Leeuwenhoek. 2012;101(3):677–682. doi: 10.1007/s10482-011-9666-y. [DOI] [PubMed] [Google Scholar]
- 34.Shafreen R. M. B., Srinivasan S., Manisankar P., Pandian S. K. Biofilm formation by Streptococcus pyogenes: modulation of exopolysaccharide by fluoroquinolone derivatives. Journal of Bioscience and Bioengineering. 2011;112(4):345–350. doi: 10.1016/j.jbiosc.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Yadav M. K., Park S. W., Chae S. W., Song J. J., Kim H. C. Antimicrobial activities of Eugenia caryophyllata extract and its major chemical constituent eugenol against Streptococcus pneumoniae . Acta Pathologica, Microbiologica et Immunologica Scandinavica. 2013;121(12):1198–1206. doi: 10.1111/apm.12067. [DOI] [PubMed] [Google Scholar]
- 36.Kamatou G. P., Vermaak I., Viljoen A. M. Eugenol—from the remote Maluku Islands to the international market place: a review of a remarkable and versatile molecule. Molecules. 2012;17(6):6953–6981. doi: 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ananda Baskaran S., Kazmer G. W., Hinckley L., Andrew S. M., Venkitanarayanan K. Antibacterial effect of plant-derived antimicrobials on major bacterial mastitis pathogens in vitro. Journal of Dairy Science. 2009;92(4):1423–1429. doi: 10.3168/jds.2008-1384. [DOI] [PubMed] [Google Scholar]
- 38.Oyedemi S. O., Okoh A. I., Mabinya L. V., Pirochenva G., Afolayan A. J. The proposed mechanism of bactericidal action of eugenol, α-terpineol and γ-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli . African Journal of Biotechnology. 2009;8(7):1280–1286. [Google Scholar]
- 39.Xu J.-S., Li Y., Cao X., Cui Y. The effect of eugenol on the cariogenic properties of Streptococcus mutans and dental caries development in rats. Experimental and Therapeutic Medicine. 2013;5(6):1667–1670. doi: 10.3892/etm.2013.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill A. O., Holley R. A. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. International Journal of Food Microbiology. 2006;111(2):170–174. doi: 10.1016/j.ijfoodmicro.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 41.Otter J. A., Vickery K., Walker J. T., et al. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. Journal of Hospital Infection. 2015;89(1):16–27. doi: 10.1016/j.jhin.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Otto M. Physical stress and bacterial colonization. FEMS Microbiology Reviews. 2014;38(6):1250–1270. doi: 10.1111/1574-6976.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nostro A., Scaffaro R., D'Arrigo M., et al. Development and characterization of essential oil component-based polymer films: a potential approach to reduce bacterial biofilm. Applied Microbiology and Biotechnology. 2013;97(21):9515–9523. doi: 10.1007/s00253-013-5196-z. [DOI] [PubMed] [Google Scholar]
- 44.Adil M., Singh K., Verma P. K., Khan A. U. Eugenol-induced suppression of biofilm-forming genes in Streptococcus mutans: an approach to inhibit biofilms. Journal of Global Antimicrobial Resistance. 2014;2(4):286–292. doi: 10.1016/j.jgar.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Mitsch P., Zitterl-Eglseer K., Köhler B., Gabler C., Losa R., Zimpernik I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poultry Science. 2004;83(4):669–675. doi: 10.1093/ps/83.4.669. [DOI] [PubMed] [Google Scholar]
- 46.Sosto F., Benvenuti C. Controlled study on thymol + eugenol vaginal douche versus econazole in vaginal candidiasis and metronidazole in bacterial vaginosis. Arzneimittel-Forschung. 2011;61(2):126–131. doi: 10.1055/s-0031-1296178. [DOI] [PubMed] [Google Scholar]