Key Clinical Message
We report a 14-year-old girl, who developed shigatoxin-producing E. coli (STEC)-HUS complicated by encephalopathy. She was successfully treated with hemodiafiltration, high-dose methylprednisolone pulse therapy, and soluble recombinant thrombomodulin under plasma exchange. von Willebrand factor multimers analysis provides potential insights into how the administered therapies might facilitate successful treatment of STEC-HUS.
Keywords: Encephalopathy, Escherichia coli O111, hemolytic uremic syndrome, plasma exchange, recombinant soluble thrombomodulin, von Willebrand factor
Introduction
Hemolytic uremic syndrome (HUS) is a life-threatening disease, characterized by microangiopathic hemolytic anemia, destructive thrombocytopenia, and renal failure 1. Most HUS occurs in association with Shiga toxin-producing Escherichia coli (STEC) infection 2. Patients with STEC-HUS generally recover with fluid therapy and hemodialysis. Mortality is high among STEC-HUS patients with encephalopathy, despite treatments including plasma exchange, steroid pulse, and more recently eculizumab 3. In recent STEC outbreaks in the United States (STEC-O111) and Germany (STEC-O104) in 2008 and 2011, respectively 4,5, STEC-HUS incidence and mortality were 16.7% and 3.8% and 22% and 3.7%, respectively.
In 2011, an outbreak of STEC-O111 and/or -O157 infection in Toyama, Japan occurred following raw meat ingestion in a barbecue restaurant chain. Overall, 181 patients were infected, of whom 34 developed STEC-HUS (18.8%) including 21 with encephalopathy (61.8%) and five deaths (14.7%; all with encephalopathy) 6–8. Ten STEC-HUS patients were aged 1–14 years, including eight with encephalopathy 7. Seven children including five with encephalopathy recovered and three died 7. We report clinical and laboratory findings for a 14-year-old girl in the Toyama series with STEC-HUS and encephalopathy.
Case Report
In April 2011, a 14-year-old girl ingested raw meat in a barbecue restaurant in Toyama, and then traveled to Osaka. Bloody diarrhea developed 5 days later. At a local hospital, levofloxacin was prescribed without improvement. Six days later after raw meat ingestion, she was transferred to Yodogawa Christian hospital. Almost simultaneously, multiple outbreaks of hemorrhagic enterocolitis due to STEC: O111 (producing both shigatoxin-1 and -2) were reported from several hospitals around Toyama. All affected patients had eaten raw meats in the same chain restaurants around Toyama. Admission laboratory findings included: white blood cell (WBC) [24,700/μl], red blood cell (RBC) [5.28 × 106/μL], hemoglobin (Hb) [16.7 g/dL], platelet [143 × 103/μL], C-reactive protein (CRP) [3.55 mg/dl], lactate dehydrogenase (LDH) [227 IU/L], blood urea nitrogen (BUN) [15.6 mg/dL], creatinine (Cr) [0.69 mg/dL], normal hemostatic tests, proteinuria, and no hematuria. Stool cultures showed normal flora, stool shigatoxin stool was negative, and both the antigens of STEC:O111 and O157 in stool were negative.
On day 3, the patient developed anemia (RBC [2.63 × 106/μL], Hb [8.2 g/dL], LDH [1148 IU/L], haptoglobin [8 mg/dl], and thrombocytopenia [12,000/μl], with an increase in BUN [26.6 mg/dL] and Cr [1.06 mg/dL] as shown in Figure1). Schistocytes were seen in the peripheral blood smear. Plasma ADAMTS13 activity levels were 43% of normal. The patient became anuric and comatose (Glasgow Coma Scale [GCS] 14). Continuous hemodiafiltration was initiated with plasma exchange. On day 5, pleural effusions developed, respiratory function worsened, and consciousness deteriorated further. Intubation was performed. Brain magnetic resonance imaging showed high intensity areas in the bilateral thalamus and basal ganglia, and part of the pontine tegmentum on T2 FLAIR images (Fig.1 Inset). Acute encephalopathy developed. STEC-HUS was diagnosed. High-dose methylprednisolone pulse therapy [500 mg/day] for days 5–7 was administered. On day 6, serum antibodies to STEC:O111 antigen were noted. On day 9, hemolysis worsened, whereas severe thrombocytopenia persisted. Plasma exchange was increased to twice daily. A second 3-day course of a high-dose methylprednisolone pulse therapy was administered. Gabexate mesilate, a synthetic anticoagulant was administered. Serum levels of fibrin/fibrinogen degradation product (FDP) and thrombin–antithrombin complex (TAT) increased to 120 μg/mL and 24.3 ng/mL, respectively. Soluble recombinant thrombomodulin (130 units/kg/day) was infused during days 9–14. Clinical and laboratory findings subsequently improved, including thrombocytopenia, hemolysis, and renal function (Fig.1). Extubation occurred on day 22. Plasma exchange was tapered, and discontinued on day 24. After rehabilitation, the patient was discharged without appreciable sequelae on day 64.
Figure 1.
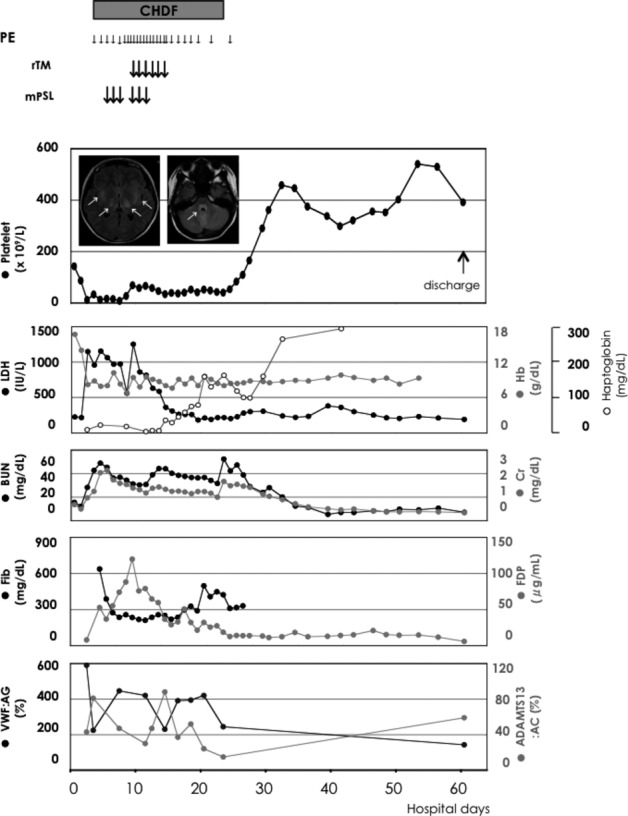
Clinical course in a 14-year-old girl with STEC-HUS complicated by acute encephalopathy after admission.
Retrospective analyses of stored plasma samples were performed. Plasma samples from admission showed that levels of the following cytokines were not elevated: interleukin (IL)-6 [4 pg/mL (normal: <4)], IL-8 [59 pg/mL (normal: <2)], and tumor necrosis factor (TNF)α [12 pg/mL (normal: <15)]. In contrast, plasma samples from admission identified elevated levels of neopterin [98 nmol/L (normal: <5)], soluble form TNF receptor type I (sTNF-RI) [13,200 pg/mL (normal: 484–1407)], sTNF-RII [18,300 pg/mL (normal: 829–2262)], and tau protein [344 pg/mL (normal: undetectable)]. Plasma samples from day 3 identified reduced plasma ADAMTS13 activity (43%) levels and high levels of plasma VWF antigen levels (605% of normal).
Retrospective analysis of plasma VWF multimer patterns using citrated plasma samples (frozen at −80°C) was also performed (Fig.2). During the acute phase, no high-to-intermediate sized VWF multimers were identified in samples taken three and 13 days prior to initiation of plasma exchange. After each plasma exchange, VWF multimer patterns were present, although high-sized VWF multimers continued to be absent. Plamsa exchange was performed once or twice daily until day 20, then tapered, and discontinued on day 24. UL-VWF multimers appeared in plasma at days 21 and 24, and disappeared at day 61 just before discharge. At discharge, plasma levels of VWF and ADAMTS13 had returned to almost normal ranges.
Figure 2.
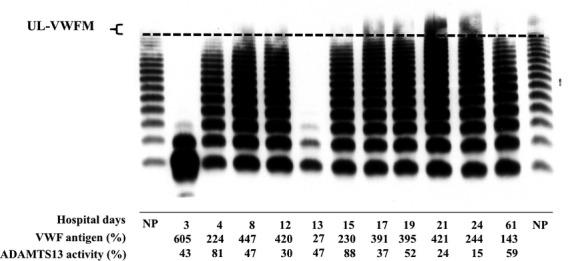
Change of VWF multimer patterns during the acute phase.
Discussion
We report a patient with STEC-HUS, mild-to-moderate reduction of plasma ADAMTS13 activity, and increased plasma levels of VWF antigen. Despite persistent thrombocytopenia in the acute phase, VWF multimers were degraded on one occasion and highly multimerized on a different occasion. Therapy with continuous hemodiafiltration, high-dose methylprednisolone pulse therapy and soluble recombinant thrombomodulin was successful and the patient was discharged without any deficits. In explaining our findings, several factors should be considered.
First, identification of UL-VWF multimers in this patient differs from the VWF pattern usually seen with STEC-HUS where the multimers are usually depleted. UL-VWFMs, stored in Weibel–Palade bodies (WPBs) of vascular endothelial cells, are released upon stimulation by inflammatory cytokines, such as IL-6, IL-8, and TNFα 9. Likewise, UL-VWFMs are released into the circulation by injured vascular endothelial cells. On admission, plasma levels of cytokines including IL-8, neopterin, TNF-RI and RII, and tau protein were high, indicating vascular injury, inflammation, and neurological cell damages 6. Also, the B-subunit of shigatoxin-1 and -2, both AB5-holotoxins, binds to globotrialosyl ceramide (Gb3) by which UL-VWFMs are released from Weibel–Palade bodies 10. Shigatoxin binds to Gb3, internalizes, and blocks protein synthesis by attachment to ribosomal RNA. Shigatoxin also directly enhances platelet aggregation under high and low shear stress at very low concentrations 11. Thus, in our patient, UL-VWFM, may have been released excessively from activated vascular endothelial cells, was involved in platelet thrombi formation, and then was consumed by proteases released from platelets and/or leucocytes.
Second, our findings may explain how plasma exchange may have had therapeutic benefit in this patient. In particular, plasma exchange might work bifunctionally: one effect was to reduce concentrations of various cytokines, UL-VWFM, and shigatoxin, and the other effect was to supply normal VWFM (for hemostasis). During the acute phase of STEC-HUS, the STEC vigorously produces shigatoxin, which consistently activates platelets, even at low concentrations (pg/ml). So, plasma exchange alone for STEC-HUS is likely to be inefficient, unless shigatoxin function is blocked. Hence, in addition to basic supportive therapy for STEC-HUS such as dialysis and fluid therapy, cytokine adsorption is favorable, and high-dose methylprednisolone pulse therapy might suppress cytokine production 12.
Third, in comparison to previous reports, the occurrence of acute encephalopathy associated with STEC-HUS in Toyama was high, and the deceased cases had encephalopathy. This toxicity is attributable to brain edema, presumably due to increased vascular permeability and/or severe vascular endothelial cell injuries mediated by shigatoxin itself and cytokines, yet the mechanism is not fully understood 13. Strains of STEC:O111 isolated in Toyama predominantly produced shigatoxin-2, which is more toxic than shigatoxin-1. However, a peculiar MRI finding on high intensity areas, often symmetrical in thalamus, basal ganglia, and pontine tegmentum, has not been favorably addressed 14.
Fourth, common therapeutic features on seven survived childhood patients in Toyama included continuous hemodiafiltration, high-dose methylprednisolone pulse therapy, and recombinant thrombomodulin. High-dose intravenous immunoglobulin infusion was administered to six of the seven survivors. Administration of recombinant thrombomodulin may have been particularly important, as this drug has been available in Japan as treatment for disseminated intravascular coagulation (DIC) since 2008 15. Recombinant thrombomodulin is a multifunctional protein. A lectin-like domain directly absorbs and neutralizes high mobility group box1 (HMGB1), which is a pro-inflammatory cytokine that acts as a lethality factor when endotoxin shock occurs 16. Also, EGF-like domains 4–6 of the recombinant thrombomodulin can bind thrombin and inactive the catalytic activity of thrombin. The thombin–recombinant thrombomodulin complex can accelerate activation of protein C and thrombin activatable fibrinolytic inhibitor (TAFI) to activated protein C and TAFIa, respectively. In turn, activated protein C generates anticoagulant action via inactivation of Va and VIIIa and TAFIa suppresses complement activation via inactivation of C3a and C5a 15. As the action of recombinant thrombomodulin on platelets remains unclear, we are unable to directly address how recombinant thrombomodulin can resolve STEC-HUS. There are at least two possibilities: one is direct inhibitory activity to platelet aggregation, and the second is to block fibrin clot formation over platelet thrombi, as suggested by significant increases of FDP and TAT during the clinical course before recombinant thrombomodulin is administered.
In conclusion, we report a novel therapy for STEC-HUS. VWF-dependent hemostatic defect that is generated in STEC-HUS appears to have been restored by plasma exchange. Hypercoagulability, presumably induced by shigatoxin or cytokine storms, appears to have been suppressed with high-dose methylprednisolone pulse therapy and recombinant thromobomodulin.
Acknowledgments
The authors wish to express our sincere gratitude to Prof. Hau C. Kwaan (Northwestern University Feinberg School of Medicine, Chicago) for his critical reading to this manuscript.
Conflict of Interest
Nothing to declare.
References
- Ruggenenti P, Noris M. Remuzzi G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 2001;60:831–846. doi: 10.1046/j.1523-1755.2001.060003831.x. [DOI] [PubMed] [Google Scholar]
- Noris M. Remuzzi G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- Nathanson S, Kwon T, Elmaleh M, Charbit M, Launay EA, Harambat J, et al. Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. 2010;5:1218–1228. doi: 10.2215/CJN.08921209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. The New England journal of medicine. 2011;365:1771–1780. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- Piercefield EW, Bradley KK, Coffman RL. Mallonee SM. Hemolytic uremic syndrome after an Escherichia coli O111 outbreak. Arch. Intern. Med. 2010;170:1656–1663. doi: 10.1001/archinternmed.2010.346. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Kuroda M, Sakashita N, Konishi M, Kaneda H, Igarashi N, et al. Cytokine profiles of patients with enterohemorrhagic Escherichia coli O111-induced hemolytic-uremic syndrome. Cytokine. 2012;60:694–700. doi: 10.1016/j.cyto.2012.07.038. [DOI] [PubMed] [Google Scholar]
- Taneichi H, Konishi M, Igarashi N, Kaneda H, Matsukura H, Ogura K, et al. An outbreak of enterohemorrhagic Escherichia coli O111 associated with the consumption of raw beef in Toyama in Japan. J. Jpn Pediatr. Soc. 2013;117:1409–1415. [Google Scholar]
- Takanashi J, Taneichi H, Misaki T, Yahata Y, Okumura A, Ishida Y, et al. Clinical and radiologic features of encephalopathy during 2011 E. coli O111 outbreak in Japan. Neurology. 2014;82:564–572. doi: 10.1212/WNL.0000000000000120. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Recombinant human protein CWEiSSsg. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Huang J, Motto DG, Bundle DR. Sadler JE. Shiga toxin B subunits induce VWF secretion by human endothelial cells and thrombotic microangiopathy in ADAMTS13-deficient mice. Blood. 2010;116:3653–3659. doi: 10.1182/blood-2010-02-271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Narita N, Matsumoto M, Sakurai Y, Ikari H, Yoshioka A, et al. Enhanced low shear stress induced platelet aggregation by Shiga-like toxin 1 purified from Escherichia coli O157. Am. J. Hematol. 2001;66:105–115. doi: 10.1002/1096-8652(200102)66:2<105::AID-AJH1025>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Fujii J, Kinoshita Y, Matsukawa A, Villanueva SY, Yutsudo T. Yoshida S. Successful steroid pulse therapy for brain lesion caused by Shiga toxin 2 in rabbits. Microb. Pathog. 2009;46:179–184. doi: 10.1016/j.micpath.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Greinacher A, Friesecke S, Abel P, Dressel A, Stracke S, Fiene M, et al. Treatment of severe neurological deficits with IgG depletion through immunoadsorption in patients with Escherichia coli O104:H4-associated haemolytic uraemic syndrome: a prospective trial. Lancet. 2011;378:1166–1173. doi: 10.1016/S0140-6736(11)61253-1. [DOI] [PubMed] [Google Scholar]
- Lobel U, Eckert B, Simova O, Meier-Cillien M, Kluge S, Gerloff C, et al. Cerebral magnetic resonance imaging findings in adults with haemolytic uraemic syndrome following an infection with Escherichia coli, subtype O104:H4. Clin. Neuroradiol. 2014;24:111–119. doi: 10.1007/s00062-013-0231-0. [DOI] [PubMed] [Google Scholar]
- Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J. Thromb. Haemost. 2007;5:31–41. doi: 10.1111/j.1538-7836.2006.02267.x. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Goto K, Ochi Y, Mizunaga S, Saikawa T, et al. Recombinant thrombomodulin prevents heatstroke by inhibition of high-mobility group box 1 protein in sera of rats. Shock. 2010;34:402–406. doi: 10.1097/SHK.0b013e3181d492e4. [DOI] [PubMed] [Google Scholar]


