Key Clinical Message
The decision for PJP prophylaxis depends on a physician's evaluation of multiple variables. The high rate of PJP infection described in this article combined with the known impaired T-cell function post Bendamustine treatment justifies considering all patients for PJP prophylaxis when they receive Bendamustine treatment.
Keywords: Bendamustine, Pneumocystis, Trimethoprim/Sulfamethoxazole
Introduction
Pneumocystis jiroveci pneumonia (PJP) has been increasingly described as a serious opportunistic infection in HIV-seronegative patients with malignancy, as a consequence of immunosuppression from chemotherapy.
The standard method for diagnosing PJP is demonstration of Pneumocystis in either bronchoalveolar lavage fluid or induced sputum. Testing with either specimen has a sensitivity ranging from 80 to 95% 1,2. Trimethoprim/sulpfamexazole (TMP/SMX) is the gold standard for treatment and prophylaxis of PJP.
The death rate due to PJP ranges between 10 and 20% in patients with HIV 3. This infection carries a worse prognosis in the HIV seronegative population with a mortality rate of 30 to 60%, possibly as a consequence of late diagnosis and delayed treatment 4.
Bendamustine is an alkylating agent with multiple unique mechanisms of action. It was first synthesized in 1963 by Ozegowski and Krebs and was available only in East Germany until 1990. It is increasingly used in the treatment of chronic lymphocytic leukemia (CLL), multiple myeloma and non-Hodgkin lymphomas (NHL). Myelosuppression is a common side effect of Bendamustine with severe T-lymphocyte depletion and increased risk of opportunistic infections.
We describe four cases of PJP in HIV negative individuals as sequelae of Bendamustine/Rituximab (BR) treatment. The use of PJP prophylaxis has been a routine practice in patients undergoing treatment with Bendamustine.
Case Presentations
Case 1
A 69-year-old male was diagnosed with stage B CLL in June 2011. One year later, cytotoxic therapy was initiated with Bendamustine (100 mg/m2) on days 1 and 2 and Rituximab (375 mg/m2) on day 1. He presented on day 13 after the third cycle of treatment with dry cough, dyspnea, and a temperature of 38°C. Examination revealed oxygen saturation (SPO2) of 93% on room air. Fine crackles and wheezes were audible at both lung bases.
Arterial blood gas (ABG) demonstrated respiratory alkalosis with hypoxemia. Oxygen partial pressure (PO2) was 9.7 kilopascal (Kpa). Blood cultures did not identify any organism. Laboratory results showed lymphopenia, elevated C-reactive protein (CRP), and lactate dehydrogenase (LDH) at 495 U/L (220 upper limit of normal). Chest X-ray (CXR) was suggestive of PJP with a perihilar and mid-zone ground glass pattern (Fig.1). Chest Computed Tomography (CT) supported the diagnosis of PJP (Fig.2).
Figure 1.
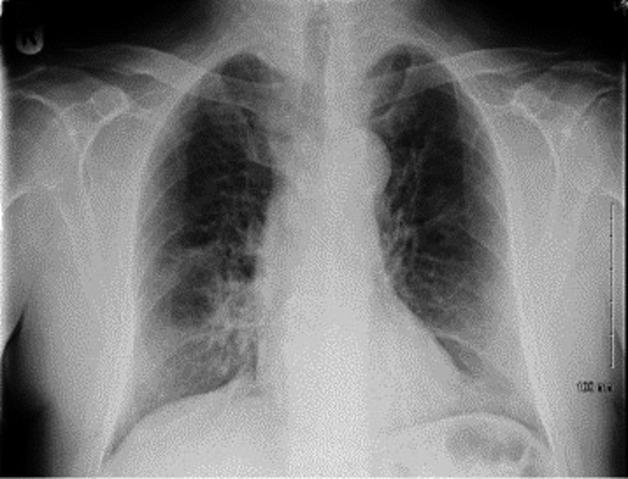
CXR Showing perihilar and mid-zone ground glass pattern.
Figure 2.
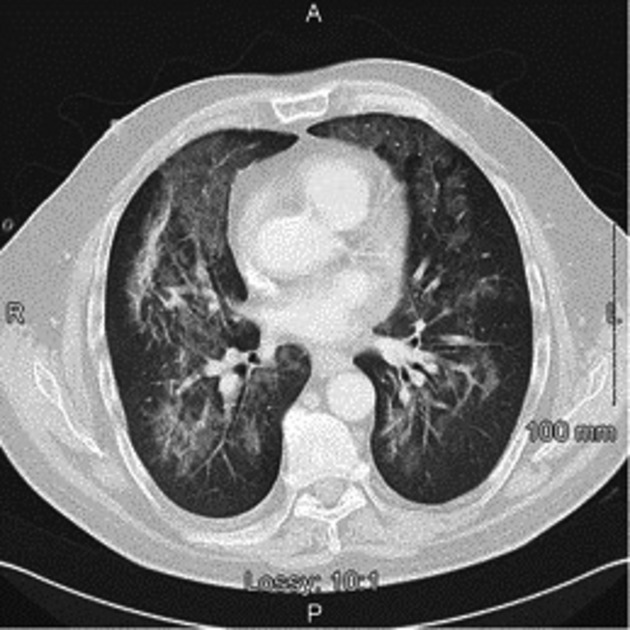
CT-Thorax demonstrating perihilar infiltrates consistent with the diagnosis of PJP.
Bronchoscopy was normal, but bronchial washings were positive for PJP polymerase chain reaction (PCR). TMP/SMX was initiated at a dosage of 20 mg/kg six hourly intravenously and continued for 21 days. The patient improved and was discharged on the prophylactic dose of TMP/SMX.
Case 2
A 73-year-old male was diagnosed with marginal zone lymphoma and was treated with Bendamustine (100 mg/m2) and Rituximab (375 mg/m2) as per standard protocol. He presented on day ten after the second cycle of chemotherapy with fever and productive cough. Examination revealed tachycardia at 105 beats per minute and SPO2 of 93% on 40% oxygen. Bronchial breathing and crackles were heard at the left lower zone.
Investigations showed neutrophil leukocytosis, lymphopenia, renal impairment, high CRP at 192 mg/L and LDH of 300 U/L. ABG demonstrated hypoxemia with PO2 of 8.9 Kpa. Lobar consolidation was evident on CXR (Fig.3). Echocardiogram revealed mild mitral regurgitation, good left ventricular function, and mild left ventricular hypertrophy.
Figure 3.
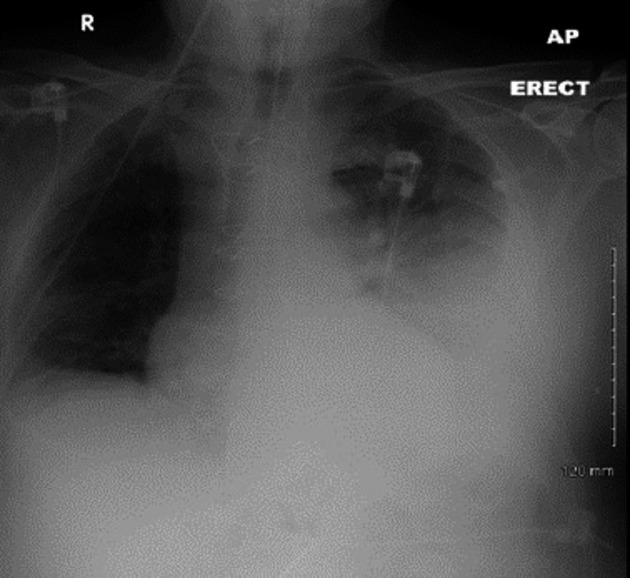
CXR showing consolidation involving the left lower lobe.
Despite treatment with Tazocin and Clarithromycin for a presumed bacterial pneumonia, he remained hypoxic. Subsequently, he was admitted to ICU for management of fast atrial fibrillation. Sputum was positive for PJP by PCR testing. Intravenous TMP/SMX was started on day six post admission at 20 mg/kg six hourly for 14 days with complete resolution of the infection.
Case 3
A 68-year-old female was diagnosed with stage IV A follicular B-cell non-Hodgkin lymphoma (FL). She was started on Bendamustine (90 mg/m2) and Rituximab (375 mg/m2) as per standard protocol. She developed fever, dry cough, and dyspnea 14 days post the third cycle of treatment. Examination revealed fever, hypotension, and tachypnea. SPO2 was 88 on 40% oxygen. Widespread wheezes were audible on chest auscultation.
CXR showed bilateral upper and mid zone opacification with sparing of both bases (Fig.4). Laboratory investigations revealed lymphopenia, high CRP and LDH of 1090 U/L. The patient received Tazocin without any beneficial effect. Twenty-four hours later, she deteriorated further and remained hypotensive, breathless, and hypoxic. She was transferred to ICU, required intubation and ventilation. PJP was identified by PCR testing in the bronchoalveolar lavage.
Figure 4.
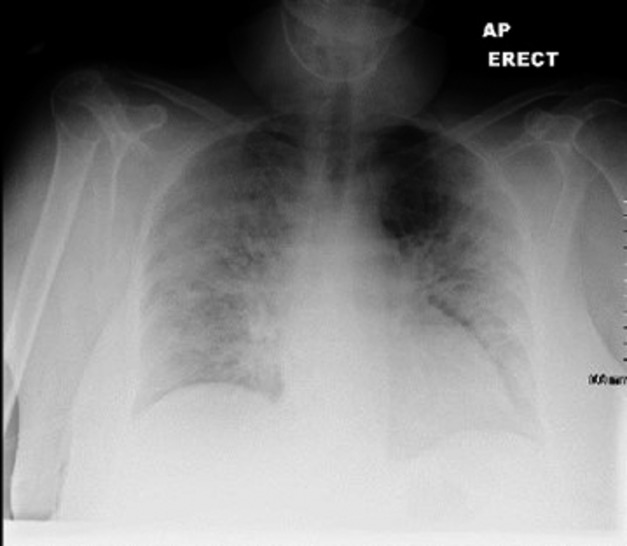
CXR showing bilateral upper and mid zone opacification with sparing of the bases.
Treatment with intravenous TMP/SMX was started on day three of admission at 20 mg/kg six hourly and continued for 15 days. She remained on mechanical ventilation for 33 days. Eventually, she was weaned successfully from the ventilator and made a complete recovery.
Case 4
A 71-year-old female with a diagnosis of CLL was treated with Bendamustine (100 mg/m2) and Rituximab (375 mg/m2) as per standard protocol. She presented to the emergency department on day ten after the first cycle with fever, rigors and vomiting for 2 days. Physical examination revealed a high-grade temperature of 39°C, tachycardia and tachypnea. Her SPO2 was 98% on room air. No other abnormalities were identified on examination.
Laboratory investigations showed lymphopenia, renal impairment, elevated CRP of 42 mg/L and LDH of 305 U/L. ABG on 40% oxygen showed hypoxemia with PO2 of 8.2 Kpa. CXR was suggestive of PJP with large volume perihilar infiltration (Fig.5).
Figure 5.
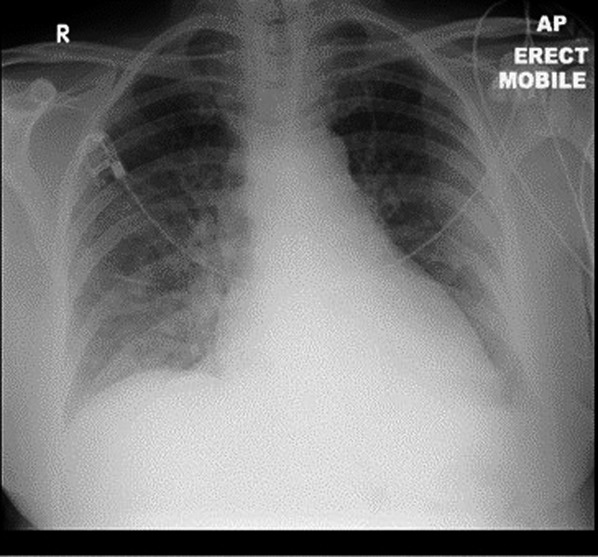
CXR revealing perihilar infiltration.
The patient was subsequently commenced on intravenous TMP/SMX at 20 mg/kg eight hourly and she was transferred to ICU as she was hypotensive and hypoxemic. Bronchoscopy was deferred as the patient was too unstable. Echocardiogram was normal with good LV function. She developed paroxysmal fast atrial fibrillation, which was managed with Amiodarone infusion. She made an excellent clinical recovery after 14 days of intravenous TMP/SMX with complete resolution of the radiological signs.
Discussion
In patients with hematological malignancies or solid tumors receiving chemotherapy or high-dose steroid therapy, clinicians should be vigilant to the possibility of PJP and consider the need for prophylaxis 5.
Like HIV patients, lymphopenia may place cancer patients at increased risk of opportunistic infections such as PJP. However, in contrast to the HIV population, there has not been agreement regarding PJP prophylaxis in HIV seronegative patients receiving immunosuppressive therapies. In a meta-analysis of randomized controlled trials of PJP prophylaxis in immunocompromised patients without HIV, the meta-analysis concludes that PJP prophylaxis is indicated when the risk of PJP is higher than 3.5% in adults 6.
All patients described received Bendamustine and Rituximab. Rituximab has rarely been associated with PJP. In the initial trials of Rituximab with combination chemotherapy, over 3000 patients were treated without any cases of PJP described 7. Case reports have described Pneumocystis pneumonia post Rituximab treatment, but the rate of infection is low 8–14.
Bendamustine has the potential to induce a reduction in CD4 lymphocyte counts causing a severe T-lymphocyte-mediated immunosuppression. Bendamustine is a recognized risk for opportunistic infection, including PJP 15–18. It may result in grade 3 to 4 lymphopenia in 74% of patients and thus, a recent article has recommended bimonthly monitoring of CD4 T-helper cells with initiation of PJP prophylaxis in patients with counts less than 200/μL 19.
A review of randomized controlled trials (RCTs) found that prophylaxis with TMP/SMX significantly reduced the occurrence of PJP by more than 90% 6. Preventive treatment was not associated with an increased risk of adverse events. Given the low rate of adverse events, which include nausea, skin rash, and myelosuppression, prophylaxis should be considered for patients at risk. According to reports, gastrointestinal symptoms and skin reactions, each occurs in about 3.5% of patients 20. The incidence and severity of the adverse reactions are generally dose dependent. Hematological cytopenias are the most serious side effect, reportedly occurring in less than 0.5% of patients 21. No differences between once daily versus thrice weekly TMP/SMX were seen 18,22.
The described patient cases provide evidence that PJP can develop in patients without significant evidence of neutropenia and infection and can occur early in treatment. Bendamustine was received by 67 patients between November 2009 and October 2014 in our practice, primarily for low-grade lymphoproliferative disorders. We describe four cases of PJP of 39 patients, who received Bendamustine before PJP prophylaxis was introduced in August 2012. Twenty-eight patients have received Bendamustine since the introduction of PJP prophylaxis without any further cases of PJP.
Current guidelines vary without clear, consistent recommendations for PJP prophylaxis in patients receiving Bendamustine. Recent studies have prescribed PJP prophylaxis for patients with prolonged grade III/IV neutropenia 23, based on local practice 24 or based on the CD4 count 19.There is increasing evidence that the majority of patients will develop significant reductions in their CD4 counts post Bendamustine. Recent publications have described PJP post Bendamustine treatment 15–18. On the basis of this series of cases and this increasing evidence of CD4 T-cell suppression, we propose that the safest practice is to ensure that all patients receive PJP prophylaxis from the beginning of treatment with Bendamustine.
Conclusion
The decision for PJP prophylaxis depends on a physician's evaluation of multiple variables, including corticosteroid, duration, and dosage of other immunosuppressive therapy, underlying pathology and comorbidities that may adversely affect the immune system. The high rate of PJP infection described in this article combined with the known impaired T-cell function post Bendamustine treatment justifies considering all patients for PJP prophylaxis when they receive Bendamustine treatment.
Conflict of Interest
None declared.
References
- Baughman RP. Current methods of diagnosis. In: Walzer PD, editor. Pneumocystis carinii Pneumonia. 2nd edn. New York: Dekker; 1994. pp. 381–401. [Google Scholar]
- Huang L, Hecht FM, Stansell JD, Montanti R, Hadley WK. Hopewell PC. Suspected pneumocystis carinii pneumonia with a negative induced sputum examination. Is early bronchoscopy useful? Am. J. Respir. Crit. Care Med. 1995;151:1866–1871. doi: 10.1164/ajrccm.151.6.7767533. [DOI] [PubMed] [Google Scholar]
- Curtis JR, Yarnold PR. Schwartz DN, Weinstein RA. Bennett CL. Improvements in outcomes of acute respiratory failure for patients with human immunodeficiency virus related pneumocystis carinii pneumonia. Am. J. Respir. Crit. Care Med. 2000;162:393–398. doi: 10.1164/ajrccm.162.2.9909014. [DOI] [PubMed] [Google Scholar]
- Su YS, Lu JJ, Perng CL. Chang FY. Pneumocystis jirovecii pneumonia in patients with and without human immunodeficiency virus infection. Journal of Microbiology, Immunology, and Infection. 2008;41:478–482. [PubMed] [Google Scholar]
- Bolle'e G, Sarfati C, Thie'ry G, Bergeron A, de Miranda S. Menotti J, et al. Clinical picture of Pneumocystis jiroveci pneumonia in cancer patients. Chest. 2007;132:1305–1310. doi: 10.1378/chest.07-0223. [DOI] [PubMed] [Google Scholar]
- Green H, Paul M, Vidal L. Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin. Proc. 2007;82:1052–1059. doi: 10.4065/82.9.1052. Sep. [DOI] [PubMed] [Google Scholar]
- García-Muñoz R, Izquierdo-Gil A, Muñoz A, Roldan Galiacho V, Rabasa P. Panizo C. Lymphocyte recovery is impaired in patients with CLL and indolent NHL treated with bendamustine and rituximab. Ann. Hematol. 2014 doi: 10.1007/s00277-014-2135-8. DOI: 10.1007/s00277-014-2135-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kobayashi Y, Asakura Y, Mori M, Azuma T, Maruyama D, et al. Pneumocystis jiroveci pneumonia in relation to CD4+ lymphocyte count in patients with B-cell non-Hodgkin lymphoma treated with chemotherapy. Leukaemia & Lymphoma. 2010;51:1816–1821. doi: 10.3109/10428194.2010.506569. [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Kaya H. Yoshida T. Two cases of Pneumocystis jiroveci pneumonia with non-Hodgkin's lymphoma after CHOP-based chemotherapy containing rituximab. J. Clin. Exp. Hematop. 2010;50:159–162. doi: 10.3960/jslrt.50.159. [DOI] [PubMed] [Google Scholar]
- Besada E. Nossent JC. Should Pneumocystis jiroveci prophylaxis be recommended with Rituximab treatment in ANCA-associated vasculitis? Clin. Rheumatol. 2013;32:1677–1681. doi: 10.1007/s10067-013-2293-4. [DOI] [PubMed] [Google Scholar]
- Kamel S, O'Connor S, Lee N, Filshie R, Nandurkar H. Tam CS. High incidence of Pneumocystis jirovecii pneumonia in patients receiving biweekly rituximab and cyclophosphamide, adriamycin, vincristine, and prednisone. Leuk. Lymphoma. 2010;51:797–801. doi: 10.3109/10428191003699860. May. [DOI] [PubMed] [Google Scholar]
- Kumar D, Gourishankar S, Mueller T, Cockfield S, Weinkauf J, Vethanayagam D, et al. Pneumocystis jirovecii pneumonia after rituximab therapy for antibody-mediated rejection in a renal transplant recipient. Transplant Infectious Disease. 2009;11:167–170. doi: 10.1111/j.1399-3062.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- Shelton E, Yong M. Cohney S. Late onset Pneumocystis pneumonia in patients receiving rituximab for humoral renal transplant rejection. Nephrology. 2009;14:696–699. doi: 10.1111/j.1440-1797.2009.01168.x. [DOI] [PubMed] [Google Scholar]
- Kamar N, Milioto O, Puissant-Lubrano B, Esposito L, Pierre MC, Ould Mohamed A, et al. Incidence and Predictive Factors for Infectious Disease after Rituximab Therapy in Kidney-Transplant Patients. American Journal of Transplantation. 2010;10:89–98. doi: 10.1111/j.1600-6143.2009.02785.x. [DOI] [PubMed] [Google Scholar]
- Klippstein A, P Schneider C, G Sayer H. Höffken K. Pneumocystis carinii pneumonia as a complication of benamustine monotherapy in a patient with advanced progressive breast cancer. J. Cancer Res. Clin. Oncol. 2003;129:316–319. doi: 10.1007/s00432-003-0441-y. [DOI] [PubMed] [Google Scholar]
- Reinbolt RE, Alam S, Layman R, Shapiro C. Lustberg M. PCP in an Atypical Host. Clin. Breast Cancer. 2012;12:138–141. doi: 10.1016/j.clbc.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SJ, Bernstein SH, Friedberg JW. Barr PM. Pneumocystis jirovecii pneumonia as a complication of bendamustine in a patient receiving bendamustine plus rituximab for marginal zone lymphoma. Leuk. Res. 2011;35:e223–e224. doi: 10.1016/j.leukres.2011.07.014. Nov. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Friedberg JW, Kahl BS, Van der Jagt RH. Tremmel L. Bendamustine produces durable responses with an acceptable safety profile in patients with rituximab-refractory indolent non-hodgkin lymphoma. Clin. Lymphoma Myeloma Leuk. 2010;10:452–457. doi: 10.3816/CLML.2010.n.079. [DOI] [PubMed] [Google Scholar]
- Wolfram B. Ghielmini M. Bendamustine in Indolent Non-Hodgkin's Lymphoma: a Practice Guide for patient management. Oncologist. 2013;18:954–964. doi: 10.1634/theoncologist.2013-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHSG. 2010. Acute Sector Empirical Antibiotic Guidelines (V2) November.
- Goedert JJ. Bower M. Impact of highly effective antiretroviral therapy on the risk for Hodgkin lymphoma among people with human immunodeficiency virus infection. Curr. Opin. Oncol. 2012;24:531–536. doi: 10.1097/CCO.0b013e3283560697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WT, Rivera GK, Schell MJ, Thornton D. Lott L. Successful intermittent chemoprophylaxis for Pneumocystis carinii pneumonitis. N. Engl. J. Med. 1987;316:1627–1632. doi: 10.1056/NEJM198706253162604. [DOI] [PubMed] [Google Scholar]
- Fischer K, Cramer P, Busch R, Böttcher S, Bahlo J. Schubert J. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. Journal of Clinical Oncology. 2012;30:3209–3216. doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- Freedberg KA, Tosteson AN, Cohen CJ. Cotton DJ. Primary prophylaxis for Pneumocystis carinii pneumonia in HIV-infected people with CD4 counts below 200/mm3: a cost-effectiveness analysis. J. Acquir. Immune Defic. Syndr. 1991;4:521. [PubMed] [Google Scholar]


