Abstract
A decrease in the number of dendritic cells (DCs) is a major cause of post-sepsis immunosuppression and opportunistic infection and is closely associated with poor prognosis. Increasing the number of DCs to replenish their numbers post sepsis can improve the condition. This therapeutic approach could improve recovery after sepsis. Eighty C57BL/6 mice were subjected to sham or caecal ligation and puncture (CLP) surgery. Mice were divided into four groups: (i) Sham + vehicle, (ii) Sham + DC, (iii) CLP + vehicle, and (iv) CLP + DC. Bone-marrow-derived DCs (BMDCs) were administered at 6, 12 and 24 hr after surgery. After 3 days, we assessed serum indices of organ function (alanine aminotransferase, aspartate aminotransferase, creatinine, amylase and lipase), organ tissue histopathology (haematoxylin and eosin staining), cytokine [interferon-γ (IFN-γ), tumour necrosis factor-α, interleukin-12p70 (IL-12p70), IL-6 and IL-10] levels in the serum, programmed death-1 (PD-1) expression on T cells, regulatory T-cell differentiation in the spleen, and the survival rate (monitored for 7 days). BMDC transfer resulted in the following changes: a significant reduction in damage to the liver, kidney and pancreas in the CLP-septic mice as well as in the pathological changes seen in the liver, lung, small intestine and pancreas; significantly elevated levels of the T helper type 1 (Th1) cytokines IFN-γ and IL-12p70 in the serum; decreased levels of the Th2 cytokines IL-6 and IL-10 in the serum; reduced expression of PD-1 molecules on CD4+ T cells; reduced the proliferation and differentiation of splenic suppressor T cells and CD4+ CD25+ Foxp3+ regulatory T cells, and a significant increase in the survival rate of the septic animals. These results show that administration of BMDCs may have modulated the differentiation and immune function of T cells and contributed to alleviate immunosuppression, hence reducing organ damage and mortality post sepsis. Hence, the immunoregulatory effect of BMDC treatment has potential for the treatment of sepsis.
Keywords: dendritic cells, programmed death-1, regulatory T cells, sepsis
Introduction
Sepsis caused by trauma, shock and infection is the leading cause of death among critically ill patients. It is difficult to treat, has a high mortality rate, and poses a challenge to practitioners of emergency medicine worldwide. The mechanism underlying sepsis is complex. Since the 1970s, an excessive inflammatory response has been considered the major cause. However, extensive anti-inflammatory therapy has neither improved the situation nor decreased the mortality rate; it may have even led to treatment failure.1 It is now generally agreed that, during the early stages of sepsis, the body combats the infection by secreting large amounts of pro-inflammatory cytokines, such as tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), which in turn induce the overriding systemic inflammatory response syndrome. The body then reacts to counter this excessive response through innate mechanisms. The aberrant development of this anti-inflammatory response causes immunosuppression, which increases vulnerability to unavoidable opportunistic infections and eventually leads to multiple organ damage and death.2
Dendritic cells (DCs) are unique cells capable of activating naive T lymphocytes. They are potent professional antigen-presenting cells, the main activators of the T-cell immune response, and the bridge connecting the innate and adaptive immune responses.3 In recent years, animal models and clinical studies have shown that the excessive inflammatory response in sepsis results in massive apoptosis-induced depletion of DCs, wherein the number of mature and activated DCs decreases significantly. Hence, the immune functions of DCs, namely antigen presentation and activation of T-lymphocyte proliferation and differentiation, are also compromised, which eventually results in suppression of the adaptive immune response.4–6 The decrease in the number of DCs is an important cause of post-sepsis immunosuppression and opportunistic infection, and it is closely associated with an unsatisfactory prognosis in sepsis.7,8 Therefore, replenishing the number of DCs during sepsis has potential as an immunoregulatory treatment for sepsis.
Toliver-Kinsky et al.9 increased the production of DCs by administering Flt3 ligand (Flt3L) and subsequently decreased infection in burn wounds and mortality rates in burn-sepsis models. Their study further showed that Flt3L could promote DC function by improving local and systemic adaptive immune responses, thereby efficiently preventing opportunistic infection and sepsis in burn victims.10 In a previous study, we administered continuous injections of Flt3L to increase the number of lung DCs, which resulted in a significant reduction in lung damage in severe sepsis models, improved lung function, and a decreased mortality rate in experimental animals.11 However, Flt3L might influence the proliferation and differentiation of other immunocytes, besides DCs. Hence, our previous study did not illustrate a direct relation between the decreased number of DCs and severe sepsis-induced immunosuppression and organ damage.
To address this issue, bone marrow cells from naive mice were induced in vitro to differentiate to bone-marrow-derived DCs (BMDCs). The BMDCs were administered to caecal ligation and puncture (CLP) septic mice. The effects of this treatment on T-cell differentiation and inflammatory responses as well as on the therapeutic outcome were determined to provide evidence that targeting DCs provides successful immunotherapy for sepsis.
Materials and methods
Animals
Adult male C57BL/6 (I-Ab MHC class II) mice (6–8 weeks old; 22–25 g) were purchased from the Laboratory Animal Centre, Beijing, China. The animals were allowed to acclimatize for at least 7 days before use in experiments. They were housed in a controlled environment and provided with standard rodent chow and water, but were fasted overnight before experiments. Animal care complied with Chinese regulations (SYXK2002-006) for the protection of animals used for experimental and other scientific purposes.
Cell preparation
The preparation of BMDCs was described previously.12 Briefly, DCs were prepared by culturing bone marrow (BM) cells obtained from C57BL/6 naive mice at 37° in a 5% CO2 atmosphere. BM cells were flushed from the femurs and tibias. After lysis of red blood cells, whole BM cells were cultured in complete medium containing murine granulocyte–macrophage colony-stimulating factor (400 U/ml; PeproTech, Rocky Hill, NJ), IL-4 (200 U/ml; PeproTech) and 10% fetal bovine serum.
The culture medium was changed after 4 days of culturing, and lipopolysaccharide (1 μg/ml; Escherichia coli 0111:B4; Sigma, St Louis, MO) was added to the medium and incubated for 2 days. The cells were then pelleted, resuspended in the complete medium, and incubated for 20 min at 4°, after which the adherent cells were removed. The cells were resuspended and counted, and the concentration was adjusted. The harvested cells were collected into a syringe at 5 × 106 cells per 200 μl for later use.
CLP model
Mice were subjected to sham or CLP surgery as previously described.13 Briefly, mice were anaesthetized with pentobarbital (40 mg/kg, intraperitoneally), a small abdominal midline incision was made, and the caecum was exposed. The caecum of CLP mice was mobilized, ligated and punctured through both surfaces once with a 22-gauge needle. The abdomen was then closed. Mice were divided randomly into four groups (20/group) as follows. (i) Sham + vehicle: Sham mice were given intraperitoneal (i.p.) injections of 200 μl of the saline solution used for BMDCs. (ii) Sham + DC: Sham mice received i.p. injections of 5 × 106 BMDCs at 6, 12 and 24 hr after surgery. (iii) CLP + vehicle: CLP mice were given i.p. injections of the saline solution used for BMDCs. (iv) CLP + DC: CLP mice were given i.p. injections of 5 × 106 BMDCs at 6, 12 and 24 hr after surgery.
Mice were killed 3 days after surgery. The blood, liver, lung and spleen were harvested for the assessment of organ injury, T-cell subsets and inflammatory factors, as described below. In another set of experiments, mice (20/group) were randomly divided as described above, and their mortality was monitored for up to 7 days after sham or CLP surgery and vehicle or BMDC transfer.
Quantification of organ function and injury
Blood samples were centrifuged (1610 g for 3 min at room temperature) to separate the serum. All serum samples were stored frozen and were analysed within 24 hr by a veterinary clinical laboratory using standard laboratory techniques. Liver injury was assessed by measuring changes in serum alanine aminotransferase (ALT; a specific marker for hepatic parenchymal injury) and aspartate aminotransferase (AST; a non-specific marker for hepatic injury). Renal dysfunction was assessed by measuring the rise in serum creatinine (an indicator of reduced glomerular filtration rate and hence of renal failure). In addition, serum levels of amylase and lipase were determined as measures of pancreatic injury.
Light microscopy
For histological examination, tissue slices of the lung, liver, small intestine and pancreas were fixed in 10% formalin and then placed in fresh formalin for an additional 24 hr. Paraffin-embedded tissue was prepared using routine histological techniques, and 5-μm-thick sections were stained with haematoxylin and eosin (H&E) for light microscopy analysis. H&E-stained tissue sections were evaluated by two senior pathologists who were blinded to the experimental conditions. Images were captured with an Olympus BX40F microscope (Olympus, Melville, NY), and digital photographs were obtained with a Sony 3CCD colour video camera (Sony, Tokyo, Japan).
Cytokine testing
Serum levels of interferon-γ (IFN-γ), TNF-α, IL-12p70, IL-6 and IL-10 were determined using a cytometric bead array (BD Biosciences, Mountain View, CA). Testing standards and cytometric capture bead assays were prepared according to the instructions provided in the kit manual. Samples were acquired and analysed using a FACSCalibur flow cytometer (BD Biosciences). Data analysis was performed using the cytometric bead array software (BD Biosciences).
Flow cytometry analysis
The spleens were cut into pieces with scissors, ground, then filtered through a 200-mesh stainless steel screen to remove blood cells for the preparation of the mononuclear cell suspension. The cell concentration was adjusted to 1 × 106 cells/ml with RPMI-1640 medium (containing 10% fetal bovine serum). Cells in one replicate tube of the mononuclear cell suspension were added to allophycocyanin-labelled anti-mouse CD3ε, FITC-labelled anti-mouse CD4, Peridinin chlorophyll protein-labelled anti-mouse CD8a and phycoerythrin-labelled anti-mouse programmed death-1 (PD-1, CD279) antibodies and incubated for 30 min at 4° before flow cytometry analysis. Mononuclear cells from the other replicate tube were mixed with FITC-labelled anti-mouse CD4 and Peridinin chlorophyll protein-Cy5.5-labelled anti-mouse CD25 antibodies and incubated for 15 min at room temperature in the dark. Membranes were permeabilized with a permeabilization medium, after which the cells were resuspended in PBS, added to allophycocyanin-labelled anti-mouse Foxp3 antibody, incubated for 30 min at 4°, and analysed using flow cytometry.
All antibodies and IgG isotypes were purchased from BD Pharmingen (San Diego, CA). A FACSCalibur cell sorter (BD Biosciences) was used for data acquisition, and FlowJo software (Tree Star Inc., Ashland, OR) was used for data analysis.
Statistical analysis
The results were presented as the mean ± standard deviation (SD). Group differences were compared by one-way analysis of variance. When a significant difference was found, multiple comparisons were made using the Bonferroni method with type I error adjustment. In a separate set of experimental mice, mortality rates among the four groups at each time-point were compared using Fisher's exact test. Bonferroni's method was used to adjust for multiple comparisons if the overall mortality rates were significantly different. In experiments involving histology or flow cytometry, the figures shown are representative of at least three experiments performed on different days. All statistical assessments were two-sided, and P-values < 0·05 were considered significant. Statistical analyses were performed using SPSS 15·0 statistics software (SPSS Inc., Chicago, IL).
Results
BMDC transfer ameliorates impaired organ function caused by CLP
Organ damage in the liver, kidney and pancreas of the experimental animals was assessed in terms of changes in the serum levels of ALT, AST, creatinine, lipase and amylase in the experimental mice. On the third day after CLP surgery, there was no significant change in the serum levels of ALT, AST, creatinine, lipase and amylase in the Sham groups. Compared with levels in the Sham + vehicle group, the serum levels of ALT, AST, lipase, amylase and creatinine were higher in the CLP + vehicle group (AST and creatinine P < 0·01; ALT, lipase and amylase P < 0·05). Compared with levels in the CLP + vehicle group, the serum levels of ALT, AST, creatinine and amylase were lower in the CLP + DC group (ALT, AST and amylase P < 0·05; creatinine P < 0·01), and the serum level of lipase was also slightly lower (P > 0·05) (Fig.1a–e). These results demonstrate that BMDC treatment reduced damage in the liver, kidney and pancreas in the CLP-sepsis mouse model.
Figure 1.
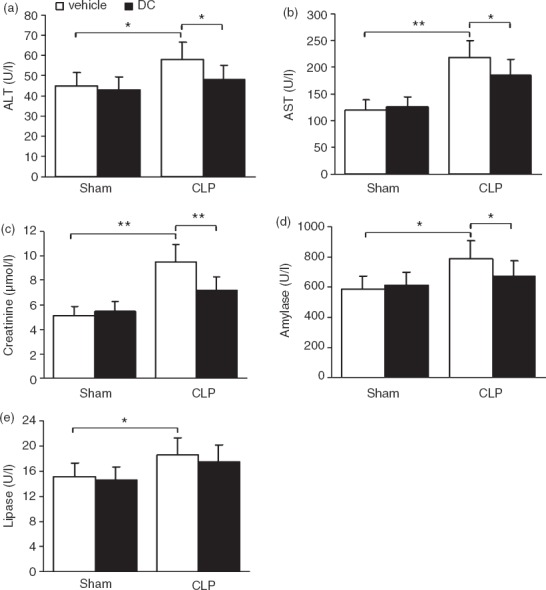
Effect of bone-marrow-derived dendritic cell (BMDC) transfer on organ function and injury. Blood samples were obtained from mice 3 days after sham or caecal ligation and puncture (CLP) surgery and vehicle or BMDC transfer. Using standard laboratory techniques, the samples were tested for: (a) alanine aminotransferase (ALT), (b) aspartate aminotransferase (AST), (c) creatinine, (d) amylase and (e) lipase. The results are presented as the mean ± SD (n = 5 for each group). Error bars indicate the SD. *P < 0·05; **P < 0·01.
BMDC transfer reduces histological organ injury caused by CLP
We histologically examined the effect of BMDC transfer on organ injury 3 days after sham or CLP surgery and vehicle or BMDC transfer using H&E-stained tissue sections (representative photomicrographs are shown in Fig.2). No evident pathological changes in the lung, liver, small intestine or pancreatic tissues were observed in the Sham groups. When compared with the Sham + vehicle group (data not shown), the CLP + vehicle group exhibited significant pathological organ changes, including pulmonary interstitial oedema; capillary tube expansion, hyperaemia and haemorrhage in alveolar cavities; partial collapse of the pulmonary alveolus; a significant decrease in pulmonary ventilation (Fig.2ai); necrosis and exfoliation of small intestinal villi; extensive and acute inflammatory cell infiltrates in intestinal mucosa; interstitial oedema in the intestinal wall (Fig.2bi); focal degeneration and extensive inflammatory cell infiltration in hepatic tissues (Fig.2ci); and focal degeneration and necrosis in pancreatic tissues (Fig.2di). The pathological changes in the organs of the CLP + DC group were less severe than those in the CLP + vehicle group: the extent of pulmonary interstitial oedema; decrease in pulmonary ventilation; and necrosis of small intestinal villi, hepatic cells and pancreatic acinar cells were all markedly reduced (Fig.2, aii–dii). These results demonstrate that BMDC treatment efficiently reduced pathological damage to the lung, liver, small intestine and pancreas in the CLP-sepsis mouse model.
Figure 2.
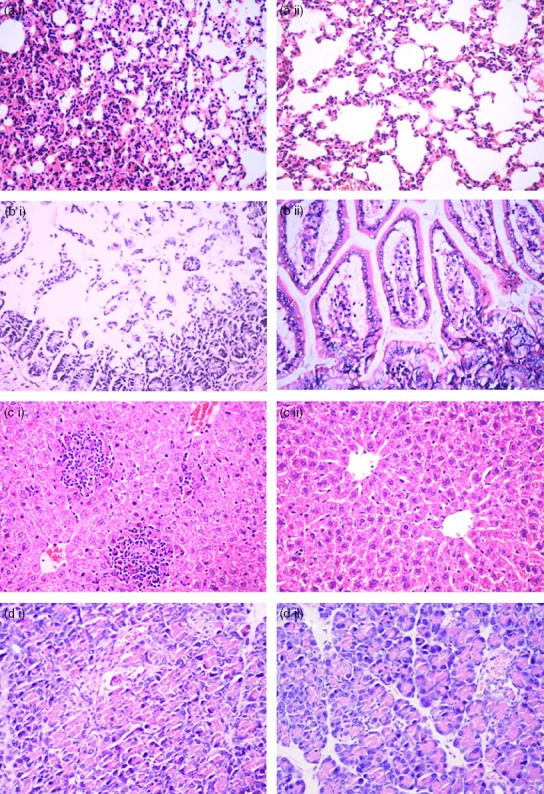
Pathological damage in organs. Tissue samples taken 3 days after surgery were prepared by routine histological techniques and stained with haematoxylin & eosin (H&E) for light microscopy analysis. Lung (a i), small intestine (b i), liver (c i), and pancreatic (d i) sections from mice in the caecal ligation and puncture (CLP) + vehicle group. Lung (a ii), small intestine (b ii), liver (c ii), and pancreatic (d ii) sections from mice in the CLP + dendritic cell (DC) group. The extent of pulmonary interstitial oedema; decrease in pulmonary ventilation; and necrosis of liver cells, small intestine villi and pancreatic acinar cells were all markedly reduced in the CLP + DC group, relative to damage in the CLP + vehicle group. Original magnification: × 400. Figures are representative of results from each experimental group.
Effect of BMDC transfer on cytokine levels in the serum following CLP
Serum levels of individual cytokines are indicative of the existing balance between pro-inflammatory and anti-inflammatory reactions. As shown in Table1, the serum levels of IFN-γ, TNF-α, IL-12p70, IL-6 and IL-10 determined 3 days after surgery in the two Sham groups were not significantly different (all P > 0·05). The serum levels of the pro-inflammatory cytokines IFN-γ and IL-12p70 and the anti-inflammatory cytokines IL-6 and IL-10 were higher in the CLP + vehicle group than in the Sham + vehicle group (all P < 0·05). The levels of IFN-γ and IL-12p70 were higher in the CLP + DC group than in the CLP + vehicle group (both P < 0·05); the levels of TNF-α were slightly higher in the CLP + DC group than in the CLP + vehicle group, although the difference was not statistically significant (P > 0·05). The levels of IL-6 and IL-10 in the CLP + DC group were significantly lower than those in the CLP + vehicle group (both P < 0·05).
Table 1.
Serum cytokine concentrations determined 3 days after surgery1
| Groups | IL-6 | IFN-γ | TNF-α | IL-12p70 | IL-10 |
|---|---|---|---|---|---|
| Sham + vehicle | 18·5 ± 5·4 | 75·8 ± 21·2 | 32·3 ± 13·5 | 82·8 ± 25·5 | 40·1 ± 20·2 |
| Sham + DC | 20·1 ± 7·8 | 91·1 ± 32·2 | 35·5 ± 16·6 | 92·1 ± 24·8 | 32·3 ± 12·6 |
| CLP + vehicle | 43·2 ± 16·8* | 115·6 ± 41·2* | 42·1 ± 21·4 | 110·1 ± 36·7* | 128·8 ± 44·1* |
| CLP + DC | 26·3 ± 15·7** | 136·5 ± 44·7** | 54·1 ± 18·4 | 135·1 ± 32·7** | 50·4 ± 30·9** |
CLP, caecal ligation and puncture; DC, dendritic cell; IFN-γ, interferon-γ; IL-6, interleukin-6; TNF-α, tumour necrosis factor-α.
Values represent the mean ± SD, n = 5 for each group; units: pg/ml.
P < 0·05, CLP + vehicle versus Sham + vehicle.
P < 0·05, CLP + DC versus CLP + vehicle.
Effect of BMDC transfer on regulatory T-cell differentiation
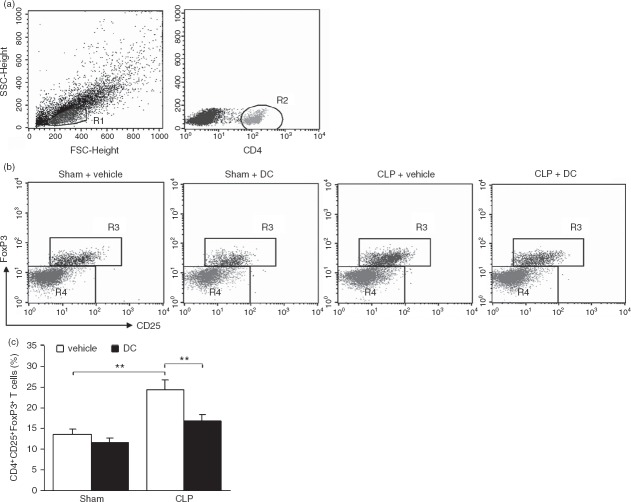
Regulatory T (Treg) cells play a major role in suppressing immune reactivity in infectious disease and injury. The differentiation of splenic CD4+ CD25+ Foxp3+ Treg cells was analysed by flow cytometry. Mononuclear cells were gated based on their size and particles within the FSC and SSC plots (Fig.3a, R1), after which CD4+ T cells were gated (Fig.3a, R2) to determine the percentage of CD4+ CD25+ Foxp3+ Treg cells (Fig.3b, R3). The percentage of CD4+ CD25+ Foxp3+ Treg cells was significantly higher (P < 0·01) in the CLP + vehicle group than in the Sham + vehicle group. The percentage of CD4+ CD25+ Foxp3+ Treg cells was significantly lower (P < 0·01) in the CLP + DC group than in the CLP + vehicle group (Fig.3c). These results show that BMDC treatment reduced the proliferation and differentiation of splenic CD4+ CD25+ Foxp3+ Treg cells in the CLP-sepsis mouse model.
Figure 3.
Effect of bone-marrow-derived dendritic cell (BMDC) transfer on regulatory T-cell differentiation. Spleen mononuclear cells were labelled with monoclonal antibodies against CD4 and CD25, permeabilized, and intracellularly labelled with monoclonal antibody against Foxp3. The percentage of positive cells was determined by flow cytometry. Representative dot plots showing spleen mononuclear cells (a, R1), CD4+ T-cell subsets (a, R2), CD4+ CD25+ Foxp3+ regulatory T (Treg) cells (b, R3), and the percentage of CD4+ CD25+ Foxp3+ Treg cells (c). Error bars indicate the SD. **P < 0·01.
Effect of BMDC transfer on inhibitory T-cell differentiation
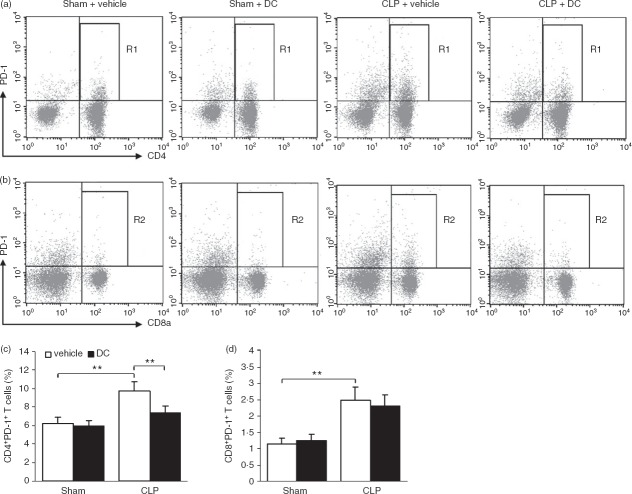
PD-1 is expressed on lymphocytes, particularly T cells, and is an inducible negative regulator of T-cell activity. The expression of PD-1 on splenic T cells was analysed with flow cytometry. Mononuclear cells were gated based on their size and particles within the forward scatter (FSC) and side scatter (SSC) plots, after which the CD3ε-positive T cells in the mononuclear cells were gated to determine the percentage of CD4+ PD-1+ (Fig.4a, R1) and CD8+ PD-1+ T cells (Fig.4b, R2) among the CD3+ T cells. Relative to percentages in the Sham + vehicle group, the percentages of CD4+ PD-1+ and CD8+ PD-1+ T cells increased significantly (all P < 0·01) in the CLP + vehicle group. Relative to the percentage in the CLP + vehicle group, the percentage of CD4+ PD-1+ T cells decreased significantly (P < 0·01) in the CLP + DC group, while the percentage of CD8+ PD-1+ T cells also decreased, but not significantly (P > 0·05) (Fig.4c,d). These results show that BMDC treatment reduced the expression of PD-1 on T cells, particularly on CD4+ T cells, and reduced the differentiation of suppressor T cells.
Figure 4.
Effect of bone-marrow-derived dendritic cell (BMDC) transfer on inhibitory T-cell differentiation. Spleen mononuclear cells were labelled with a mixture of monoclonal antibodies against CD3ε, CD4, CD8a and programmed death 1 (PD-1). The percentage of positive cells was determined by flow cytometry. Representative dot plots showing CD4+ PD-1+ (a, R1) and CD8+ PD-1+ (b, R2) T-cell subsets and the percentage of CD4+ PD-1+ (c) and CD8+ PD-1+ (d) T cells. Error bars indicate the SD. **P < 0·01.
Effect of BMDC transfer on CLP-induced mortality
As described in the Materials and methods section, an additional 80 mice were divided into the four treatment groups (20 mice/group) and monitored for mortality for up to 7 days after sham or CLP surgery and vehicle or BMDC transfer. In the two sham groups (vehicle or BMDC transfer), all mice survived until the end of the observation period. Fatality in mice from the CLP + vehicle group was observed on day 1, and only 30% of the mice survived to day 7. On the other hand, the survival rate of mice in the CLP + DC group was 60% on day 7, and the mortality rate from day 3 to day 7 in this group was much lower than that in the CLP + vehicle group (all P < 0·05) (Fig.5). Hence, BMDC transfer markedly increased the survival rate in the CLP-sepsis mouse model.
Figure 5.
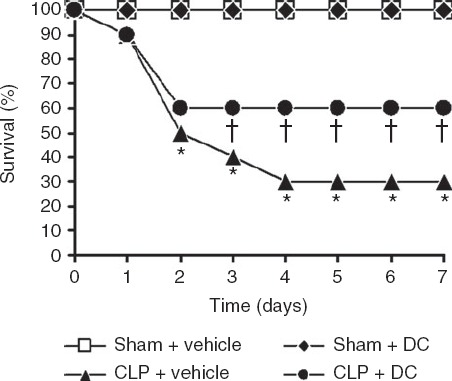
Effect of bone-marrow-derived dendritic cell (BMDC) transfer on caecal ligation and puncture (CLP) -induced mortality. Mortality in the different experimental groups (20 mice/group) was monitored for up to 7 days. The survival rate for the CLP + DC group was significantly higher than that for the CLP + vehicle group. *P < 0·05, CLP + vehicle versus Sham + vehicle/DC; †P < 0·05, CLP + DC versus CLP + vehicle.
Discussion
The current study demonstrated that on day 3 after the onset of sepsis (the most severe stage observed in the mouse model), when the host displayed an excessive inflammatory response, there were significant pathological changes or dysfunction in the lung, liver, kidney, small intestine and pancreas. The expression of the negative regulator PD-1 on T cells increased, and the differentiation of CD4+ CD25+ Foxp3+ Treg cells also increased significantly at the same time. These changes contributed to the suppressed immune reaction. After the administration of BMDCs to CLP-sepsis mice, the levels of the T helper type 1 (Th1) cytokines IFN-γ and IL-12p70 increased, while the levels of the Th2 cytokines IL-6 and IL-10 decreased significantly. More importantly, the expression of the immune suppressor molecule PD-1 on T cells decreased significantly, particularly on the CD4+ T cells. The differentiation of suppressor T cells was then reduced significantly, as were the proliferation and differentiation of CD4+ CD25+ Foxp3+ Treg cells, which alleviated pathological changes and organ damage and markedly increased the survival rate.
Because DCs bridge the innate and adaptive immune responses, a decrease in DCs can suppress the immune response. Studies have shown that apoptosis is the major cause of the decrease in mature DCs during sepsis, wherein the antigen-presenting function of DCs as well as their promotion of T-lymphocyte proliferation and activation both decrease, so further inducing the suppression of the adaptive immune response, which is closely associated with poor prognosis after sepsis. Guisset et al.7 noticed a decrease in circulating blood DCs in patients with sepsis and much higher DC numbers in survivors of sepsis than in deceased patients. Scumpia et al.14 depleted CD11c+ DCs in mice by injecting diphtheria toxin, which blocked the expression of the CD11c gene, and found that mortality was significantly higher than in normal mice. Therefore, maintaining an adequate number of DCs plays a critical role in improving recovery after sepsis and increasing the survival rate.
In this study, we used a murine CLP-sepsis model because it is the most widely used model for experimental sepsis and is currently considered the gold standard in sepsis research. In this model, sepsis originates from a polymicrobial infectious focus within the abdominal cavity. This is followed by bacterial translocation into the blood compartment, which then triggers a systemic inflammatory response.13 Related research has shown that the numbers of CD11c+ DCs in the spleen and lymph nodes of the CLP-sepsis model mice decrease significantly between 12 and 36 hr, a peak phase of animal mortality.15,16 In the current study, injection of BMDCs into mice at 6, 12 and 24 hr efficiently replenished the number of DCs and so reduced the extent of sepsis-induced organ damage, increased the survival rate and achieved good clinical outcome.
The host immune response during sepsis is not a simple excessive inflammatory reaction. Research has shown that over 80% of patients with sepsis who survive the early stage of the disease eventually die of a secondary infection caused by the immunosuppressed state.17 In laboratory tests, immunosuppression was observed in patients with sepsis as significantly increased levels of IL-10 in blood plasma, compromised T-cell function, and massive depletion of immunocytes, all of which were indicative of the negative regulation of the immune response during the development of sepsis.18 In addition to the decrease in DC number, DC functions also changed in patients with sepsis. Research has shown that the production of the pro-inflammatory cytokine IL-12 by DCs decreases significantly during sepsis, while the production of the anti-inflammatory cytokine IL-10 increases significantly. This pushes the Th1/Th2 balance towards Th2-produced anti-inflammatory cytokines such as IL-4, IL-10 and IL-13, which enhance immunosuppression.19 The current study using the CLP-sepsis model showed that the levels of the pro-inflammatory cytokines IFN-γ, TNF-α and IL-12p70 in the serum increased on day 3 and that an excessive inflammatory response existed. Meanwhile, the levels of IL-6 and IL-10 increased significantly, moving the Th1/Th2 balance towards Th2, which induced anti-inflammatory reactions and immunosuppression.
Recent studies have shown that the number of DCs decreases significantly during the course of sepsis and that DCs appear to induce immune tolerance under the influence of apoptotic cell antigens, so promoting the proliferation and differentiation of Treg cells and suppressor T cells, the major cause of immunodeficiency.20,21 PD-1 is expressed on lymphocytes, particularly T cells, and is an inducible negative regulator of T-cell activity. Ligation of PD-1 by its ligand PD-L1 induces a co-inhibitory signal in activated T cells and promotes T-cell apoptosis, anergy and functional exhaustion.22,23 Huang et al.24 reported that abdominal bacterial clearance in PD-1−/− septic mice was much higher than in wild-type mice. Additionally, the anti-PD-1 antigen enhanced the expression of an apoptosis-suppressing gene (Bcl-xL) and reduced T-cell apoptosis during sepsis.25 Zhang et al.26 showed that expression of PD-1 on T cells and PD-L1 on monocytes was up-regulated in septic mice. Therefore, an effective blockade of PD-1 and PD-L1 could reduce the mortality rate.
Treg cells are a subset of lymphocyte T cells with negative-regulatory functions. The proliferation of Treg cells is closely associated with sepsis-induced immunosuppression. Monneret et al.27 first observed that sepsis increases CD4+ CD25+ T cells in the peripheral blood of septic patients. Scumpia et al.,28 in a murine model of polymicrobial sepsis, observed an increased percentage of Treg cells in the spleen in comparison with sham mice. Here, although the absolute number of Treg cells was not changed after sepsis, the cells did express a higher level of Foxp3 mRNA, and they were more efficient than the Treg cells from sham mice in altering the proliferative capacity of effector T cells. In a similar study, Wisnoski et al. observed that polymicrobial sepsis was associated with an increased percentage of Treg cells in blood and spleen and with an increase in Foxp3 expression in the spleen. This increase was not present in IL-10-deficient mice, whereas it was enhanced in IL-6-deficient mice, suggesting that the increase was related to the development of Tr1 cells.29
The current study showed the following: the ratio of CD4+ PD-1+ and CD8+ PD-1+ T cells increased significantly in the septic spleen; the differentiation of suppressor T cells was enhanced; the number of CD4+ and CD8+ effector T cells, in which PD-1 was not expressed, decreased; and the proliferation of Treg cells, in which Foxp3 was expressed, was markedly enhanced, indicating an immunosuppressive function of T cells. BMDC treatment efficiently inhibited the induction of the immunosuppression function of the remaining DCs in septic mice, reduced the proliferation and differentiation of Treg cells and suppressor T cells, enhanced the immune clearance of sepsis-causing pathogens, and eventually reduced organ damage thereby improving therapy outcome.
In summary, BMDC treatment affected the differentiation and immune function of T cells, so reducing sepsis-induced immunodeficiency and organ damage caused by secondary infection. The treatment had an immunoregulatory effect on sepsis. However, it remains unknown whether the injected BMDCs distribute back to the septic host and improve sepsis-induced immunosuppression in the long term. Regulation of the host immune system is complex, and the effect of DC differentiation, maturation and migration as well as information transfer via other cytokines during sepsis has yet to be explored. Therefore, the time, dose and safety aspects of DC treatment require further study.
Acknowledgments
This study was supported, in part, by grants from the National Natural Science Foundation of China (Project No. 81000848 and No. 81302538) and the Capital Health Research and Development of Special of China (Project No. 2011-5002-01 and No. 2011-5002-02).
Disclosure
The authors declare that they have no competing interests.
References
- Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–44. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl M, Chung CS, Garber M, Huang X, Ayala A. Contribution of anti-inflammatory/immune suppressive processes to the pathology of sepsis. Front Biosci. 2006;11:272–99. doi: 10.2741/1797. [DOI] [PubMed] [Google Scholar]
- Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–47. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–22. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- Wen H, Hogaboam CM, Gauldie J, Kunkel SL. Severe sepsis exacerbates cell-mediated immunity in the lung due to an altered dendritic cell cytokine profile. Am J Pathol. 2006;168:1940–50. doi: 10.2353/ajpath.2006.051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Fujimi S, Lederer JA, Hubbard WJ, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J Immunol. 2006;177:4514–20. doi: 10.4049/jimmunol.177.7.4514. [DOI] [PubMed] [Google Scholar]
- Guisset O, Dilhuydy MS, Thiébaut R, et al. Decrease in circulating dendritic cells predicts fatal outcome in septic shock. Intensive Care Med. 2007;33:148–52. doi: 10.1007/s00134-006-0436-7. [DOI] [PubMed] [Google Scholar]
- Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C. Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit Care. 2009;13:R119. doi: 10.1186/cc7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by Fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J Immunol. 2005;174:404–10. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with Fms-like tyrosine Kinase-3 ligand after burn injury enhances global immune responses to infection. J Immunol. 2008;180:3038–48. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- Wang HW, Yang W, Lu JY, Tian G, Li F, Wang XH, Kang JR, Yang Y. Treatment with Fms-like tyrosine kinase 3 ligand reverses lung dendritic cell immunoparalysis and ameliorates zymosan-induced secondary lung injury in mice. Clin Exp Immunol. 2012;170:156–66. doi: 10.1111/j.1365-2249.2012.04641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamim CF, Lundy SK, Lukacs NW, Hogaboam CM, Kunkel SL. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood. 2005;105:3588–95. doi: 10.1182/blood-2004-08-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina E. Murine model of polymicrobial septic peritonitis using cecal ligation and puncture (CLP) Methods Mol Biol. 2010;602:411–5. doi: 10.1007/978-1-60761-058-8_23. [DOI] [PubMed] [Google Scholar]
- Scumpia PO, McAuliffe PF, O'Malley KA, et al. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol. 2005;175:3282–6. doi: 10.4049/jimmunol.175.5.3282. [DOI] [PubMed] [Google Scholar]
- Ding Y, Chung CS, Newton S, Chen Y, Carlton S, Albina JE, Ayala A. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock. 2004;22:137–44. doi: 10.1097/01.shk.0000131194.80038.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron PA, Martins A, Minnich D, et al. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. J Immunol. 2004;173:3035–43. doi: 10.4049/jimmunol.173.5.3035. [DOI] [PubMed] [Google Scholar]
- Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TS, Deng JC. Molecular and cellular aspects of sepsis-induced immunosuppression. J Mol Med (Berl) 2008;86:495–506. doi: 10.1007/s00109-007-0300-4. [DOI] [PubMed] [Google Scholar]
- Shubin NJ, Monaghan SF, Ayala A. Anti-inflammatory mechanisms of sepsis. Contrib Microbiol. 2011;17:108–24. doi: 10.1159/000324024. [DOI] [PubMed] [Google Scholar]
- Faivre V, Lukaszewicz AC, Alves A, Charron D, Payen D, Haziot A. Human monocytes differentiate into dendritic cells subsets that induce anergic and regulatory T cells in sepsis. PLoS ONE. 2012;7:e47209. doi: 10.1371/journal.pone.0047209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastille E, Didovic S, Brauckmann D, Rani M, Agrawal H, Schade FU, Zhang Y, Flohé SB. Modulation of dendritic cell differentiation immunosuppression after polymicrobial in the bone marrow mediates sustained sepsis. J Immunol. 2011;186:977–86. doi: 10.4049/jimmunol.1001147. [DOI] [PubMed] [Google Scholar]
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Venet F, Wang YL, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA. 2009;106:6303–8. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–40. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou Y, Lou J, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–71. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- Scumpia PO, Delano MJ, Kelly KM, et al. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006;177:7943–9. doi: 10.4049/jimmunol.177.11.7943. [DOI] [PubMed] [Google Scholar]
- Wisnoski N, Chung CS, Chen Y, Huang X, Ayala A. The contribution of CD4+ CD25+ T-regulatory-cells to immune suppression in sepsis. Shock. 2007;27:251–7. doi: 10.1097/01.shk.0000239780.33398.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]