Abstract
Extracellular signal-regulated kinase 3 (ERK3 )is an atypical member of the mitogen-activated protein kinase (MAPK) family. We have previously shown that ERK3 is expressed during thymocyte differentiation and that its expression is induced in mature peripheral T cells following activation of ERK1/2 by T-cell receptor (TCR) signalling. Herein, we have investigated whether ERK3 expression is required for proper T-cell selection. Using a knock-in mouse model in which the coding sequence of ERK3 is replaced by the gene encoding for the β-galactosidase reporter, we show that ERK3 is expressed by double-positive (DP) thymocytes undergoing positive selection. In ERK3-deficient mice with a polyclonal TCR repertoire, we observe a decrease in positive selection. This reduction in positive selection was also observed when ERK3-deficient mice were backcrossed to class I- and class II-restricted TCR transgenic mice. Furthermore, the response of DP thymocytes to in vitro TCR stimulation was strongly reduced in ERK3-deficient mice. Together, these results show that ERK3 expression following TCR signalling is critical for proper thymic positive selection.
Keywords: mitogen-activated protein kinases, positive selection, thymus
Introduction
The generation of T cells in the thymus is mediated by a complex biological mechanism that combines differentiation, proliferation, death, selection and lineage commitment. Stem cells seed the thymus and differentiate into immature CD4− CD8− double-negative (DN) thymocytes. These must first rearrange successfully the T-cell receptor β (TCR-β) locus and express the pre-TCR (β-selection) in order to differentiate into CD4+ CD8+ double-positive (DP) thymocytes.1 The DP thymocytes rearrange the TCR-α genes and start expressing the conventional TCR. As TCR rearrangement creates a wide diversity of TCR sequences, DP thymocytes must undergo an education process to preserve thymocytes expressing a useful TCR (positive selection) while eliminating thymocytes with an autoreactive TCR (negative selection). Properly educated DP thymocytes will then further differentiate into CD4+ or CD8+ single-positive (SP) thymocytes depending on their MHC specificity. SP thymocytes exit as naive T cells into the peripheral lymphoid organs.2,3
Positive selection is the result of DP thymocytes perceiving an MHC-restricted signal via their TCR. As a consequence these will up-regulate TCR expression levels. They will also increase the expression of CD69 and CD5. Therefore, using CD5 expression, DP thymocytes can be divided into three DP thymocyte subsets.4 DP1 thymocytes, which express low levels of the TCR and CD5, represent pre-selection thymocytes. The DP1 thymocytes that will receive a positive selection signal will become DP2 thymocytes that bear a high level of CD5 and intermediate level of the TCR. Some of these DP2 will become DP3 thymocytes expressing high levels of TCR and intermediate levels of CD5. Interestingly, DP1 and DP2 thymocytes can generate both CD4SP and CD8SP thymocytes while DP3 thymocytes will only give rise to CD8 T cells. This suggests that a different window and signalling threshold exists for the selection of thymocytes into the CD4 or CD8 lineage.4
The mitogen-activated protein kinases (MAPKs) extracellular signal regulated kinase 1 (ERK1) and ERK2 are activated upon stimulation of cells with a broad range of extracellular signals including antigens.5,6 Activated ERK1/2 translocate to the nucleus to mediate the phosphorylation of transcription factors allowing cellular responses to occur.7 The ERK1/2 MAPKs are rapidly phosphorylated in thymocytes and mature T cells following TCR activation. The activation of ERK1/2 is essential for proper T-cell differentiation events, as mice lacking ERK1 and ERK2 show a severe thymic differentiation defect.8,9 Indeed, ERK1 and ERK2 double-deficient thymocytes show a blockade at the β-selection checkpoint, indicating that ERK1/2 activation by the pre-TCR is essential for the transition from DN to DP.8 Furthermore, deletion of ERK1 and ERK2 in DP thymocytes severely impairs positive selection and the subsequent generation of SP thymocytes and mature T cells.8 Moreover, differential strength and kinetics of ERK1/2 activation allow for the discrimination between positively and negatively selecting ligands by DP thymocytes.3,10,11 Low and sustained ERK1/2 activation occurs during positive selection whereas high and short ERK1/2 activation is observed during negative selection.3,10,11 In contradiction to these observations, it was shown that ERK1/2 activation is not necessary for negative selection to occur.12
Other members of the ERK family have been described13 but their roles in T-cell differentiation are ill-defined. ERK3 is another member of the MAPK family with highest homology to ERK1/2.13,14 ERK3, and its paralogous protein ERK4, are considered to be atypical MAPKs because they lack the conserved Thr-Xaa-Tyr motif in the activation loop and possess a long C-terminal extension.13,14 The signalling events leading to ERK3 activation and its substrates or partners are still largely unknown. ERK3 is constitutively phosphorylated by group I p-21-activated kinases15,16 in resting cells and its phosphorylation status does not change in response to various extracellular signals.17
We have previously shown that ERK3 expression is induced via ERK1/2 in mature T cells following TCR stimulation.18 We also reported that Erk3 is transcribed during thymic T-cell differentiation with the highest level of expression in DN and DP thymocytes.19 Inactivation of the Erk3 gene leads to a reduction in thymic cell number that is caused by a twofold reduction in DP thymocyte number because of their reduced survival during the rearrangement of the TCR-α chain.19 Indeed, introduction of a transgene encoding for a rearranged TCR rescues DP thymocyte number.19 However, in this study, we did not evaluate the contribution of Erk3 to thymic selection events.
The induction of ERK3 expression by the classical MAPK ERK1/2 and the known key roles of ERK1/2 during thymic T-cell differentiation and positive selection have led us to investigate whether ERK3 expression is induced following thymic positive selection and if its induction is required for proper T-cell selection. We show that ERK3 is expressed by pre- and post-selection DP thymocytes. In the absence of ERK3 expression, we observe a decrease in positive selection both in mice with a polyclonal repertoire of TCRs and in TCR transgenic mice. Together these results show that ERK3 expression by DP thymocytes is critical for proper thymic positive selection.
Material and methods
Mice
Erk3-deficient mice were described previously.20 Erk3+/− mice on a 129/SvEv background and Erk3+/− mice on a C57BL/6 background were bred together to obtain Erk3+/− F1 mice. These F1 mice were then bred with one another to obtain Erk3+/+ and Erk3−/− F2 embryos. OT-I21 and OT-II22 TCR transgenic mice on a C57BL/6 and Rag1−/− background, referred to respectively as OT-I and OT-II mice, were provided by Taconic (Hudson, NY). These mice were bred with Erk3+/− mice on a C57BL/6 background. The progeny was inter-crossed to obtain Erk3+/+ or Erk3−/− OT-I and OT-II Rag1-deficient E18.5 embryos.
Antibodies and flow cytometry
Anti-CD4 (RM4-5), anti-CD8 (53-6.7) and anti-CD69 (H1.2F3) antibodies were bought from BD Biosciences (Mississauga, ON, Canada). Anti-TCR-β (H57-597) and anti-CD5 (53-7.3) antibodies were purchased from Biolegend (San Diego, CA).
Thymi from E18.5 embryos were dissociated and cell staining was performed as previously described23 followed by acquisition on a FACSCanto (BD Biosciences). Data analysis was performed using FlowJo (TreeStar, Ashland, OR).
β-galactosidase staining
Fluorescein digalactopyranoside (FDG; Sigma-Aldrich, Oakville, ON, Canada) staining was performed as previously described.18 Briefly, cells were surface stained and re-suspended in PBS. Warmed FDG (7·5 mm) was added to cells while gently vortexing. The reaction was stopped by adding ice-cold PBS and cells were kept on ice for 5 min. After centrifugation, cells were re-suspended in PBS supplemented with 10% horse serum and incubated at 15° for 15–20 min to enhance β-galactosidase activity before flow cytometry analysis.
In vitro thymocyte stimulation
Twenty-four-well plates were coated with 1 μg/ml of purified anti-CD3 antibodies (Cedarlane, Burlington, ON, Canada; clone 145-2C11); 2 × 106 thymocytes were stimulated for 24 hr. Cells were harvested and stained with corresponding antibodies.
Statistical analysis
Statistical analyses for differences between groups were performed using a Mann–Whitney U-test. Data are presented as mean ± SEM. All tests were two sided, and P < 0·05 was considered statistically significant.
Results
ERK3 is expressed during positive selection
We have recently shown that ERK3 expression is induced following TCR stimulation in mature T cells18 and that ERK3 is expressed during thymocyte differentiation.19 We therefore investigated whether ERK3 was expressed in thymocytes undergoing positive selection during thymic differentiation. To do so, we took advantage of a mouse in which the Erk3 coding sequence is replaced by the LacZ reporter.20 To identify cells transcribing Erk3, we measured β-galactosidase activity using the fluorescent substrate FDG. This approach correlates well with data obtained by RT-quantitative PCR (not shown). As shown in Fig.1, Erk3 transcription is detectable in DP thymocytes, the differentiation stage in which positive and negative selection occurs. More precisely, Erk3 expression was found in cells that have undergone positive selection, which can be identified based on their increased levels of TCR, CD69 or CD5 expression. Therefore ERK3 expression during DP thymocyte positive selection indicates that it may play a role in this process.
Figure 1.
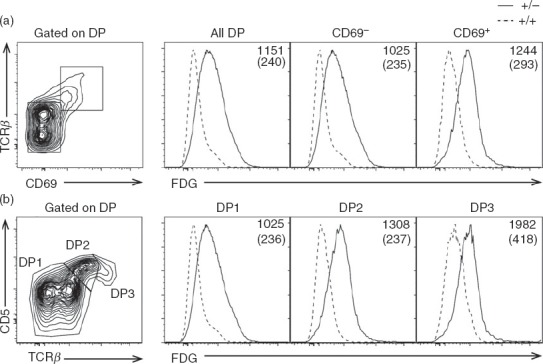
Erk3 expression is induced during positive selection. Erk3+/− thymocytes were stained with fluorescein digalactopyranoside (FDG) to detect Erk3 transcription. Erk3+/+ thymocytes served as negative controls. (a) Histograms show FDG expression on total double-positive (DP) thymocytes, pre-selection DP thymocytes (CD69−) and post-selection DP thymocytes (CD69+). Representative of three independent experiments. (b) The expression of extracellular signal-regulated kinase (ERK3; FDG) is shown for DP1 (TCRlo CD5lo), DP2 (TCRint CD5hi) and DP3 (TCRhi CD5int) thymocytes. Bold numbers on histograms represent the FDG mean fluorescence intensity (MFI) of Erk3+/− thymocytes while numbers in parenthesis represent the FDG MFI of Erk3+/+ thymocytes.
Reduced positive selection of polyclonal thymocytes in the absence of ERK3
The expression of ERK3 during thymocyte positive selection led us to evaluate whether ERK3 deficiency affects thymic selection events. Since Erk3−/− mice die at birth,20 we used thymi from E18.5 embryos to study thymic T-cell selection. The use of immature thymi explains their unusual TCR, CD69 and CD5 profile (compare Fig.1, where adults were used, to Fig.2). Moreover, no CD8SP thymocytes are detectable in E18.5 thymi (Fig.2a) and the CD8+ CD4lo thymocytes represent immature intermediate SP thymocytes (Fig.2a) that are on their way to become DP thymocytes and as such they do not express the TCR (data not shown). We have previously published that ERK3 deficiency reduces DP and CD4SP thymocyte numbers.19 However, in these experiments, we did not evaluate whether ERK3 influences positive selection of thymocytes. To test if positive selection is impaired in absence of ERK3, we analysed the expression of cell surface markers that are associated with positive selection. As shown in Fig.2(b) and (d), ERK3-deficient DP, CD4+ CD8low and CD4SP thymocytes show reduced up-regulation of TCR and CD69 expression. Furthermore, we also observed a statistically significant reduction in the production of DP2 post-selection thymocytes in the absence of ERK3 and a tendency (P = 0·134) for a reduction in DP3 thymocytes (Fig.2c and e), again illustrating a defect in positive selection. These observations suggest that ERK3 may contribute to positive selection events.
Figure 2.
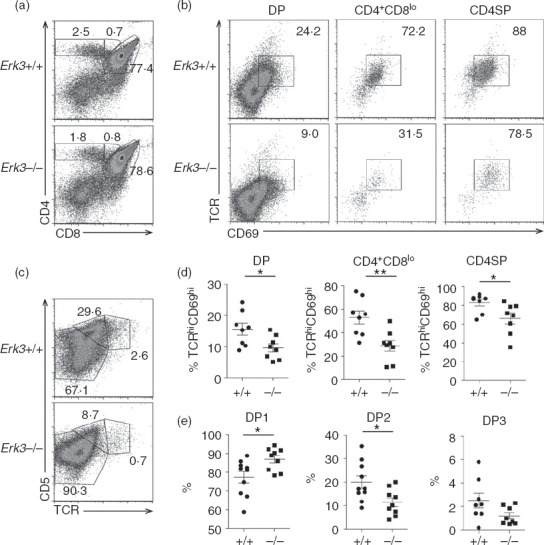
Defective positive selection of extracellular signal-regulated kinase 3 (ERK3) -deficient polyclonal thymocytes. (a) Representative CD4/CD8 thymic profile of Erk3+/+ and Erk3−/− E18.5 thymi. The percentage of cells within the different thymic subsets is indicated on the FACS dot plots. (b) T-cell receptor (TCR) and CD69 expression is shown for double-positive (DP), CD4+ CD8lo and CD4 single-positive (SP) Erk3+/+ and Erk3−/− thymocytes from E18.5 embryos. The percentage of TCR+ CD69+ cells is indicated on the FACS profile. (c) TCR and CD5 expression on DP thymocytes is shown for Erk3+/+ and Erk3−/− mice. The percentage of DP1 (TCRlo CD5lo), DP2 (TCRint CD5hi) and DP3 (TCRhi CD5int) thymocytes is indicated on the FACS dot plot. (d) Compilation of the frequency of Erk3+/+ and Erk3−/− TCRhi CD69+ thymocytes within the different thymocyte subsets. (e) Compilation of the frequency of DP1, DP2 and DP3 thymocytes in Erk3+/+ and Erk3−/− thymi. The line represents the mean and the error bars are the standard error of the mean (SEM). Each dot represents one individual mouse. The statistical significance was determined with a Mann–Whitney U-test (*P < 0·05; **P < 0·01). The results of three independent experiments are shown.
Impaired positive selection of MHC class II-restricted OT-II TCR transgenic thymocytes in absence of ERK3
Although the results presented above suggested a role for ERK3 during positive selection, it was important to rule out any other possible defect that could lead to a reduction in the generation of SP thymocytes. We have previously shown that ERK3 is necessary for the proper survival of DP thymocytes to allow them to test different TCR-α chain rearrangements.19 This could directly influence positive selection by limiting the chance of a DP thymocyte to express a TCR permissive to positive selection. To bypass this problem, we have introduced already rearranged TCR-α and TCR-β transgenes into the ERK3-deficient background. As previously shown,19 introduction of the OT-II TCR specific for ovalbumin in the context of the MHC class II molecule I-Ab restores thymic cellularity and DP thymocyte number allowing us to study further steps in T-cell differentiation (Fig.3). One striking effect of ERK3 deficiency was to reduce the generation of OT-II CD4SP thymocytes (Fig.3). To test the possibility that this was the result of impaired positive selection, we evaluated the expression on DP thymocytes of cell surface markers that are induced following positive selection. As shown in Fig.4(a) and (c), ERK3-deficient OT-II DP thymocytes show a severe reduction in the TCRhi CD69hi subsets, indicating that ERK3 affects positive selection of DP thymocytes. We also confirmed that ERK3 deficiency affects positive selection by measuring CD5 up-regulation (Fig.4b and d). These results indicate that ERK3 expression is required for proper positive selection of MHC class II-restricted thymocytes.
Figure 3.
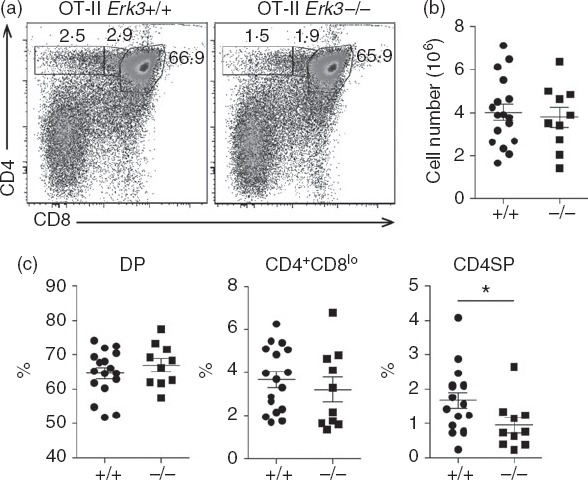
Impaired generation of CD4 single-positive (SP) thymocytes expressing the MHC class II-restricted OT-II T-cell receptor (TCR) in the absence of extracellular signal-regulated kinase 3 (ERK3). CD4/CD8 FACS profiles (a) are shown for OT-II Erk3+/+ and OT-II Erk3−/− mice. The percentage of each gated population is indicated on the dot plot. A representative FACS profile out of six independent experiments is shown. Total thymocyte numbers (b), double-positive (DP) thymocytes, CD4+ CD8lo thymocytes and CD4SP thymocytes frequencies (c) are shown for OT-II+/+ and OT-II−/− mice. The line represents the mean and the error bars are the standard error of the mean (SEM). Each dot represents one individual mouse. The statistical significance was determined with a Mann–Whitney U-test (*P < 0·05). The results of three independent experiments are shown.
Figure 4.
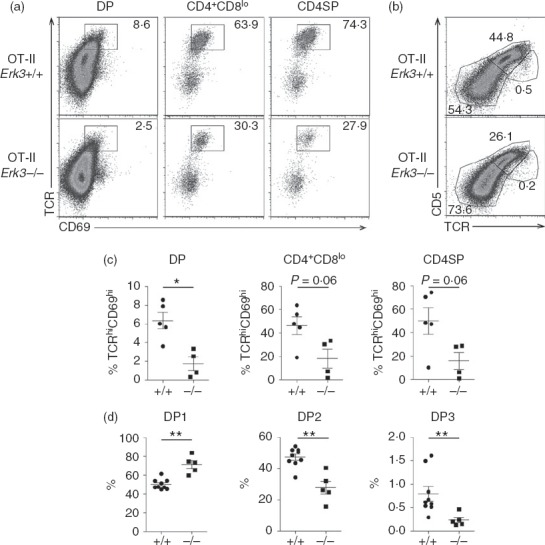
Impaired positive selection of extracellular signal-regulated kinase 3 (ERK3) -deficient OT-II thymocytes. (a) T-cell receptor (TCR) and CD69 expression is shown for double-positive (DP), CD4+ CD8lo and CD4 single-positive (SP) Erk3+/+ and Erk3−/− OT-II thymocytes. The percentages of TCR+ CD69+ cells are indicated on the FACS profiles. (b) TCR and CD5 expression on OT-II DP thymocytes are shown for Erk3+/+ and Erk3−/− mice. The percentages of DP1 (TCRlo CD5lo), DP2 (TCRint CD5hi) and DP3 (TCRhi CD5int) thymocytes are indicated on the FACS dot plot. (c) Compilation of the frequency of OT-II Erk3+/+ and Erk3−/− TCRhi CD69+ thymocytes within the different thymocyte subsets. (d) Compilation of the frequency of DP1, DP2 and DP3 thymocytes in OT-II Erk3+/+ and Erk3−/− thymi. The line represents the mean and the error bars are the standard error of the mean (SEM). Each dot represents one individual mouse. The statistical significance was determined with a Mann–Whitney U-test (*P < 0·05; **P < 0·01). The results of three independent experiments are shown.
Positive selection of MHC class I-restricted OT-I TCR transgenic thymocytes is strongly reduced by ERK3 deficiency
We then investigated whether ERK3 was also required for the positive selection of MHC class I-restricted thymocytes. To do this, the ERK3 deficiency was introduced into the OT-I TCR transgenic mice, which express a TCR specific for ovalbumin in the context of the MHC class I molecule Kb. The introduction of an already rearranged TCR transgene accelerated thymic differentiation, allowing us to study CD8SP thymocyte differentiation in E18.5 thymi. As shown in Fig.5, the generation of CD8SP thymocytes was severely impaired in the absence of ERK3 and as a consequence the proportion of DP thymocytes was increased. The few CD8SP thymocytes that were present did not express the TCR (Fig.6a) and so probably represent cells at the intermediate step of differentiation between DN and DP. To further demonstrate that the reduced generation of CD8SP thymocytes was a consequence of defective thymocyte-positive selection, we have analysed the expression of markers that are induced following positive selection. As shown in Fig.6(a) and (c), DP thymocytes from Erk3−/− mice were severely impaired in their ability to up-regulate TCR-β and CD69, indicating that they did not properly perceive the positive selection signal. Moreover, there was also a severe reduction in the generation of DP2 and DP3 thymocytes (Fig.6b and d), again indicating defective positive selection in the absence of ERK3. In conclusion, ERK3 is necessary for the proper positive selection of both MHC class I- and class II-restricted thymocytes.
Figure 5.
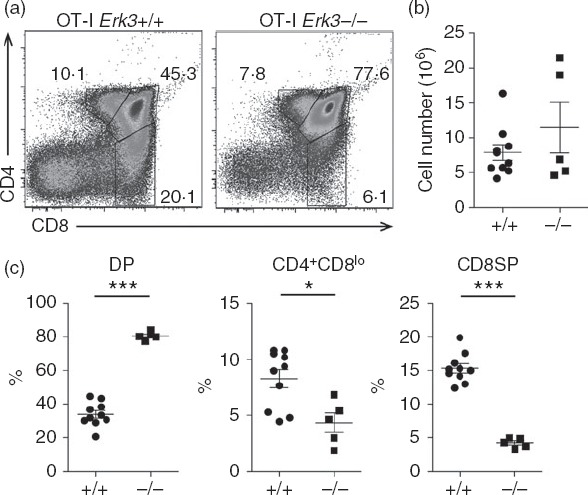
Generation of SP8 thymocytes from the MHC class I-restricted OT-I T-cell receptor (TCR) transgenic mice is impaired in absence of extracellular signal-regulated kinase 3 (ERK3). CD4/CD8 FACS profiles (a) are shown for OT-I Erk3+/+ and OT-I Erk3−/− mice. The percentage of each gated population is indicated on the dot plot. Representative results out of five experiments are shown. Total thymocytes numbers (b), double-positive (DP) thymocytes, CD4+ CD8lo thymocytes and CD8 single-positive (SP) thymocytes frequencies (c) are shown for OT-I Erk3+/+ and OT-I Erk3−/− mice. The line represents the mean and the error bars are the standard error of the mean (SEM). Each dot represents one individual mouse. The statistical significance was determined with a Mann–Whitney U-test (*P < 0·05; ***P < 0·001). The results of four independent experiments are shown.
Figure 6.
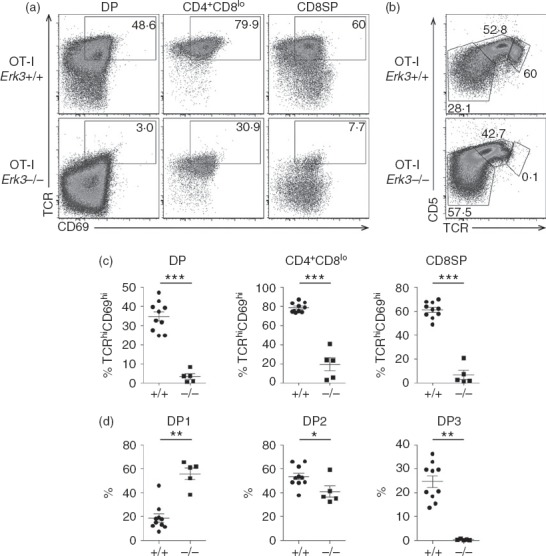
Impaired positive selection of extracellular signal-regulated kinase 3 (ERK3) -deficient OT-I thymocytes. (a) T-cell receptor (TCR) and CD69 expression is shown for double-positive (DP), CD4+ CD8lo and CD8 single-positive (SP) OT-I Erk3+/+ and Erk3−/− thymocytes. The percentage of TCR+ CD69+ cells is indicated each FACS profile. (b) TCR and CD5 expression on DP thymocytes is shown for OT-I Erk3+/+ and Erk3−/− mice. The percentages of DP1 (TCRlo CD5lo), DP2 (TCRint CD5hi) and DP3 (TCRhi CD5int) thymocytes are indicated on each FACS dot plot. (c) Compilation of the frequency of OT-I Erk3+/+ and Erk3−/− TCRhi CD69+ thymocytes within the different thymocyte subsets. (d) Compilation of the frequency of DP1, DP2 and DP3 thymocytes in OT-I Erk3+/+ and Erk3−/− thymi. The line represents the mean and the error bars are the standard error of the mean (SEM). Each dot represents one individual mouse. The statistical significance was determined with a Mann–Whitney U-test (*P < 0·05; **P < 0·01; ***P < 0·001). The results of four independent experiments are shown.
The response of DP and SP thymocytes to TCR stimulation is decreased in the absence of ERK3
The experiments described above were done in germline Erk3−/− mice in which all thymic cell types are ERK3 deficient. Hence, it was possible that ERK3 deficiency was affecting thymocyte differentiation in a non-thymocyte autonomous fashion. For example, ERK3 deficiency could affect the composition of thymic antigen-presenting cells or their ability to present self-peptide MHC ligands to thymocytes. However, preliminary characterization of the thymic epithelial cells did not show any difference in MHC class I and class II expression between Erk3+/+ and Erk3−/− mice (data not shown). Although these data suggest that the defective positive selection that we observe in the absence of ERK3 was not the result of an impairment of thymic epithelial cells, we further evaluated the response of isolated thymocytes to in vitro TCR stimulation. This was possible because DP thymocytes placed at 37° in single cell suspensions increase surface TCR expression24 both in Erk3+/+ and Erk3−/− mice (see Supporting information, Fig. S1), compensating for TCR expression differences observed in vivo (Fig.6). OT-I thymocytes from Erk3+/+ and Erk3−/− mice were stimulated with anti-CD3 antibodies for 24 hr and then stained to analyse the up-regulation of activation markers. As shown in Fig.7, DP and CD8SP thymocytes deficient for ERK3 failed to properly up-regulate the expression of CD5 and CD69 following TCR triggering. Similar results were obtained after stimulation with anti-CD3 antibodies of OT-II Erk3−/− thymocytes (see Supporting information, Fig. S2). These results indicate that an intrinsic ERK3 deficiency in thymocytes leads to an impairment in their ability to respond to TCR signalling, which then decreases thymic positive selection.
Figure 7.
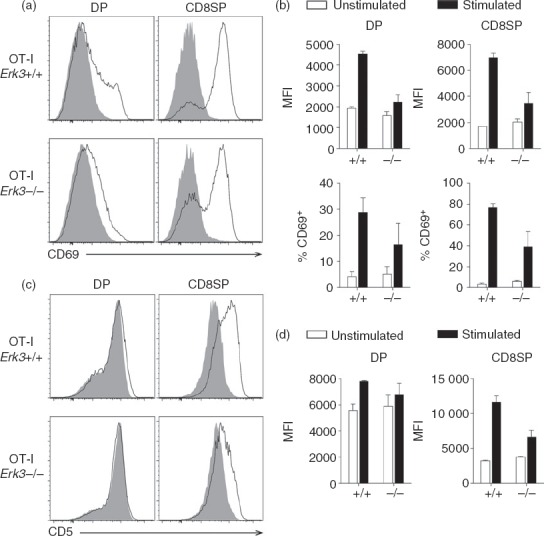
Defective up-regulation of CD69 and CD5 expression in OT-I T-cell receptor (TCR) transgenic thymocytes lacking extracellular signal-regulated kinase 3 (ERK3) after TCR stimulation. OT-I thymocytes were incubated for 24 hr with (black line) or without (filled grey histogram) anti-CD3 antibodies for stimulation. The expression of CD69 (a) and CD5 (c) is shown for double-positive (DP) and CD8 single-positive (SP) thymocytes. (b) Compilation of the mean fluorescence intensity (MFI) of CD69 on CD69+ thymocytes (top panel) and percentage of CD69+ cells (bottom panel). (d) Compilation of the MFI of CD5 expression by unstimulated and stimulated cells is shown. The bar graphs represent the mean and the error bars are the standard error of the mean (SEM). The results of two independent experiments are shown.
Discussion
In this study, we have demonstrated that ERK3 deficiency affects thymic positive selection, a critical step of T-cell differentiation for the generation of a useful repertoire of mature T cells. Although positive selection was affected in Erk3−/− mice with a polyclonal repertoire, ERK3 deficiency did not completely abolish the generation of CD4SP thymocytes. This suggests that another molecule might be playing a similar role during thymic T-cell differentiation. The lack of detectable expression of ERK4 (M. Marquis, S. Meloche and N. Labrecque, unpublished results), the closest homologue of ERK3, rules out functional redundancy between ERK3 and ERK4 during thymic differentiation. Furthermore, defects in positive selection are often more difficult to observe in a polyclonal repertoire. Indeed, change in the affinity of the selected repertoire might occur in ERK3-deficient mice to compensate for the lack of this signalling pathway. Moreover, our study of positive selection using TCR transgenic mice shows an almost complete block in T-cell differentiation, indicating that ERK3 is a critical effector of positive selection and further supporting the possibility of a change in the repertoire selected in non-TCR transgenic Erk3−/− mice. Therefore, our results identify ERK3 as a new key player involved in the positive selection of thymocytes.
We have previously shown that ERK3 deficiency affects the survival of DP thymocytes.19 The reduced DP thymocyte survival is probably not responsible for the lack of positive selection because the introduction of TCR transgenes restores DP thymocyte survival.19 This is also illustrated by the fact that in TCR transgenic mice, ERK3 deficiency did not affect thymic cell number. However, it is still possible that ERK3 promotes the survival of positively selected thymocytes. We believe that this is unlikely because we have not observed a difference in OT-I and OT-II thymocyte survival following 24 hr of in vitro culture with or without anti-CD3 stimulation between Erk3+/+ and Erk3−/− mice (data not shown). Further studies are required to definitively rule out a role for ERK3 in the survival of post-selected thymocytes.
We have previously shown that ERK3 expression is induced by the classical MAPK ERK1/2 in mature T cells.18 The fact that ERK3 is already expressed in DP thymocytes, even within the DP thymocytes that have not undergone positive selection, suggests that its expression is induced by pre-TCR signalling, which activates ERK1/2. The fact that ERK1/2 signalling is essential18 for the induction of ERK3 expression suggests that ERK3 is an effector of the ERK1/2 signalling pathway during T-cell differentiation in the thymus. Moreover, the phenotype of ERK3-deficient mice parallels the one observed in ERK1/2 double-deficient mice where positive selection is severely impaired by the absence of ERK1/2.8 Hence, it is tempting to speculate that ERK3 is one of the effectors mediating the lack of positive selection in ERK1 and ERK2 knock-out mice. Further studies are needed to address that very important question because only one effector (Early growth response protein 1) of the ERK1/2 signalling pathway during thymocyte differentiation has been identified.8
Previous work from our laboratory has also shown that the thymic epithelium contributes to the decreased generation of SP thymocytes in the absence of ERK3.19 However, we do not think that ERK3 deficency in the epithelium is responsible for the defective positive selection phenotype because we could recapitulate the results with in vitro stimulation of thymocytes. Furthermore, the reduced response of ERK3-deficient thymocytes in vitro to anti-TCR stimulation suggests that ERK3 expression controls the outcome of TCR signalling during thymic selection events. As anti-TCR stimulation probably induces a strong TCR signal, this raises the possibility that ERK3 will also contribute to TCR signalling during negative selection. Further studies should reveal whether ERK3 directly contribute to early TCR signalling events or if it only contributes as a downstream effector of the classical MAPK signalling pathway.
In conclusion, our results demonstrate that the atypical MAPK ERK3 controls thymocyte positive selection and they suggest that ERK3 might act as a direct downstream effector of the ERK1/2 signalling pathway. A better understanding of the molecular pathway controlling the positive selection of the T-cell repertoire is essential to improve T-cell reconstitution in immune-compromised patients.
Acknowledgments
We thank members of the laboratory for helpful discussion and the staff of the Animal Facility for mouse care. We thank Heather Melichar for critical reading of the manuscript. JS, JFD and MM performed the experiments; JS, SM and NL designed the study; JS, JFD, SB and NL prepared the manuscript. This work was supported by grants from the Natural Sciences and Engineering Council of Canada (NSERC; grant number 262146-2009) to NL and the Canadian Institutes of Health Research to SM. MM was supported by the Cole Foundation and the University of Montreal and SB by a fellowship from the Canadian Institutes of Health Research. SM holds the Canada Research Chair in Cellular Signaling.
Glossary
- Ab
antibody
- DN
double negative
- DP
double positive
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- MHC
Major Histocompatibility Complex
- PBS
Phosphate Buffered Saline
- SP
single positive
- TCR
T-cell receptor
Disclosures
The authors have no financial conflicts of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. T-cell receptor expression is up-regulated on OT-I Erk3+/+ and OT-I Erk3−/− double-positive thymocytes after incubation at 37°.
Figure S2. Defective up-regulation of CD69 and CD5 expression in OT-II T-cell receptor (TCR) transgenic thymocytes lacking extracellular signal-regulated kinase3 after TCR stimulation.
References
- Aifantis I, Mandal M, Sawai K, Ferrando A, Vilimas T. Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol Rev. 2006;209:159–69. doi: 10.1111/j.0105-2896.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- Labrecque N, Baldwin T, Lesage S. Molecular and genetic parameters defining T-cell clonal selection. Immunol Cell Biol. 2011;89:16–26. doi: 10.1038/icb.2010.119. [DOI] [PubMed] [Google Scholar]
- Saini M, Sinclair C, Marshall D, Tolaini M, Sakaguchi S, Seddon B. Regulation of Zap70 expression during thymocyte development enables temporal separation of CD4 and CD8 repertoire selection at different signaling thresholds. Sci Signal. 2010;3:ra23. doi: 10.1126/scisignal.2000702. [DOI] [PubMed] [Google Scholar]
- Whitehurst CE, Boulton TG, Cobb MH, Geppert TD. Extracellular signal-regulated kinases in T cells. Anti-CD3 and 4 β-phorbol 12-myristate 13-acetate-induced phosphorylation and activation. J Immunol. 1992;148:3230–7. [PubMed] [Google Scholar]
- Whitehurst CE, Geppert TD. MEK1 and the extracellular signal-regulated kinases are required for the stimulation of IL-2 gene transcription in T cells. J Immunol. 1996;156:1020–9. [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–43. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Pages G, Guerin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouysségur J. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–7. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- Werlen G, Hausmann B, Palmer E. A motif in the αβ T-cell receptor controls positive selection by modulating ERK activity. Nature. 2000;406:422–6. doi: 10.1038/35019094. [DOI] [PubMed] [Google Scholar]
- Daniels MA, Teixeiro E, Gill J, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–9. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- McGargill MA, Ch'en IL, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. Cutting edge: extracellular signal-related kinase is not required for negative selection of developing T cells. J Immunol. 2009;183:4838–42. doi: 10.4049/jimmunol.0902208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P, Meloche S. Atypical mitogen-activated protein kinases: structure, regulation and functions. Biochim Biophys Acta. 2007;1773:1376–87. doi: 10.1016/j.bbamcr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Turgeon B, Saba-El-Leil MK, Meloche S. Cloning and characterization of mouse extracellular-signal-regulated protein kinase 3 as a unique gene product of 100 kDa. Biochem J. 2000;346:169–75. [PMC free article] [PubMed] [Google Scholar]
- Deleris P, Trost M, Topisirovic I, Tanguay PL, Borden KL, Thibault P, Meloche S. Activation loop phosphorylation of ERK3/ERK4 by group I p21-activated kinases (PAKs) defines a novel PAK-ERK3/4-MAPK-activated protein kinase 5 signaling pathway. J Biol Chem. 2011;286:6470–8. doi: 10.1074/jbc.M110.181529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Mota-Peynado A, Chernoff J, Beeser A. Identification of the atypical MAPK Erk3 as a novel substrate for p21-activated kinase (Pak) activity. J Biol Chem. 2011;286:13603–11. doi: 10.1074/jbc.M110.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleris P, Rousseau J, Coulombe P, Rodier G, Tanguay PL, Meloche S. Activation loop phosphorylation of the atypical MAP kinases ERK3 and ERK4 is required for binding, activation and cytoplasmic relocalization of MK5. J Cell Physiol. 2008;217:778–88. doi: 10.1002/jcp.21560. [DOI] [PubMed] [Google Scholar]
- Marquis M, Boulet S, Mathien S, et al. The non-classical MAP kinase ERK3 controls T cell activation. PLoS ONE. 2014a;9:e86681. doi: 10.1371/journal.pone.0086681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis M, Daudelin JF, Boulet S, et al. The catalytic activity of the mitogen-activated protein kinase extracellular signal-regulated kinase 3 is required to sustain CD4+ CD8+ thymocyte survival. Mol Cell Biol. 2014b;34:3374–87. doi: 10.1128/MCB.01701-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger S, Turgeon B, Levesque K, Wood GA, Aagaard-Tillery KM, Meloche S. Loss of Erk3 function in mice leads to intrauterine growth restriction, pulmonary immaturity, and neonatal lethality. Proc Natl Acad Sci USA. 2009;106:16710–5. doi: 10.1073/pnas.0900919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J Immunol. 2005;175:4400–7. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- Nakayama T, June CH, Munitz TI, Sheard M, McCarthy SA, Sharrow SO, Samelson LE, Singer A. Inhibition of T cell receptor expression and function in immature CD4+ CD8+ cells by CD4. Science. 1990;249:1558–61. doi: 10.1126/science.2120773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. T-cell receptor expression is up-regulated on OT-I Erk3+/+ and OT-I Erk3−/− double-positive thymocytes after incubation at 37°.
Figure S2. Defective up-regulation of CD69 and CD5 expression in OT-II T-cell receptor (TCR) transgenic thymocytes lacking extracellular signal-regulated kinase3 after TCR stimulation.


