Abstract
Key message
A salicylic acid-inducible WRKY gene, PtrWRKY73, from Populus trichocarpa , was isolated and characterized. Overexpression of PtrWRKY73 in Arabidopsis thaliana increased resistance to biotrophic pathogens but reduced resistance against necrotrophic pathogens.
Abstract
WRKY transcription factors are commonly involved in plant defense responses. However, limited information is available about the roles of the WRKY genes in poplar defense. In this study, we isolated a salicylic acid (SA)-inducible WRKY gene, PtrWRKY73, from Populus trichocarpa, belonging to group I family and containing two WRKY domains, a D domain and an SP cluster. PtrWRKY73 was expressed predominantly in roots, old leaves, sprouts and stems, especially in phloem and its expression was induced in response to treatment with exogenous SA. PtrWRKY73 was localized to the nucleus of plant cells and exhibited transcriptional activation. Overexpression of PtrWRKY73 in Arabidopsis thaliana resulted in increased resistance to a virulent strain of the bacterial pathogen Pseudomonas syringae (PstDC3000), but more sensitivity to the necrotrophic fungal pathogen Botrytis cinerea. The SA-mediated defense-associated genes, such as PR1, PR2 and PAD4, were markedly up-regulated in transgenic plants overexpressing PtrWRKY73. Arabidopsis non-expressor of PR1 (NPR1) was not affected, whereas a defense-related gene PAL4 had reduced in PtrWRKY73 overexpressor plants. Together, these results indicated that PtrWRKY73 plays a positive role in plant resistance to biotrophic pathogens but a negative effect on resistance against necrotrophic pathogens.
Electronic supplementary material
The online version of this article (doi:10.1007/s00299-015-1745-5) contains supplementary material, which is available to authorized users.
Keywords: Populus, WRKY, Transcription factor, Pathogen, SA
Introduction
Plants are constantly challenged by a variety of microbial pathogens like fungi and bacteria. Complex defense mechanisms have evolved in plants to protect themselves against the attack of pathogens. Upon pathogen infection, plants rapidly activate defense responses mediated by multiple signal transduction pathways (Durrant and Dong 2004; Jones and Dangl 2006). Phytohormones are important components of different signaling pathways involved in plant defense (Rivas-San Vicent and Plasencia 2011). In many cases, pathogen-induced accumulation of salicylic acid (SA) leads to a rapid increase in the levels of reactive oxygen species (ROS) (Chen et al. 1993; Baker and Orlandi 1995; Lamb and Dixon 1997; Klessig et al. 2000). The change in the cellular redox potential resulted in the translocation of NONEXPRESSOR OF PR1 (NPR1) into the nucleus and enhanced transcriptional co-activator level, such as TGA transcription factors which activated SA-responsive pathogenesis-related genes (PRs) to trigger systemic acquired resistance (SAR) against biotrophic pathogen (Uknes et al. 1992; Sticher et al. 1997; Dong 2004; Zheng et al. 2006; Loake and Grant 2007). In other cases, necrotrophic pathogens such as Alternaria brassicicola or Botrytis cinerea can induce defense responses characterized by jasmonic acid (JA)- and ethylene (ET)-dependent signal transduction pathways (Thomma et al. 1999). It has been well demonstrated that these signaling pathways are not separate cascades, but can interact with each other (Reymond and Farmer 1998; Kunkel and Brook 2002). For example, the JA-signaling mutants, mpk4 and coi1, show enhanced SA accumulation and signaling (Petersen et al. 2000; Kloek et al. 2001), whereas blocking SA accumulation in pathogen-infected plants can improve JA signaling (Spoel et al. 2003).
Plant defense responses are involved in defense-response gene activation upon pathogen infection (Rushton and Somssich 1998). In SA-mediated defense signaling pathway, expression of certain PR genes are induced to increase resistance to biotrophic and hemibiotrophic pathogens (Kunkel and Brook 2002). NPR1, whose nuclear localization is required for SA signaling, mediates pathogen resistance in many plants (Spoel et al. 2003). The expression of the JA-responsive genes LOX2, PDF1.2, and VSP was up-regulated in response to infection by Pseudomonas syringae pv. tomato DC3000 (PstDC3000) in the plants unable to accumulate SA (Penninckx et al. 1998). In Arabidopsis, PDF1.2 is used as a marker for JA defense signaling pathways and is often correlated with resistance to necrotrophic pathogens (Thomma et al. 1998). In planta, products of pathogen-induced genes include key enzymes for phytoalexin biosynthesis (Ferrari et al. 2007; Ren et al. 2008) and those encoding transcription regulatory factors involved in signal perception and transduction of plant defense responses. Between them, transcriptional regulation of plant genes is a central step in plant defense responses. Therefore, elucidation of the complex regulatory mechanisms for controlling defense gene expression among plant species is important to understanding the molecular basis of plant–pathogen interactions.
WRKY transcription factors have been extensively studied with regard to their roles in regulation of genes associated with plant defense responses (Ulker and Somssich 2004; Eulgem and Somssich 2007). This gene family had a lineage-specific expansion during the course of plant evolution, and consists of 45 members in barley (Mangelsen et al. 2008), 74 in Arabidopsis (Ulker and Somssich 2004), 81 in rice (Xie et al. 2005) and at least 100 in Populus trichocarpa (He et al. 2012; Jiang et al. 2014). All products of WRKY genes contained either one or two WRKY domains, which comprised of a highly conserved amino acid sequence, WRKYGQK, following a zinc-finger motif CX4–7-CX23–28-HX1–2-(H/C) (Eulgem et al. 2000). Further, phylogenetic analysis of WRKY domains suggests that they evolved from an ancestral group IIc-like WRKY early in the eukaryote lineage (Brand et al. 2013). The conservation of the WRKY domain interacted with a remarkable conservation of the cis-acting W-box [(T)(T)TGAC(C/T)] containing the invariant TGAC core, which is required for function and WRKY binding (Eulgem et al. 2000).
Increasing evidence demonstrates that WRKY proteins participate widely in plant immunity. In Arabidopsis, 49 of the 74 AtWRKY genes were modulated in plants infected by P. syringae or treated by SA (Dong et al. 2003). W-boxes distribute in the promoters of target genes correlated with plant defense mediated by SA and JA signaling pathway, such as NPR1, PR2, PR10, VSP1 and VSP2 (Despres et al. 1995; Rushton et al. 1996; Yu et al. 2001; Li et al. 2004). Interestingly, W-boxes were also found in the promoters of some WRKY genes. For example, a cluster of W-boxes in the promoter of AtWRKY18 acted as negative regulatory elements for its inducible expression (Chen and Chen 2002). Furthermore, some WRKY genes regulate the expression of genes involved in the biosynthesis of defense-related phytohormones. WRKY28 and WRKY46 are transcriptional activators of ISOCHORISMATE SYNTHASE 1 (ICS1) and AVR PPHB SUSCEPTIBLE 3 (PBS3), which are associated with SA synthesis and SA-glucoside accumulation in Arabidopsis, respectively (van Verk et al. 2011). On the contrary, AtWRKY70 and AtWRKY54 play roles as negative regulators of SA biosynthesis (Wang et al. 2006). WRKY proteins also regulate antagonistic relationships between different defense pathways. For instance, mutation of AtWRKY33 resulted in enhanced susceptibility to the necrotrophic fungal pathogens, Botrytis cinerea and Alternaria brassicicola. Ectopic overexpression of AtWRKY33 not only increased resistance to necrotrophic fungi, but also made plants more susceptible to biotrophic pathogen P. syringae (Zheng et al. 2006). AtWRKY70 also functions as a repressor of JA-responsive genes and an activator of SA-induced genes, integrating signals from the two antagonistic pathways to modulate resistance to different races of pathogens (Li et al. 2004). In addition, strawberry FaWRKY1 is associated with a strong oxidative burst and glutathione-S-transferase (GST) induction to increase resistance to avirulent strains of P. syringae (Encinas-Villarejo et al. 2009). In Populus, PtWRKY23 affected resistance to Melampsora infection possibly by deregulation of genes that disrupt redox homeostasis and cell wall metabolism (Levée et al. 2009). In a previous study, we found several defense-related cis-elements distributed in the promoters of poplar WRKY genes and about 60 WRKY genes were induced or down-regulated by treatments of SA, MeJA and Marssonina brunnea (Jiang et al. 2014). However, the functional characterization of these WRKY genes in Populus, especially their roles in plant immune responses, is limited.
In this study, we isolated and characterized an SA-inducible gene PtrWRKY73 from P. trichocarpa. Amino acid sequence alignment and phylogenetic analyses showed that PtrWRKY73 is a group I member of the WRKY gene family and is similar to tobacco NtWRKY1 and AtWRKY33. Gene expression profiling in wild-type plants showed that transcripts of PtrWRKY73 accumulated in roots, old leaves, sprouts and stems (especially phloem). Overexpression of PtrWRKY73 in Arabidopsis resulted in increased resistance to P. syringae but more susceptibility to B. cinerea. In transgenic lines, overexpressing PtrWRKY73, the genes involved in SA signaling pathway, such as PR1, PR2, WRKY70, PAD4 and CPR5 were markedly up-regulated, whereas expression of the phenylalanine ammonia-lyase gene PAL4, an SA biosynthesis-related gene was decreased. These results indicate that PtrWRKY73 might regulate the SA-mediated defense pathway to mediate resistance to different pathogens in Arabidopsis.
Methods and materials
Plant material and treatments
Populus trichocarpa Torr. and A. Gray and P. tomentosa Carr. (clone 741) were grown in a greenhouse at 25 °C under a 14 h light and 10 h dark cycle with supplemental light (4,500 lux). 2-month-old poplars were employed for gene expression analyses. Seeds of Arabidopsis thaliana (Columbia 0 ecotype) were kept at 4 °C for at least 2 days before placement in a growth environment to facilitate uniform germination. Seedlings were transferred to pots after 2 weeks of germination on MS plates (Murashige and Skoog 1962). Plants were grown on a 1:1 mixture of vermiculite and peat in an illumination incubator at 22 °C, 80 % relative humidity and a 16 h photoperiod with supplemental light.
SA (5 mM in water) was applied to whole plant of poplar and Arabidopsis, respectively. The treated plants were immediately covered with a transparent lid and the leaves were collected after treatments (Li et al. 2004).
Botrytis cinerea were cultured, incubated and plant inoculation was performed as described previously (Zheng et al. 2006). Semi-quantitative RT-PCR was performed to quantify the ACTIN of Botrytis using the specific primers (F: 5′-CGCCCCTGCATTCTACGTCTC-3′; R: 5′-CAAGCTGGAGGATTGACTGGC-3′).
Pathogen inoculations were performed by infiltration of leaves of at least six plants for each treatment with the Pseudomonas syringae pv. tomato DC3000 strain (OD600 = 0.001 in 10 mM MgCl2). Inoculated leaves were harvested 3 d after infiltration and homogenized in 10 mM MgCl2. Diluted leaf extracts were plated on King’s B medium supplemented with rifampicin (100 μg/mL), incubated at 25 °C for 2 days before counting the colony-forming units.
Cloning of PtrWRKY73, vector construction and transformation of Arabidopsis
The cDNA fragment encoding PtrWRKY73 was PCR amplified with gene-specific primers (PtrWRKY73-F: 5′-ATGGCTGCTTCTTCAGGGAGC-3′; PtrWRKY73-R: 5′-CCAAGAACTCCTACGTGCTACG-3′) based on the transcript sequence (Potri.013G153400.1) from the P. trichocarpa genome. The PCR was performed using pfu DNA polymerase (Takara, Dalian, China) in a total volume of 50 μL. Cycling conditions were 94 °C for 3 min; 32 cycles of 94 °C for 45 s, 60 °C for 1 min and 72 °C for 2 min, followed by a final extension of 72 °C for 10 min. The PCR products were cloned into the plant binary vector pCXSN (Chen et al. 2009). The resulting vector p35S:PtrWRKY73, with the PtrWRKY73 open reading frame was driven by the cauliflower mosaic virus (CaMV) 35S promoter and the hygromycin phosphotransferase (Hpt) gene conferring resistance to hygromycin. The genomic DNA fragment containing an approximate 1.5 kb upstream sequence of PtrWRKY73 was amplified by the primers: Pro-PtrWRKY73 (F: 5′-GGATCAGTCAAAGAACAAGCTG-3′; R: 5′-GAAGAGGTCATGAAAGGGTAG-3′). The Pro-PtrWRKY73:GUS construct in the binary vector pCXGUS-P (Chen et al. 2009) was made by ligating the PCR-amplified genomic DNA fragments. Finally, these two constructs were transferred into Agrobacterium tumefaciens strains EHA105 by the freeze–thaw method. Transformation of A. thaliana plants was carried out by the floral dip method (Clough and Bent 1998). Transformants were selected on MS plates supplemented with 30 mg/mL of hygromycin and 50 mg/mL of carbenicillin.
Sequence comparisons and phylogenetic analysis
Amino acid sequence alignments were performed with DNAMAN software. The phylogenetic tree was constructed by the neighbor-joining method using MEGA 4.1 software (LynnonBiosoft, Quebec, Canada). The accession numbers of the WRKY proteins were kept in Supplementary Table 1.
Molecular analysis of transgenic plants
Genomic DNA was extracted from 300 mg of both untransformed control and transgenic plants using a CTAB method (Jia et al. 2010). Each PCR mixture (10 μL) contained 5.5 μL GoTaqRGreen Master Mix (Promega, Beijing, China), 0.25 μL of each primer, 0.5 μL cDNA and 3.5 μL nuclease-free water. PCR analysis was carried out with gene-specific primers that were positioned on the encoding sequence of PtrWRKY73 and the NOS terminal of pCXSN vector. PCR products were resolved on a 1 % (w/v) agarose gel and visualized after ethidium bromide staining under UV light.
RNA extraction and quantitative real-time PCR and semi-quantitative RT-PCR
Total RNA from fresh tissues of plants was extracted using RNA RNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions with a modification as reported previously (Jia et al. 2010). Extraction of total RNA from the xylem and phloem tissues was performed as Tian et al. (2013). Samples from at least three plants were pooled for analysis. The total RNA before the cDNA synthesis was treated with RNase-free DNase (TaKaRA, Dalian, China), following the manufacturer’s instructions to avoid any genomic DNA contamination. First-strand cDNA was synthesized from 2 µg RNA with RT-AMV transcriptase (TaKaRa, Dalian, China) in a total volume of 20 µL using oligo (dT)18 at 42 °C for 30 min. 18S rRNA was used as an internal control. The amplification products of RT-PCR were resolved by 1 % (w/v) agarose gel electrophoresis and visualized with ethidium bromide under UV light to test the expression level of PtrWRKY73 under various treatments and defense-related genes in transgenic plants. Quantitative real-time PCR and data analysis were performed in a 25 μL reaction volume containing 12.5 μL of SYBR Premix ExTaqTM (TaKaRa, Dalian, China). Differences of genes expression, expressed as fold change relative to control, were calculated using the [Δ][Δ]Ct = 2[Δ]Ct,18S − [Δ]Ct method. The gene-specific primers used for semi-quantitative RT-PCR and quantitative real-time PCR analysis were shown in Supplementary Table 2. Real-time PCR analysis was based on at least two biological replicates of each sample and three technical replicates of each biological replicate.
Subcellular localization
The PCR-amplified cDNA of PtrWRKY73 using primers of PtrWRKY73-pCX-DG-F and PtrWRKY73-R were ligated into pCX-DG (Chen et al. 2009) to generate the PtrWRKY73-GFP construct. The resulting vector was induced into onion epidermal cells by Gene Gun (GJ-1000, SCIENTZ, China). The onion skin was stained with DAPI, and photographed under a light microscope (Olympus BX53).
Transactivation assay
The ORF of PtrWRKY73 was amplified by PCR with gene-specific primers and cloned into NcoRI/BamHI-digested pGBKT7 vector. The resulting vector, positive control pGBKT7-PtrWRKY73 and negative control pGBKT7 (empty vector) were transformed into yeast strain Saccharomyces cerevisiae Gold2. Transformants were grown on synthetic dropout medium (SD medium) lacking tryptophan (Trp) for positive clone selection and then on SD medium lacking tryptophan (Trp), histidine (His) and adenine (Ade) for the transactivation assay, according to the method described previously (Tian et al. 2013).
GUS staining
GUS staining of T3 lines was performed by immersing seedlings in a staining solution (0.1 M sodium phosphate buffer, pH 7, 2 mM K4Fe(CN)6, 2 mM K3Fe(CN)6, 0.2 % Triton X-100, 10 m MEDTA, 2 mM X-Gluc) in 50 mL Falcon tubes for 4 h at 37 °C in the dark and washed with 70 % ethanol to remove chlorophyll (Weigel and Glazebrook 2002).
Statistical analysis
The Student’s t test program (http://www.graphpad.com/quickcalcs/ttest1.cfm) was used for statistical analysis of the data in the experiments of quantitative real-time PCR. In all these experiments, it was found that the quantitative differences between the two groups of data for comparison were statistically significant (P < 0.001).
Results
Expression of PtrWRKY73 is induced rapidly by various stresses
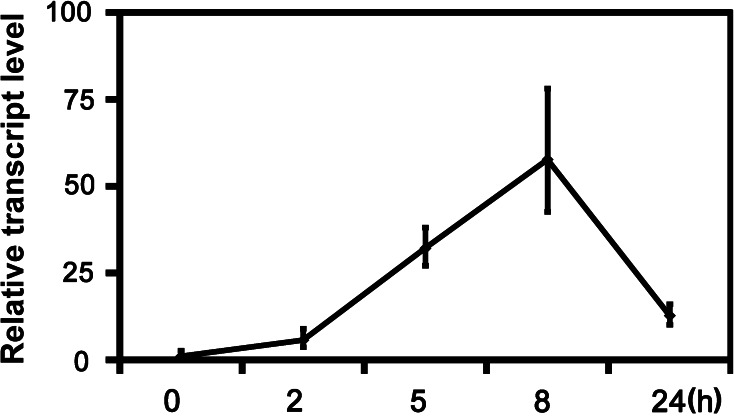
In a previous study, the functions of all poplar WRKY transcription factor family members have been characterized in plant defense and response to various biotic and abiotic stresses (Jiang et al. 2014). A putative WRKY gene, named PtrWRKY73, was identified in the P. trichocarpa genome, which was significantly induced by the fungus M. brunnea f. sp. multigermtubi and SA treatment. To confirm whether PtrWRKY73 was involved in biotic and abiotic stresses in P. trichocarpa, expression patterns of PtrWRKY73 were determine by quantitative real-time PCR over a time course of 24 h after treatment with SA. The PtrWRKY73 transcript level was significantly increased at 5 h and with a peak at 8 h (Fig. 1). These results indicated that the expression of PtrWRKY73 could be induced by defense signals and may play an important role in plant resistance to pathogens.
Fig. 1.
Response of PtrWRKY73 to exogenous salicylic acid treatments in Populus trichocarpa over time. Expression values are relative to time point 0 h. Error bars represent three biological replicates
Isolation and characterization of PtrWRKY73
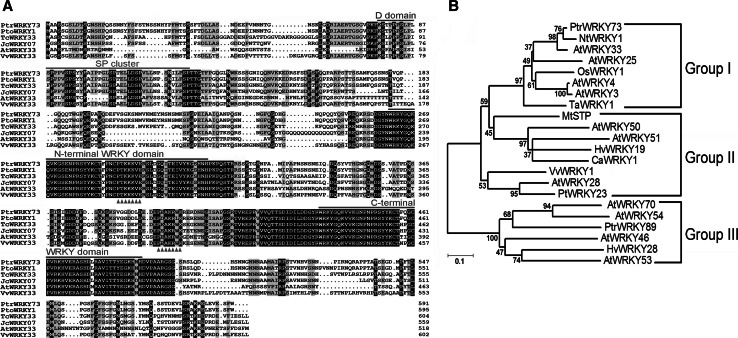
A cDNA fragment encoding PtrWRKY73 was isolated from P. trichocarpa using gene-specific primers designed based on the sequences of the P. trichocarpa genome database. The length of the nucleotide sequence of PtrWRKY73 was 1,776 bp, encoding a protein of 591 amino acid residues with a predicted theoretical isoelectric point and protein molecular weight of 7.2 and 64.93 kD. Sequence analysis showed that the deduced amino acid sequence of PtrWRKY73 contains two WRKY domains in N-terminus and C-terminus followed by a C-terminal Cys2/His2-type zinc-finger motif (Fig. 2a) and belonged to group I WRKY members. Previous studies have demonstrated that the D domain, which includes a cluster of basic residues in the upstream of the LxL motif ([K/R]1-2-x2-6-[L/I]-x-[L/I]), is a MAPK-docking site considered to define the selectivity by interacting with MAPKs (Reményi et al. 2006; Ishihama and Yoshioka 2012), and clustered serine–proline residue (SP cluster) is a minimal consensus motif for MAPK phosphorylation (Sharrocks et al. 2000). The D domain and SP cluster are conserved in the N-terminal regions of several group I WRKYs, inferring a functional importance (Sharrocks et al. 2000; Reményi et al. 2006). A deduced D domain was found in amino acid positions 78–87 of PtrWRKY73 protein and an SP cluster was adjacent to the N-terminal side of the D domain (Fig. 2a). Compared with other WRKY proteins, PtrWRKY73 had high identity with group I WRKY proteins from hybrid poplar [(P. tomentosa × P. bolleana) × P. tomentosa] (PtoWRKY1, 97.65 %), Theobroma cacao (TcWRKY33, 69.47 %), Jatropha curcas (JcWRKY07, 68.70 %) and Vitis vinifera (VvWRKY33, 65.27 %), but shared low sequence identity with AtWRKY33 (38.40 %) (Fig. 2a).
Fig. 2.
Alignment and phylogenetic analysis of PtrWRKY73 with other WRKY amino acid sequences. a Sequence alignment PtrWRKY73 and the other WRKYs. Sequences were from [(P. tomentosa × P. bolleana) × P. tomentosa] (PtoWRKY1), Theobroma cacao (TcWRKY33), J. curcas (JcWRKY07), V. vinifera (VvWRKY33) and A. thaliana (ATWRKY33). Identical amino acids were indicated by white letters on a black background and conservative amino acids by black on a dark gray background. The N-terminal and C-terminal WRKY domains were highlighted by green lines. D domain and SP cluster are highlighted by blue and red lines, respectively. The putative nuclear localization signals (NLS) were predicted and pointed out by the orange triangles at the bottom. b Phylogenetic relationships of PtrWRKY73 proteins from P. trichocarpa and selected species. Sequences were from N. tabacum (NtWRKY1), N. attenuate (NaWRKY3), Hordeum vulgare (HvWRKY19, HvWRKY28), V. vinifera (VvWRKY1), P. tremula x P. Alba (PtWRKY23), Medicago truncatula (MtSTP), Oryza sativa (OsWRKY1), Triticum aestivum (TaWRKY1), Capsicum annuum (CaWRKY1), P. trichocarpa (PtrWRKY89), A. thaliana (AtWRKY3, AtWRKY4, ATWRKY25, AtWRKY28, ATWRKY33, AtWRKY46, AtWRKY50, AtWRKY51, AtWRKY53, ATWRKY54, ATWRKY70). And the accession numbers were described in Supplementary Table 1 (color figure online)
The phylogenetic tree based on the classification method of Eulgem et al. (2000) showed that PtrWRKY73 belongs to group I of the WRKY superfamily (Fig. 2b). PtrWRKY73 was allocated to group I with the closest members being NtWRKY1 from tobacco (Nicotiana tabacum) and AtWRKY33 from Arabidopsis, which showed involvement of plant defense responses (Yamamoto et al. 2004; Zheng et al. 2006). These results suggested that PtrWRKY73 might also play a role in resistance to pathogens in poplar.
Tissue-specific expression patterns of PtrWRKY73 in poplar
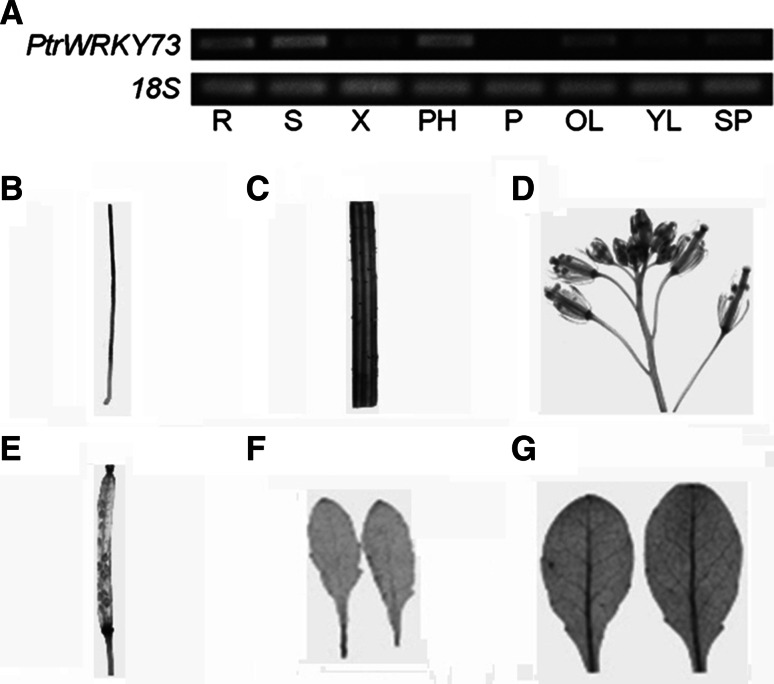
PtrWRKY73 expression in different tissues of P. trichocarpa was analyzed by semi-quantitative reverse transcription (RT)-PCR. Accumulation of PtrWRKY73 mRNA was detected in all tissues examined except in petiole (Fig. 3a). The expression levels in roots, stems and phloem were higher than in xylem, sprouts and leaves. Interestingly, PtrWRKY73 preferentially accumulated in old leaves (the leaves from the 5–6th node) rather than in young leaves (the leaves from the 2–3rd node).
Fig. 3.
Expression profiles of PtrWRKY73 in P. trichocarpa. a Semi-quantitative PCR examined-PtrWRKY73 transcript levels in different tissues. Poplar 18S expression was used as a control. Total RNA was isolated from roots (R), stems (S), xylem (X), phloem (PH), petioles (P), old leaves (OL), young leaves (YL) and sprouts (SP). PtrWRKY73 promoter driving GUS expression in various tissues of A. thaliana including roots (b), stems (c), inflorescence (d), siliques (e), young leaves (f) and old leaves (g) of 4-week-old plants
To further investigate the spatial and temporal expression patterns of PtrWRKY73, a 1,447 bp PtrWRKY73 promoter fragment was isolated from the genome of P. trichocarpa to fuse with GUS reporter gene of the pCXGUS-P vector (Chen et al. 2009) to generate the pPtrWRKY73:GUS construct, which was introduced into wild-type A. thaliana. Histochemical GUS staining showed that GUS activity was detected in roots, stems, flowers, siliques and leaves of transformed plants (Fig. 3b–g). As shown in Fig. 3b, GUS expression was detected in roots except the tips. GUS activity was also found in various floral organs such as the calyx, stigmatic papillae and abscission zone (Fig. 3d). At the late stages of silique development, GUS activity staining was slightly maintained in stigmatic papillae and abscission zone (Fig. 3e). In addition, during the leaf aging process, PtrWRKY73 transcript levels also gradually increased up to the point of leaf senescence (Fig. 3f, g).
PtrWRKY73 is localized in the nucleus and functions as a potential transcriptional activator
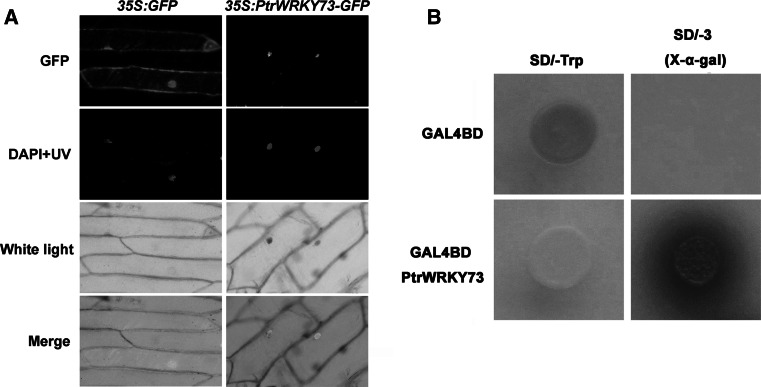
Sequence analysis using PSORT II Prediction (http://psort.hgc.jp/form2.html) showed that the predicted PtrWRKY73 contains two putative nuclear localization signals (299PTKKKVE306 and 392PEAKRWK398) (Fig. 2a). To verify its subcellular localization, PtrWRKY73 protein was fused to green fluorescent protein (GFP) under the control of the constitutive CaMV 35S promoter. The resulting vector was transformed into onion epidermal cells by Agrobacterium-mediated transformation and the result showed that the fusion protein was localized in the nucleus (Fig. 4a). In contrast, the 35S:GFP protein occurred throughout the cells including cytoplasm and the nucleus (Fig. 4a). These results indicated that PtrWRKY73 has a nuclear localization.
Fig. 4.
Nuclear localization and transcriptional activity of PtrWRKY73. a 35S::GFP was served as control. Bars 100 mm. b Transcriptional activation analysis of PtrWRKY73 fused with the GAL4 DNA-binding domain (GAL4BD) showed that PtrWRKY73 had the ability to activate the expression of the Trp and α-Gal reporter genes in yeast
To further examine whether PtrWRKY73 has transcriptional activity in vivo, PtrWRKY73 ORF was fused with the GAL4 DNA-binding domain in the pGBKT7 and transformed into Y2HGold yeast (Saccharomyces cerevisiae) cells. All the transformants containing pGBKT7-PtrWRKY73 grew well on selective medium without tryptophan (SD/-Trp) and SD/-Trp/-Ade/-His mediums, and these yeast cells were stained blue in X-gal solution. In contrast, the yeast cells with the negative control pGBKT7 only grew on SD/-Trp medium (Fig. 4b). These results demonstrated that PtrWRKY73 functions as a transcriptional activator.
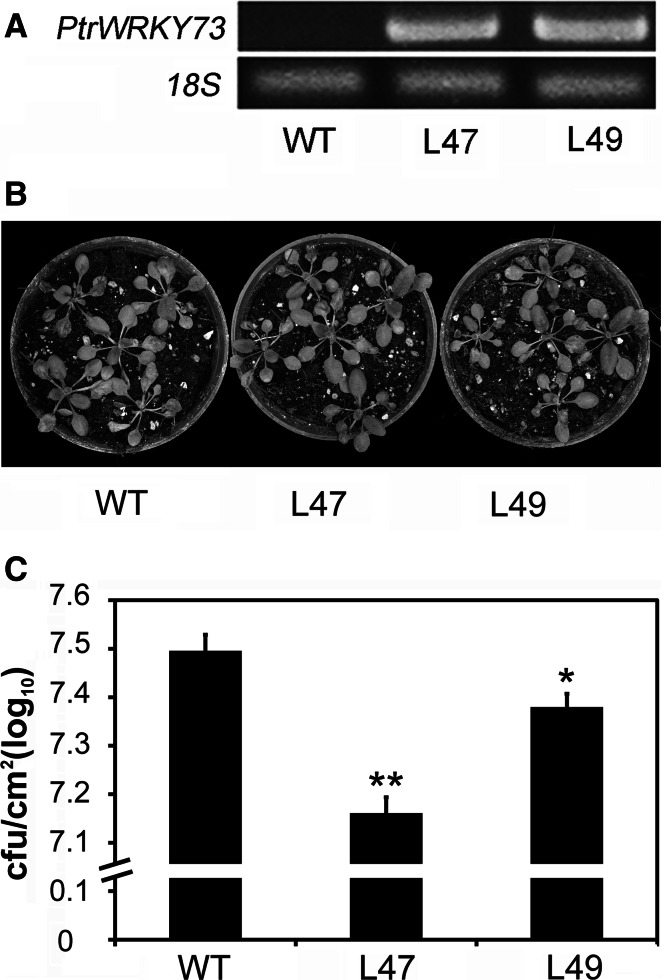
Constitutive expression of PtrWRKY73 in Arabidopsis resulted in enhanced resistance to PstDC3000 but more susceptibility to Botrytis
To examine the role of PtrWRKY73 in biotic plant defense, transgenic Arabidopsis plants overexpressing PtrWRKY73 were examined. Two independent lines (L47 and L49) with high PtrWRKY73 expression were selected and their homozygous progenies containing a single insert were used for further experiments (Fig. 5a). To elucidate the molecular basis of PtrWRKY73 in disease resistance in Arabidopsis, we examined resistance of the transgenic plants to P. syringae pv tomato strain DC3000 (PstDC3000). Three days after infection, transgenic lines exhibited weakly enhanced resistance to PstDC3000 as compared to wild-type plants (Fig. 5b). However, growth of PstDC3000 was significantly lower in leaves of transgenic lines than that of the wild-type control (Fig. 5c).
Fig. 5.
PtrWRKY73 overexpressing Arabidopsis plants showing resistance to PstDC3000. a PtrWRKY73 expression in transgenic and wild-type plants. b Phenotypes of wild-type and transgenic plants after 3 days of PstDC3000 infection. Red arrows indicate the inoculated leaves. c Growth of PstDC3000 in planta 3 days after inoculation. Values are means of three replicates. Error bars indicate standard deviation. Asterisks indicate a statistically significant difference between control and transgenic plants (P < 0.05 by Student’s t test) (color figure online)
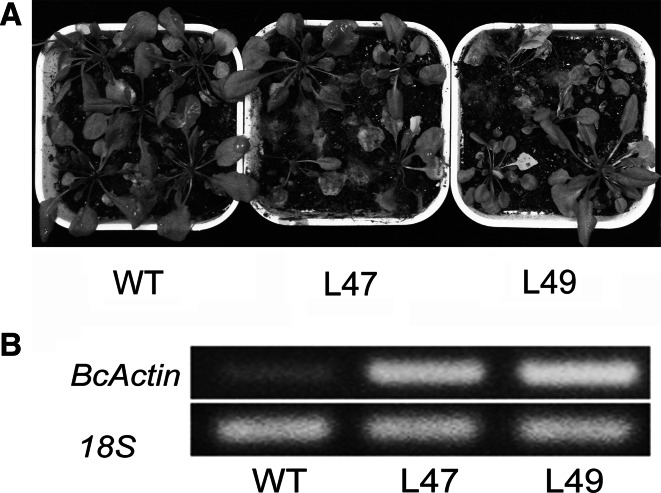
We also analyzed the responses of transgenic plants overexpressing PtrWRKY73 to the necrotrophic fungal pathogen B. cinerea. Disease symptoms in plants were quantified 10 days after inoculation. Transgenic lines tested displayed severe necrosis or death of the leaf tissue and the hyphae of B. cinerea flourished on the decayed tissues, whereas only several dead leaves were found in wild-type plants (Fig. 6a). Because the Botrytis Actin gene was previously shown to be constitutively expressed during plant infection (Benito et al. 1998), transcript levels were used indicative of the rate of fungal growth in planta. Fungal biomass in leaves was also quantified for wild-type and transgenic 35S:PtrWRKY73 plants (Fig. 6b). Semi-quantitative RT-PCR demonstrated that significantly increased levels of fungal DNA were detected from transgenic 35S:PtrWRKY73 plants compared with the wild-type control. These results indicated that overexpression of PtrWRKY73 enhanced sensitivity to Botrytis in Arabidopsis.
Fig. 6.
PtrWRKY73 overexpressing Arabidopsis plants showing susceptibility to Botrytis. a Disease response of inoculated plants at 10 days after Botrytis treatment. b Accumulation of the Botrytis Actin mRNA in inoculated plants. 18S was included as a control
PtrWRKY73 regulating SA-related genes affecting plant defense
PtrWRKY73 was induced by exogenous SA and overexpression of PtrWRKY73 enhanced resistance to PstDC3000, but susceptibility to Botrytis, indicating that PtrWRKY73 is involved in SA-mediated defense pathway. Semi-quantitative RT-PCR was used to determine the expression levels of pathogenesis-related genes in PtrWRKY73 overexpressor lines. Results show that PR1 and PR2 mRNAs accumulated to directly enhance resistance to PstDC3000 (Fig. 7). Although NPR1 acts as a modulator of PR gene expression, overexpression of PtrWRKY73 did not affect the accumulation of NPR1 transcripts. WRKY70 which acts as an activator of PRs (Li et al. 2004) had higher expression levels in L47 and L49 compared with wild-type plants. PAD4, required for SA signaling amplification (Jirage et al. 1999), had increased transcript levels in transgenic plants. In addition, CPR5 whose mutation showed constitutive expression of systemic acquired resistance (SAR) (Bowling et al. 1997) also exhibited a higher level of mRNA in transgenic 35S:PtrWRKY73 lines than the wild-type control (Fig. 7). Interestingly, the expression of SA biosynthesis-related gene PAL4 was reduced in the transgenic lines (Fig. 7). These results indicate that overexpression of PtrWRKY73 can impact transgenic plant defense through regulating of SA-related gene expression.
Fig. 7.
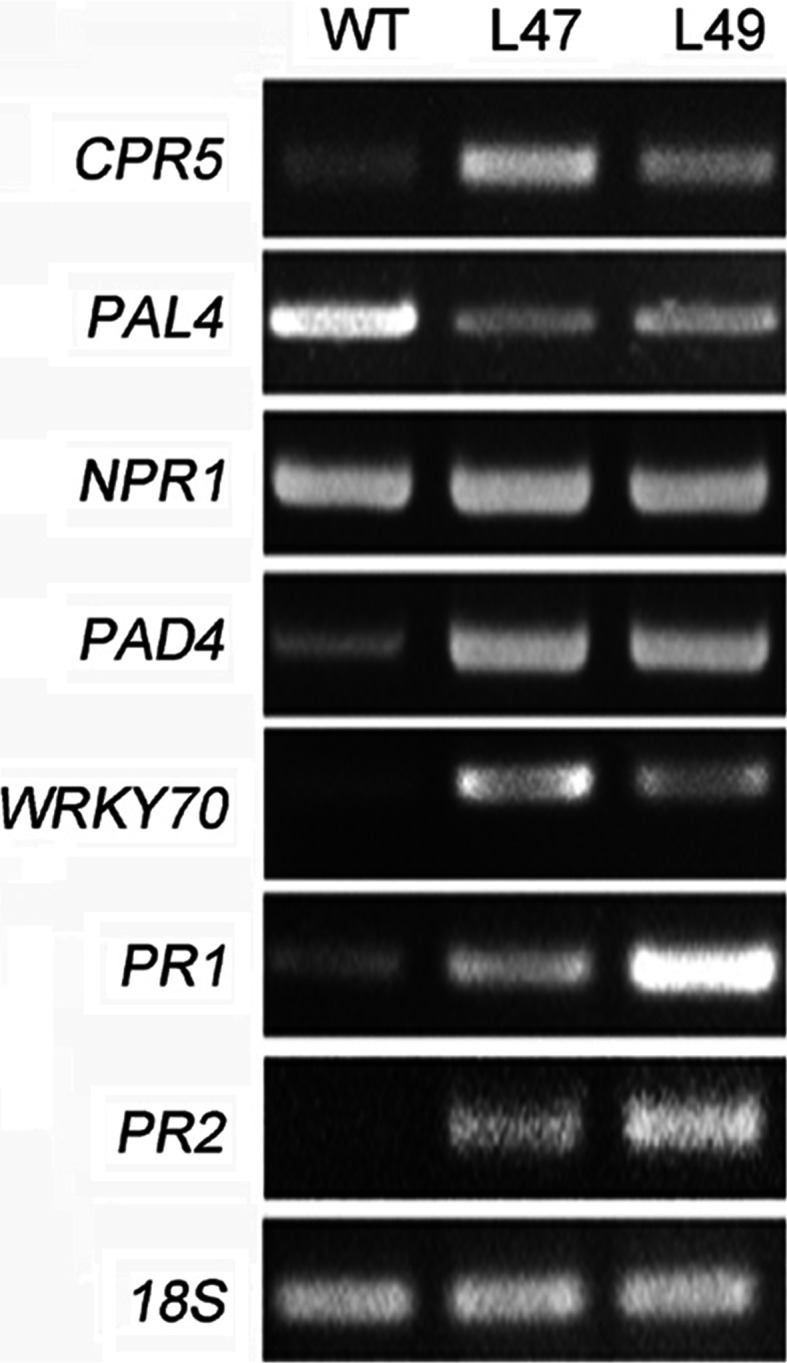
Expression of genes involved in SA signaling pathways in Arabidopsis overexpressing PtrWRKY73 as compared to wild-type plants. 18S expression was used as a control
Discussion
Recently, poplar genome sequence that has been completed (Tuskan et al. 2006) includes 100 putative WRKY genes (Jiang et al. 2014). Some WRKY genes have been cloned and characterized, for example, misexpression of PtWRKY23 from poplar (P. tremula × P. alba) resulted in more susceptibility to Melampsora infection in transgenic plants (Levée et al. 2009). An SA-induced PtoWRKY60 enhanced resistance to the fungus, Dothiorella gregaria, in transgenic Chinese white poplars (Ye et al. 2014). Our previous work showed PtrWRKY73, a pathogen-inducible gene (Jiang et al. 2014), encoded a typical WRKY protein with two WRKY domains (Fig. 2a) belonging to group I (Eulgem et al. 2000). In agreement with conserved clustered proline-directed serines (SP cluster) in group I WRKYs (Ishihama and Yoshioka 2012), the existence of the D domain and SP cluster on the PtrWRKY73 might provide an MAPK-docking site and phosphorylation targets of mitogen-activated protein kinase (MAPK) (Reményi et al. 2006;Ishihama and Yoshioka 2012), suggesting that PtrWRKY73 activity could be regulated by phosphorylation (Fig. 2a). In addition, two predicted nuclear localization signals (NLS) (Fig. 2a) strongly suggests that PtrWRKY73 protein is translocated into the nucleus to regulate gene expression. The following subcellular localization assay also supports this hypothesis (Fig. 4a).
Although the phylogenetic tree indicates that the closest homologue of PtrWRKY73 in Arabidopsis is AtWRKY33 (Fig. 2b) with both being inducible by SA (Zheng et al. 2006), the identity between these two proteins was only 38.40 % (Fig. 2a) and the most similar region mainly distributed at the C-terminal (Data not shown). Therefore, we assume that PtrWRKY73 and AtWRKY33 might play different roles in plant pathogen resistance. It has been demonstrated that AtWRKY33 was required for resistance to necrotrophic fungal pathogens (Zheng et al. 2006). Whereas our findings showed that overexpression of PtrWRKY73 could enhance the resistance to the biotrophic bacterial leaf pathogen PstDC3000 (Fig. 5), but be impaired in defense responses to the necrotrophic fungal pathogen Botrytis (Fig. 6). These results suggested that PtrWRKY73 might be involved in SA-mediated plant defense.
According to their lifestyles, plant pathogens are generally divided into necrotrophs and biotrophs. In addition, many plant pathogens exhibit both lifestyles depending on the stage of their life cycle and are referred to as hemibiotrophs (Pieterse et al. 2009). SA-mediated signaling pathways have been demonstrated to play critical roles in resistance to biotrophic and hemibiotrophic pathogens, such as P. syringae pv tomato strain DC3000 (PstDC3000). When plants are invaded by biotrophs and hemibiotrophs, some lipase-like genes such as PAD4 participate in a positive regulatory loop that increases SA levels (Jirage et al. 1999). Pathogen-induced SA accumulation changed the cellular redox potential by rapidly increasing levels of ROS, resulting in translocation of NPR1 to activate pathogen resistance genes, such as PRs (Uknes et al. 1992; Chen et al. 1993; Baker and Orlandi 1995; Lamb and Dixon 1997; Klessig et al. 2000; Dong 2004; Loake and Grant 2007). In Arabidopsis, AtWRKY70, an activator downstream of NPR1, positively regulated the SA signaling pathway associated with induction of PR1, PR2 and PR5 to enhance resistance to PstDC3000 (Li et al. 2004). In this study, PtrWRKY73 overexpressors had high PAD4 transcript levels compared with wild-type plants (Fig. 7), indicating that PtrWRKY73 positively regulates the SA amplification loop. Constitutive expression of PtrWRKY73 did not lead to increased NPR1 transcript levels but enhanced accumulation of WRKY70 mRNA (Fig. 7), contributing to PR1 and PR2 accumulation (Fig. 7). Taken together, these results suggest that PtrWRKY73 positively modulates defense signal transduction pathways independent of NPR1.
Overexpression of defense-related genes probably requires high energy levels and nutrients, resulting in morphological changes in plants. In Arabidopsis, overexpression of WRKY70 resulted in plants smaller in size than the controls, and transgenic plants also exhibited changes in morphology with lancet shaped and slightly twisted leaves (Li et al. 2004). In transgenic 35S:PtrWRKY73 plants, SA-related genes such as PRs were significantly induced but no change in morphology was found in transgenic plants compared with wild-type plants (data not shown). At the molecular level, the phenylalanine ammonia-lyase gene (PAL4), involved in SA biosynthesis, had reduced significantly (Fig. 7). In addition, CPR5, whose mutation constitutively expressed systemic acquired resistance (SAR) (Bowling et al. 1997), was activated in transgenic 35S:PtrWRKY73 plants (Fig. 7). These results indicate that a feedback mechanism might exist, that inhibits excessive SA signaling by accumulation of CPR5 mRNA and at the same time, a reduction in PAL4 expression level impairs the negative effect of energy and nutrition consumption in transgenic plants.
Author contributionstatement
YJ and KL designed the research. YD, YJ, SY, ZL, YH and SY performed the experiments. AK, YJ and KL wrote the paper. All authors discussed the results and approved the final manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank Prof. Guoliang Wang (Hunan Agricultural University, Changsha 410128, China) for providing the plant binary vector pCXSN. This work was supported by the National Natural Science Foundation of China (31370672, 31171620), the National Key Project for Research on Transgenic Plant (2011ZX08010-003), the program for New Century Excellent Talents in University (NCET-11-0700) and the “One Hundred Talents Program” of the Chinese Academy of Sciences.
Conflict of interest
The authors declare that there is no conflict of interest in the present investigation.
Footnotes
Y. Duan and Y. Jiang contributed equally to this work.
References
- Baker CJ, Orlandi EW. Active oxygen in plant pathogenesis. Ann Rev Phytopathol. 1995;33:299–321. doi: 10.1146/annurev.py.33.090195.001503. [DOI] [PubMed] [Google Scholar]
- Benito EP, ten Have A, van’t Klooster JW, van Kan JAL. Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea. Eur J Plant Pathol. 1998;104:207–220. doi: 10.1023/A:1008698116106. [DOI] [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand LH, Fischer NM, Harter K, Kohlbacher O, Wanke D. Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Res. 2013;41:9764–9778. doi: 10.1093/nar/gkt732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen Z. Potentiation of developmentally regulated plant defense response by AtWRKY18, a pathogen-induced Arabidopsis transcription factor. Plant Physiol. 2002;129:706–716. doi: 10.1104/pp.001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993;262:1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- Chen S, Songkumarn P, Liu J, Wang GL. A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol. 2009;150:1111–1121. doi: 10.1104/pp.109.137125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Despres C, Subramaniam R, Matton DP, Brisson N. The activation of the potato PR-l0a gene requires the phosphorylation of the nuclear factor PBF-1. Plant Cell. 1995;7:589–598. doi: 10.1105/tpc.7.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X. NPR1, all things considered. Curr Opin Plant Biol. 2004;7:547–552. doi: 10.1016/j.pbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Dong J, Chen C, Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 2003;51:21–37. doi: 10.1023/A:1020780022549. [DOI] [PubMed] [Google Scholar]
- Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- Encinas-Villarejo S, Maldonado AM, Amil-Ruiz F, de los Santos B, Romero F, Pliego-Alfaro F, Muñoz-Blanco J, Caballero JL. Evidence for a positive regulatory role of strawberry (Fragaria × ananassa) FaWRKY1 and Arabidopsis At WRKY75 proteins in resistance. J Exp Bot. 2009;60:3043–3065. doi: 10.1093/jxb/erp152. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Somssich IE. Networks of WRKY transcription factors in defense signaling. Curr Opin Plant Biol. 2007;10:366–371. doi: 10.1016/j.pbi.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/S1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Galletti R, Denoux C, De Lorenzo G, Ausubel FM, Dewdney J. Resistance toBotrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol. 2007;144:367–379. doi: 10.1104/pp.107.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Dong Q, Shao Y, Jiang H, Zhu S, Cheng B, Xiang Y. Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 2012;31(7):1199–1217. doi: 10.1007/s00299-012-1241-0. [DOI] [PubMed] [Google Scholar]
- Ishihama Nobuaki, Yoshioka Hirofumi. Post-translational regulation of WRKY transcription factors in plant immunity. Curr Opin Plant Biol. 2012;15:1–7. doi: 10.1016/j.pbi.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Jia Z, Sun Y, Yuan L, Tian Q, Luo K. The chitinase gene (Bbchit1) from Beauveria bassiana enhances resistance to Cytospora chrysosperma in Populus tomentosa Carr. Biotechnol Lett. 2010;32:1325–1332. doi: 10.1007/s10529-010-0297-6. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Duan Y, Yin J, Ye S, Zhu J, Zhang F, Lu W, Fan D, Luo K. Genome-wide identification and characterization of the Populus WRKY transcription factor family and analysis of their expression in response to biotic and abiotic stresses. J Exp Bot. 2014;65(22):6629–6644. doi: 10.1093/jxb/eru381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM, Glazebrook J. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc Natl Acad Sci USA. 1999;96(23):13583–13588. doi: 10.1073/pnas.96.23.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang S, Kachroo P, Trifa Y, Pontier D, Lam E, Silva H. Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci USA. 2000;97:8849–8855. doi: 10.1073/pnas.97.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, Klessig DF, Kunkel BN. Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J. 2001;26:509–522. doi: 10.1046/j.1365-313x.2001.01050.x. [DOI] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 2002;5:325–331. doi: 10.1016/S1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Ann Rev Plant Physiol Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Levée V, Major I, Levasseur C, Tremblay L, MacKay J, Séguin A. Expression profiling and functional analysis of PopulusWRKY23 reveals a regulatory role in defense. New Phytol. 2009;184:48–70. doi: 10.1111/j.1469-8137.2009.02955.x. [DOI] [PubMed] [Google Scholar]
- Li J, Brader G, Palva ET. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004;16:319–331. doi: 10.1105/tpc.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loake G, Grant M. Salicylic acid in plant defence—the players and protagonists. Curr Opin Plant Biol. 2007;10:466–472. doi: 10.1016/j.pbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Mangelsen E, Kilian J, Berendzen KW, Kolukisaoglu UH, Harter K, Jansson C, Wanke D. Phylogenetic and comparative gene expression analysis of barley (Hordeum vulgare) WRKY transcription factor family reveals putatively retained functions between monocots and dicots. BMC Genom. 2008;9:194. doi: 10.1186/1471-2164-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–479. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, Sharma SB, Klessig DF, Martienssen R, Mattsson O, Jensen AB, Mundy J. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–1120. doi: 10.1016/S0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- Pieterse CM, Leon-Reyes A, Van der Ent S, Van Wees SC. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5(5):308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- Reményi A, Good MC, Lim WA. Docking interactions in protein kinase and phosphatase networks. Curr Opin Struct Biol. 2006;16:676–685. doi: 10.1016/j.sbi.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Ren D, Liu Y, Yang K-Y, Han L, Mao G, Glazebrook J, Zhang S. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc Natl Acad Sci USA. 2008;105:5638–5643. doi: 10.1073/pnas.0711301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1:404–411. doi: 10.1016/S1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- Rivas-San Vicente M, Plasencia J. Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot. 2011;62(10):3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE. Transcriptional control of plant genes responsive to pathogens. Curr Opin Plant Biol. 1998;1(4):311–315. doi: 10.1016/1369-5266(88)80052-9. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 1996;15:5690–5700. [PMC free article] [PubMed] [Google Scholar]
- Sharrocks AD, Yang SH, Galanis A. Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem Sci. 2000;25(9):448–453. doi: 10.1016/S0968-0004(00)01627-3. [DOI] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, Van Loon LC, Dong X, Pieterse CM. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell. 2003;15:760–770. doi: 10.1105/tpc.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticher L, Mauch-Mani B, Métraux JP. Systemic acquired resistance. Annu Rev Phytopathol. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BP, Broekaert WF. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95(25):15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Tierens KFMJ, Broekaert WF. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;121:1093–1101. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Wang X, Li C, Lu W, Yang L, Jiang Y, Luo K. Functional characterization of the poplar R2R3-MYB transcription factor PtoMYB216 involved in the regulation of lignin biosynthesis during wood formation. PLoS ONE. 2013;8(10):e76369. doi: 10.1371/journal.pone.0076369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A, Schein J, Sterck L, Aerts A, Bhalerao RR, Bhalerao RP, Blaudez D, Boerjan W, Brun A, Brunner A, Busov V, Campbell M, Carlson J, Chalot M, Chapman J, Chen GL, Cooper D, Coutinho PM, Couturier J, Covert S, Cronk Q, Cunningham R, Davis J, Degroeve S, Déjardin A, Depamphilis C, Detter J, Dirks B, Dubchak I, Duplessis S, Ehlting J, Ellis B, Gendler K, Goodstein D, Gribskov M, Grimwood J, Groover A, Gunter L, Hamberger B, Heinze B, Helariutta Y, Henrissat B, Holligan D, Holt R, Huang W, Islam-Faridi N, Jones S, Jones-Rhoades M, Jorgensen R, Joshi C, Kangasjärvi J, Karlsson J, Kelleher C, Kirkpatrick R, Kirst M, Kohler A, Kalluri U, Larimer F, Leebens-Mack J, Leplé JC, Locascio P, Lou Y, Lucas S, Martin F, Montanini B, Napoli C, Nelson DR, Nelson C, Nieminen K, Nilsson O, Pereda V, Peter G, Philippe R, Pilate G, Poliakov A, Razumovskaya J, Richardson P, Rinaldi C, Ritland K, Rouzé P, Ryaboy D, Schmutz J, Schrader J, Segerman B, Shin H, Siddiqui A, Sterky F, Terry A, Tsai CJ, Uberbacher E, Unneberg P, Vahala J, Wall K, Wessler S, Yang G, Yin T, Douglas C, Marra M, Sandberg G, Van de Peer Y, Rokhsar D. The genome of black cottonwood Populus trichocarpa (Torr. & Gray) Science. 2006;313:1596. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J. Acquired resistance in Arabidopsis. Plant Cell. 1992;4:645–656. doi: 10.1105/tpc.4.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulker B, Somssich IE. WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol. 2004;7:491–498. doi: 10.1016/j.pbi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- van Verk MC, Bol JF, Linthorst HJ. WRKY transcription factors involved in activation of SA biosynthesis genes. BMC Plant Biol. 2011;11:89. doi: 10.1186/1471-2229-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Amornsiripanitch N, Dong X. A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2006;2(11):e123. doi: 10.1371/journal.ppat.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J. Arabidopsis: a laboratory manual. NY, USA: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- Xie Z, Zhang ZL, Zou X, Huang J, Ruas P, Thompson D, Shen QJ. Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 2005;137:176–189. doi: 10.1104/pp.104.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nakano T, Suzuki K, Shinshi H. Elicitor-induced activation of transcription via W box-related cis-acting elements from a basic chitinase gene by WRKY transcription factors in tobacco. Biochim Biophys Acta. 2004;1679(3):279–287. doi: 10.1016/j.bbaexp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Ye S, Jiang Y, Duan Y, Karim A, Yang L, Zhao X, Yin J, Luo K. Constitutive expression of the poplar WRKY transcription factor PtoWRKY60 enhances resistance to Dothiorella gregaria Sacc. in transgenic plants. Tree physiol. 2014;34(10):1118–1129. doi: 10.1093/treephys/tpu079. [DOI] [PubMed] [Google Scholar]
- Yu D, Chen C, Chen Z. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell. 2001;7:1527–1540. doi: 10.1105/tpc.13.7.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Qamar SA, Chen Z, Mengiste T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006;48:592–605. doi: 10.1111/j.1365-313X.2006.02901.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.