Abstract
Background
Restenosis after percutaneous coronary intervention (PCI) is a remained clinical problem which limits long-term success of PCI. Although there was recognition that probucol in treating restenosis after percutaneous transluminal coronary angioplasty, the efficacy of probucol on restenosis after stent-implantation is controversial. So this meta-analysis was conducted to investigate the association between probucol and late restenosis.
Methods
Articles were assessed by four trained investigators, with divergences resolved by consensus. PubMed, EMBASE, ScienceDirect and the Cochrane Central Register of clinical trials were searched for pertinent studies. Inclusion criteria were random allocated to treatment and a comparison of probucol-treated patients and control patients (not treated with lipid-lowering drug) undergoing PCI.
Results
Fifteen studies with 859 subjects were analyzed. Major outcome, binary angiographic restenosis defined as >50% stenosis upon follow-up angiography, was significantly decreased with probucol treatment (RR = 0.59 [0.43, 0.80] among vessels, P = 0.0007; and RR = 0.52 [0.40, 0.68] among patients, P<0.00001). Probucol also increased the minimal luminal diameter (SMD = 0.45 [0.30, 0.61], P<0.00001) and decreased late loss upon follow-up after 6 months (SMD = -0.41 [-0.60, -0.22], P<0.0001). Moreover, there was a significantly lower incidence of major adverse cardiac events (MACE) in the probucol group than control group (RR = 0.69 [0.51, 0.93], P = 0.01).
Conclusion
Probucol is more than a lipid-lowering drug. It is also effective in reducing the risk of restenosis and incidence of MACE after PCI.
Introduction
Coronary heart disease (CHD) is the leading cause of death and disability worldwide. Percutaneous coronary intervention (PCI) with stent placement is the standard nonsurgical treatment for CHD. However, restenosis after PCI remains an important clinical problem that occurs in patients who have undergone either percutaneous transluminal coronary angioplasty (PTCA) (30%-50%)[1, 2] or stent-implantation (15%-20%)[3, 4].
Post angioplasty restenosis is thought to involve vessel elastic recoil, negative remodeling[5], smooth muscle cell migration and proliferation[6] and excessive extracellular matrix production[7]. Success of stenting contributes to reducing acute elastic recoil and long-term vessel remodeling[8]. Smooth muscle cell migration and proliferation and excessive extracellular matrix production appears to be main causes of post PCI restenosis.
Probucol has demonstrated its ability in inhibiting vascular smooth muscle cell proliferation after balloon injury in different animal models[9, 10]. In some clinical trials, probucol was effective in reducing restenosis after percutaneous balloon angioplasty. However, its role in the prevention of restenosis after stenting has not been demonstrated in human. Moreover, results have been contradictory and controversial in clinical trials. These inconsistent results may be clarified via a meta-analysis of randomized controlled trials. Thus, we designed a meta-analysis to assess whether treatment with probucol reduced diameter restenosis in patients after PCI.
Methods
Search strategy and selection criteria
This meta-analysis was performed in accordance with the PRISMA (Preferred Reporting Items for Systemic Reviews and Meta-Analyses) recommendations[11]. Electronic databases (PubMed, EMBASE, ScienceDirect and the Cochrane Central Register of clinical trials) were searched using the following subject terms: “coronary artery disease”, “cardiovascular disease”, “percutaneous coronary intervention”, “percutaneous transluminal coronary angioplasty”, “stent”, “probucol”, “restenosis”, “minimal luminal diameter” and “late loss”, through January 30, 2015. The search was limited to randomized controlled trials and human studies without language restrictions. We also hand-searched the reference lists of studies, including reviews of probucol and other types of articles related to our primary subject matter, to ensure other relevant articles.
Only randomized controlled trials (RCTs) comparing probucol with control treatments (placebo, standard care without any lipid-lowering drugs) for patients with coronary atherosclerosis disease who underwent PCI were included. Follow-up coronary arteriography was conducted 6 months later to evaluate lumen diameter of PCI segments. Our study’s major outcome was binary angiographic restenosis defined as a diameter stenosis>50% at follow-up. Other studies containing minimal diameter (MLD) or late loss were also our interests. The second outcome was incidence of major adverse cardiac events (MACE), including death, myocardial infarction (MI), repeated angioplasty and coronary artery bypass surgery (CABG). If several groups were included in a single study, only the probucol group and the control group were included in our meta-analysis.
Data collection and study quality
Three researchers (JC Liu, MH Li, H Lu) independently reviewed references and abstracts retrieved by the search, assessed the completeness of the data abstraction and confirmed quality rating. The quality of each study was independently evaluated by using the Risk of Bias Table from the Cochrane Collaboration. Each factor was rated as ‘‘low risk” of bias (e.g., random sequence generation was computer generated), ‘‘high risk” of bias (e.g., participants and personnel were not blinded) or ‘‘unclear risk” of bias (e.g., methods used for allocation concealment were not described in the manuscript). We redefined low risk, high risk and unclear risk of bias in incomplete outcome data as less than 20%, more than 40% and 20–40% censored data. Any disagreement regarding data collection or the quality assessment was adjudicated by the fourth reviewer (ZG Guo).
Statistical analyses
The meta-analysis was conducted by combining the risk ratio of individual studies into a pooled risk ratio for dichotomous outcomes. And the continuous outcomes were analyzed by standard mean difference. Overall estimated effects were calculated with either fix-effects model or with a random-effects model when heterogeneity could not be explained[12]. Heterogeneity[13, 14] among studies was evaluated by the Cochran Q test (considered significant if P<0.1), the Chi-squared test, the I 2 test and tau2. Funnel plots were generated for subjectively assessment of publication bias[15].
To verify the consistency of results, the influence of each individual study on the summary effect estimate was assessed by the 1-study removed sensitivity analysis. Subgroup analyses of MLD were conducted according to ethnicity (Asian vs non-Asian), probucol dosage (>500 mg daily vs ≤500 mg daily), stent-implantation (stent vs non-stent) and the duration of drug use before PCI (≥30days vs ≤14days) to explore the source of heterogeneity.
Analyses were performed with RevMan 5.2 software (Review Manager (RevMan) [Computer program]. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.)
Results
Selected studies and characteristics
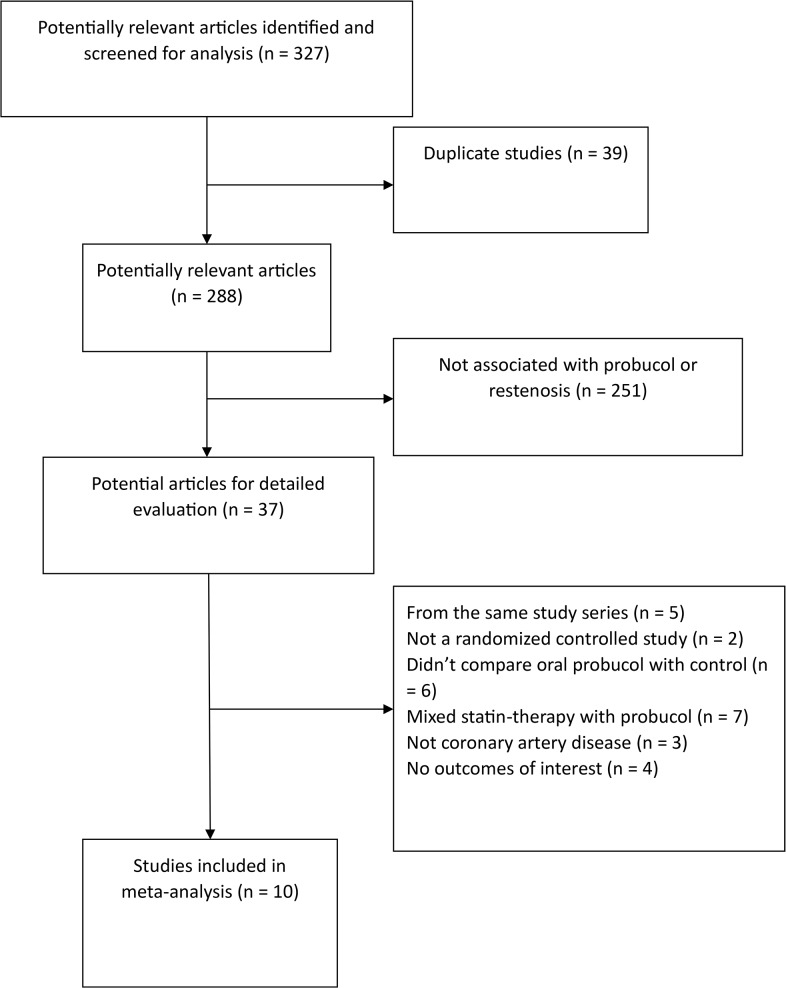
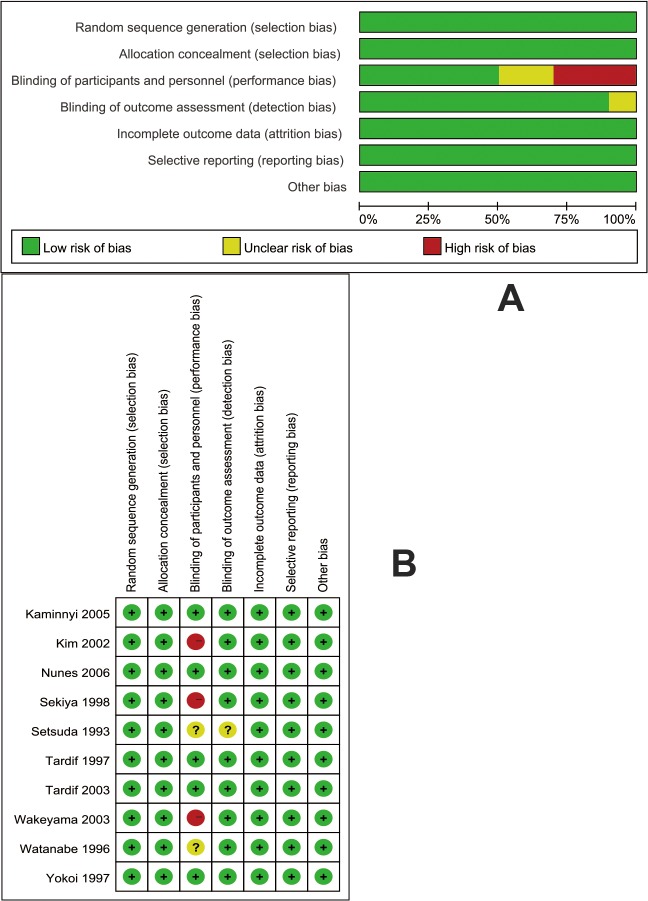
The process for the selection of studies for inclusion in the meta-analysis is shown in Fig 1. Our search strategy generated 327 results, 39 of which represented duplicate studies were removed. 37 articles were examined in detail. Of these 37 studies, 27 studies were subsequently excluded: 5 trials were from same study series; 2 trials were not randomized controlled studies; 6 trials did not compare oral probucol with conventional treatments; 7 trials were mixed statin-therapy with probucol-therapy, 3 trials included patients who did not suffer coronary artery disease, and 4 trials contained no outcomes of interest. 10 studies[16–25] were included in the analyses which examined a total of 859 patients. The characteristics of each study are listed in S1 Table. The risk of bias assessment is displayed in Fig 2. Three unblinded studies were determined to be high risk (Kim 2002, Sekiya 1998 and Wakeyama 2003).
Fig 1. Flowchart of articles included in the meta-analysis.
Fig 2. Risk of bias.
(A) Risk of bias graph. (B) Risk of bias summary.
Primary outcome
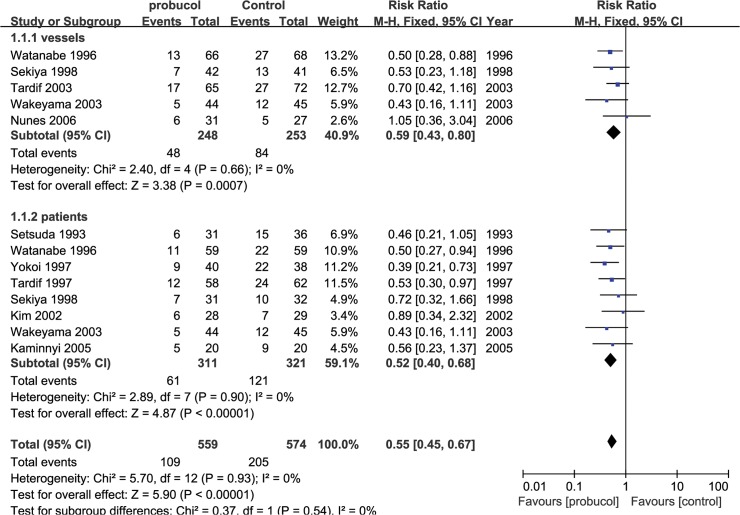
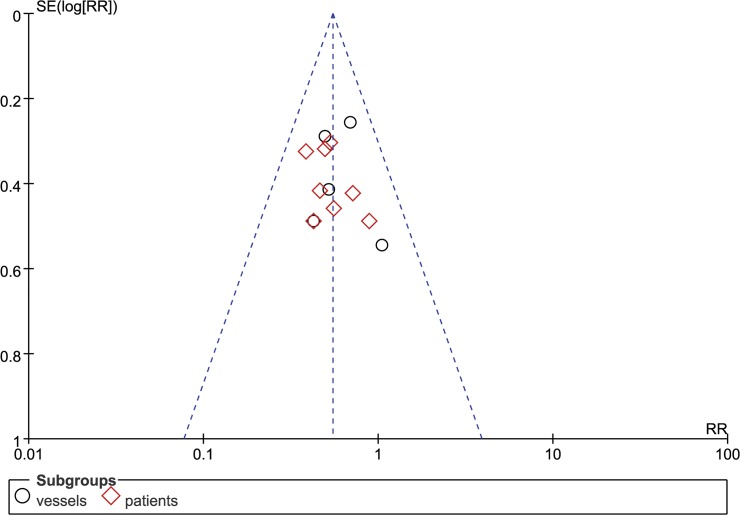
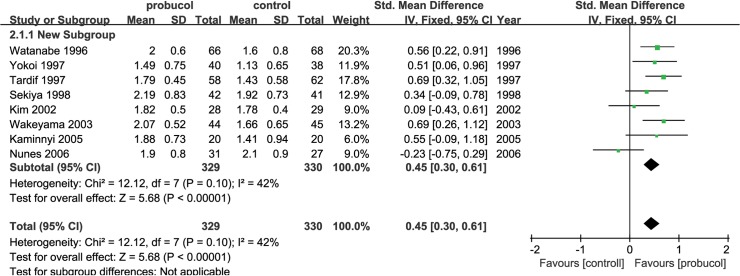
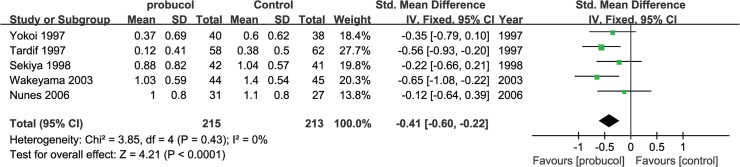
Our primary outcome was percentage of restenosis, which was measured in 10 studies. The primary outcome was measured in following two ways: the occurrence of restenosis in the PCI segments and the occurrence of restenosis among patients. Probucol reduced the risk ratio of restenosis among both lesions (RR: 0.59 [0.43, 0.80], P = 0.0007, I2 = 0) and among patients (RR: 0.52 [0.40, 0.68], P<0.00001, I2 = 0) (Fig 3) using a fix-effects model. Probucol nonetheless reduced the frequency of restenosis among patients who underwent stent implantation (RR = 0.66 [0.50, 0.88], P = 0.004) as much as it did among patients who underwent PTCA (RR = 0.47 [0.34, 0.65], P<0.00001). No evidence of publication bias was identified based on funnel plots (Fig 4). An increase in MLD was observed with probucol compared with the control group (SMD = 0.45 [0.30, 0.61], P<0.00001, I2 = 42%) (Fig 5), whereas a significant reduction in luminal late loss is shown in Fig 6 (SMD = -0.41 [-0.60, -0.22], P<0.0001, I2 = 0%).
Fig 3. Pooled outcomes of restenosis among PCI segments and patients.
Fig 4. Funnel plot of restenosis.
Fig 5. Pooled outcomes of MLD.
Fig 6. Luminal late loss upon 6-month follow-up.
Secondary outcomes
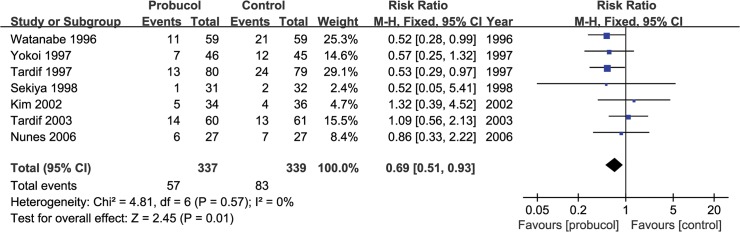
A total of 676 patients in 7 studies were considered in the analysis of MACE. 57 patients in probucol group and 87 patients in control group suffered from cardiac events (death, MI, repeat revascularization and CABG). Treatment of probucol reduced incidence of MACE by 31 percent compared with standard treatment after PCI (RR = 0.69 [0.51, 0.93], P = 0.01, I2 = 0%) (Fig 7).
Fig 7. Pooled outcomes of clinical outcomes (death, myocardial infarction, repeat revascularization, coronary artery bypass grafting).
Subgroup analyses and sensitivity analysis
A sensitivity analysis was conducted to determine the consistency of each outcome. Three high-risk studies were included in the sensitivity analysis. Adding these studies to the pooling only minimally altered the pooled MLD from 0.52 [0.36, 0.68] to 0.40 [0.23, 0.57]. Subgroup analyses of MLD based on ethnicity, dosage and duration of drug use before PCI appeared to have no effect on the results in each group (S2 Table). However, the effect of probucol on MLD among patients who underwent PTCA appeared to be more significant upon follow-up at 6months than the effect among patients who instead underwent stent implantation. (SMD = 0.59 [0.38, 0.80] vs 0.28 [0.04, 0.51], P = 0.05).
Discussion
This meta-analysis showed that probucol, which has been available for many years, may be useful in a new context. Although the sizes of the populations in many of the trials examined in our analysis were small and the results were conflicting, our meta-analysis nonetheless showed that probucol reduced the risk of restenosis by nearly 50%. Moreover, the incidence of MACE was reduced after probucol treatment without heterogeneity.
Regarding the heterogeneity in MLD that was observed across studies, probucol appeared to be less effective in patients who underwent stent implantation than in patients who underwent balloon angioplasty. This result was most likely due to the difference in the physiopathology of the restenosis that occurred after balloon angioplasty compared with stent replacement. Many studies have demonstrated that restenosis after balloon angioplasty results from early elastic recoil, negative remodeling and neointimal hyperplasia[26]. And the stent virtually eliminated early elastic recoil and negative remodeling. However, the incidence of neointimal hyperplasia increased with balloon angioplasty[27]. Because of these differences, the anti-restenotic effects of probucol on patients who have undergone either PTCA or stent should not be expected to be identical. A meta-analysis of 11 randomized studies found that significantly fewer restenosis occurred following stenting than following PTCA (25.8% vs34.2%)[28]. Stent-implantation reduced restenosis and increase MLD to a larger extent than has been noted in patients who undergo PTCA. Thus, adding probucol therapy, as a treatment for patients who underwent stent-implantation, may not be as effective as this treatment in patients who underwent PTCA. Nonetheless, probucol affected the genesis of intimal hyperplasia and reduced restenosis in patients who underwent stent-implantation.
Besides its antioxidant function, other properties of probucol should be noticed. Properties included promoting endothelial cell growth and functional re-endothelialization[29], inducing heme oxygenase-1 (HO-1) mRNA and HO-1 activity in vascular smooth muscle cell (SMC) to inhibit proliferation of SMCs and intimal thickening[30, 31]. Probucol improved endothelium-dependent arterial relaxation[32] and functional re-endothelialization following aortic balloon injury, as measured via extent of re-endothelialization, nitric oxide (NO) production and nitric oxide-mediated vasodilation in animals. In some clinical trials, plasma NO and flow-mediated vasodilation during reactive hyperemia (FMD) were improved[33], as well as carotid artery intima-media thickness (IMT) and the rate of IMT increased[34]. In addition, probucol may also benefit patients with type 2 diabetes. Some study demonstrated that probucol could reduce blood glucose and preserve β-cell function in some animal models of type 2 diabetes[35, 36]. Probucol protects β cells of the pancreas through its strong anti-free radical and antioxidant effects, thereby neutralizing reactive oxygen species and alleviating oxidative stress. A novel microencapsulated formulation of probucol is being developed and may have potential as an anti-diabetic drug.
Although these data suggest that probucol is effective in reducing the rate of restenosis among patients who have undergone PCI, concerns have been raised about its high-density lipoprotein cholesterol-lowering effect and prolongation of QT interval[37]. Recent trials have indicated that probucol’s HDL-C lowering side-effect did not hamper its ability to alleviate atherosclerosis. More and more studies found that functional HDL subfractions shifted to dysfunctional HDL subfractions during ACS[38]. The assessment of functional HDL had become a novel target to investigate the association between HDL and coronary artery disease risk[39]. Previous studies from our group[40] and from other institutions have demonstrated that probucol reduced HDL-C levels but promoted enhanced reverse cholesterol transport (RCT) via activation of CETP and scavenger receptor class B type I (SR-BI)[41, 42]. Moreover, some studies suggested that probucol may have beneficial effect on the plaque stability in vivo[43, 44]. However, QT interval prolongation will limit its long-term use. New probucol analogues or preparations may alleviate this side effect. AGI-1067, the mono succinic acid ester of probucol, is a metabolically stable compound that has greater intracellular antioxidant efficacy in vitro than probucol without its QT-interval prolonging effect[45, 46]. Similarly to probucol, AGI-1067 significantly reduced the in-stent restenosis compared with placebo in the CART-1 study[22]. However, AGI-1067 did not reduce the study’s primary endpoint, i.e. the time to the first occurrence of cardiovascular death, stroke, coronary revascularization in the ARISE study[47]. In addition, a novel polymer-free sirolimus- and probucol-eluting stent demonstrated both its safety on QT-interval prolonging issues and anti-restenotic efficacy in several clinical trials[48, 49]. These new formulations of probucol may represent new directions for its clinical use.
Limitation
Our meta-analysis included 10 RCTs to acquire as much information as possible. However, our study had several limitations. First, we had no access to individual patient data, including data regarding the underlying diseases from which each patient suffered, the stent types, the specific conventional drugs used in control group, base-line blood lipid values, or reference luminal diameters (RLDs) where either stents or balloons had been used. Each of these issues may have affected the results of our analysis and may have been the cause of the heterogeneity observed in MLD. Some clinical trials suggested that coronary stenting in smaller vessels was associated with an increased risk of subacute thrombosis[50]. Rodés et al demonstrated that probucol reduced both lumen loss and restenosis after balloon angioplasty in small coronary arteries (vessels with RLD<3.0 mm)[51]. Second, our meta-analysis considered a follow-up period of 3–6 months, the period during which restenosis most often occurred2. A follow-up period of 6 months seemed to be too short to determine with certainty the effect of probucol on restenosis or MACE after PCI. The PART study extended the observation period to 1 year and found that probucol still facilitated a significant reduction in the incidence of repeat revascularization[52]. Moreover Kasail[53] et al conducted a propensity analysis and found that probucol therapy improved long-term (>10-year) survival after complete revascularization. Despite these findings, more large scale clinical trials are required to clarify the long-term benefits of probucol.
Conclusion
In conclusion, the available data demonstrated that probucol significantly reduced the restenosis and late luminal loss, and increased MLD upon follow-up at 3-6-month in patients who underwent PCI compared with patients who were not treated with any lipid-lowering drugs. Moreover treatment with probucol reduced the incidence of MACE and probably increased the long-term survival rate after PCI.
Supporting Information
(DOC)
(DOCX)
Note: M, men; P, probucol group (standard drug treatment plus only probucol); C, control group (usual drug treatment without any lipid-lowering drugs); Duration*: duration of drug-use before PC
(DOCX)
(DOCX)
Acknowledgments
We are grateful to Dr. Yang Bai (Department of Gastroenterology, Nanfang Hospital, Southern Medical University), Dr. Yuli Huang (Division of Cardiology, Nanfang Hospital, Southern Medical University) and Prof. Xin Chen (Department of Foreign Languages, Nanchang Normal University) for their kind assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project was supported by the National Natural Science Foundation of China (81370380), the Natural Science Foundation of Guangdong Province of China (S2013010014739), and the Science and Technology Foundation of Guangdong Province of China (2012B091100155). The funders had no role in study design, data collection and analysis, decision to publish, or the preparation of the manuscript.
References
- 1. Fanelli C, Aronoff R. Restenosis following coronary angioplasty. Am Heart J 1990,119:357–368. [DOI] [PubMed] [Google Scholar]
- 2. Califf RM, Fortin DF, Frid DJ, Harlan WR 3rd, Ohman EM, Bengtson JR, et al. Restenosis after coronary angioplasty: an overview. J Am Coll Cardiol 1991,17:2B–13B. [DOI] [PubMed] [Google Scholar]
- 3. Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994,331:489–495. [DOI] [PubMed] [Google Scholar]
- 4. Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med 1994,331:496–501. [DOI] [PubMed] [Google Scholar]
- 5. Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Wong C, et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation 1996,94:35–43. [DOI] [PubMed] [Google Scholar]
- 6. Pratt RE, Dzau VJ. Pharmacological strategies to prevent restenosis: lessons learned from blockade of the renin-angiotensin system. Circulation 1996,93:848–852. [DOI] [PubMed] [Google Scholar]
- 7. Grewe PH, Deneke T, Machraoui A, Barmeyer J, Muller KM. Acute and chronic tissue response to coronary stent implantation: pathologic findings in human specimen. J Am Coll Cardiol 2000,35:157–163. [DOI] [PubMed] [Google Scholar]
- 8. Hoffmann R, Mintz GS, Dussaillant GR, Popma JJ, Pichard AD, Satler LF, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation 1996,94:1247–1254. [DOI] [PubMed] [Google Scholar]
- 9. Schneider JE, Berk BC, Gravanis MB, Santoian EC, Cipolla GD, Tarazona N, et al. Probucol decreases neointimal formation in a swine model of coronary artery balloon injury. A possible role for antioxidants in restenosis. Circulation 1993,88:628–637. [DOI] [PubMed] [Google Scholar]
- 10. Ferns GA, Forster L, Stewart-Lee A, Nourooz-Zadeh J, Anggard EE. Probucol inhibits mononuclear cell adhesion to vascular endothelium in the cholesterol-fed rabbit. Atherosclerosis 1993,100:171–181. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009,62:1006–1012. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 12. Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 2007,26:53–77. [DOI] [PubMed] [Google Scholar]
- 13. Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999,18:2693–2708. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003,327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997,315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Setsuda M, Inden M, Hiraoka N, Okamoto S, Tanaka H, Okinaka T, et al. Probucol therapy in the prevention of restenosis after successful percutaneous transluminal coronary angioplasty. Clin Ther 1993,15:374–382. [PubMed] [Google Scholar]
- 17. Watanabe K, Sekiya M, Ikeda S, Miyagawa M, Hashida K. Preventive effects of probucol on restenosis after percutaneous transluminal coronary angioplasty. American Heart Journal 1996,132:23–29. [DOI] [PubMed] [Google Scholar]
- 18. Tardif JC, Cote G, Lesperance J, Bourassa M, Lambert J, Doucet S, et al. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med 1997,337:365–372. [DOI] [PubMed] [Google Scholar]
- 19. Yokoi H, Daida H, Kuwabara Y, Nishikawa H, Takatsu F, Tomihara H, et al. Effectiveness of an antioxidant in preventing restenosis after percutaneous transluminal coronary angioplasty: the Probucol Angioplasty Restenosis Trial (PART). J Am Coll Cardiol 1997,30:855–862. [DOI] [PubMed] [Google Scholar]
- 20. Sekiya M, Funada J, Watanabe K, Miyagawa M, Akutsu H. Effects of probucol and cilostazol alone and in combination on frequency of poststenting restenosis. American Journal of Cardiology 1998,82:144–147. [DOI] [PubMed] [Google Scholar]
- 21. Kim MH, Cha KS, Han JY, Kim HJ, Kim JS. Effect of antioxidant probucol for preventing stent restenosis. Catheter Cardiovasc Interv 2002,57:424–428. [DOI] [PubMed] [Google Scholar]
- 22. Tardif JC, Gregoire J, Schwartz L, Title L, Laramee L, Reeves F, et al. Effects of AGI-1067 and probucol after percutaneous coronary interventions. Circulation 2003,107:552–558. [DOI] [PubMed] [Google Scholar]
- 23. Wakeyama T, Ogawa H, Iida H, Takaki A, Iwami T, Mochizuki M, et al. Effects of candesartan and probucol on restenosis after coronary stenting: results of insight of stent intimal hyperplasia inhibition by new angiotensin II receptor antagonist (ISHIN) trial. Circ J 2003,67:519–524. [DOI] [PubMed] [Google Scholar]
- 24. Kaminnyi AI, Lankin VZ, Samko AN, Sozykin AL, Provatorov SI, Konovalova GG, et al. Low daily dose of antioxidant probucol decreases incidence and severity of restenosis after transluminal coronary balloon angioplasty. Bull Exp Biol Med 2005,139:183–185. [DOI] [PubMed] [Google Scholar]
- 25. Nunes GL, Abizaid AC, Theodoro MP, Brito FS Jr., Caixeta A, da Silva LF, et al. Role of probucol in inhibiting intimal hyperplasia after coronary stent implantation: a randomized study. Am Heart J 2006,152:914.e911–917. [DOI] [PubMed] [Google Scholar]
- 26. Kuntz RE, Baim DS. Defining coronary restenosis. Newer clinical and angiographic paradigms. Circulation 1993,88:1310–1323. [DOI] [PubMed] [Google Scholar]
- 27. Dussaillant GR, Mintz GS, Pichard AD, Kent KM, Satler LF, Popma JJ, et al. Small stent size and intimal hyperplasia contribute to restenosis: a volumetric intravascular ultrasound analysis. J Am Coll Cardiol 1995,26:720–724. [DOI] [PubMed] [Google Scholar]
- 28. Moreno R, Fernandez C, Alfonso F, Hernandez R, Perez-Vizcayno MJ, Escaned J, et al. Coronary stenting versus balloon angioplasty in small vessels: a meta-analysis from 11 randomized studies. J Am Coll Cardiol 2004,43:1964–1972. [DOI] [PubMed] [Google Scholar]
- 29. Lau AK, Leichtweis SB, Hume P, Mashima R, Hou JY, Chaufour X, et al. Probucol promotes functional reendothelialization in balloon-injured rabbit aortas. Circulation 2003,107:2031–2036. [DOI] [PubMed] [Google Scholar]
- 30. Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, et al. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med 2006,203:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deng YM, Wu BJ, Witting PK, Stocker R. Probucol protects against smooth muscle cell proliferation by upregulating heme oxygenase-1. Circulation 2004,110:1855–1860. [DOI] [PubMed] [Google Scholar]
- 32. Keaney JF Jr., Xu A, Cunningham D, Jackson T, Frei B, Vita JA. Dietary probucol preserves endothelial function in cholesterol-fed rabbits by limiting vascular oxidative stress and superoxide generation. J Clin Invest 1995,95:2520–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tagawa T, Urabe Y, Kimura Y, Suzuki S, Ono H, Takeda K. Long-term treatment with probucol improves endothelial function in patients with coronary artery disease. Hypertens Res 2004,27:311–318. [DOI] [PubMed] [Google Scholar]
- 34. Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, et al. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol 2002,39:610–616. [DOI] [PubMed] [Google Scholar]
- 35. Drash AL, Rudert WA, Borquaye S, Wang R, Lieberman I. Effect of probucol on development of diabetes mellitus in BB rats. Am J Cardiol 1988,62:27B–30B. [DOI] [PubMed] [Google Scholar]
- 36. Gorogawa S, Kajimoto Y, Umayahara Y, Kaneto H, Watada H, Kuroda A, et al. Probucol preserves pancreatic beta-cell function through reduction of oxidative stress in type 2 diabetes. Diabetes Res Clin Pract 2002,57:1–10. [DOI] [PubMed] [Google Scholar]
- 37. Reinoehl J, Frankovich D, Machado C, Kawasaki R, Baga JJ, Pires LA, et al. Probucol-associated tachyarrhythmic events and QT prolongation: importance of gender. Am Heart J 1996,131:1184–1191. [DOI] [PubMed] [Google Scholar]
- 38. Tan Y, Liu TR, Hu SW, Tian D, Li C, Zhong JK, et al. Acute coronary syndrome remodels the protein cargo and functions of high-density lipoprotein subfractions. PLoS One 2014,9:e94264 10.1371/journal.pone.0094264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo ZG, Li C, Zhong JK, Tu Y, Xie D. Laboratory investigation of dysfunctional HDL. Chem Phys Lipids 2012,165:32–37. 10.1016/j.chemphyslip.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 40. Zhong JK, Guo ZG, Li C, Wang ZK, Lai WY, Tu Y. Probucol alleviates atherosclerosis and improves high density lipoprotein function. Lipids Health Dis 2011,10:210 10.1186/1476-511X-10-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Franceschini G, Sirtori M, Vaccarino V, Gianfranceschi G, Rezzonico L, Chiesa G, et al. Mechanisms of HDL reduction after probucol. Changes in HDL subfractions and increased reverse cholesteryl ester transfer. Arteriosclerosis 1989,9:462–469. [DOI] [PubMed] [Google Scholar]
- 42. Yamamoto S, Tanigawa H, Li X, Komaru Y, Billheimer JT, Rader DJ. Pharmacologic suppression of hepatic ATP-binding cassette transporter 1 activity in mice reduces high-density lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation 2011,124:1382–1390. 10.1161/CIRCULATIONAHA.110.009704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li S, Liang J, Niimi M, Bilal Waqar A, Kang D, Koike T, et al. Probucol suppresses macrophage infiltration and MMP expression in atherosclerotic plaques of WHHL rabbits. J Atheroscler Thromb 2014,21:648–658. [DOI] [PubMed] [Google Scholar]
- 44. Li JF, Chen S, Feng JD, Zhang MY, Liu XX. Probucol via inhibition of NHE1 attenuates LPS-accelerated atherosclerosis and promotes plaque stability in vivo. Exp Mol Pathol 2014,96:250–256. 10.1016/j.yexmp.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 45. Sundell CL, Somers PK, Meng CQ, Hoong LK, Suen KL, Hill RR, et al. AGI-1067: a multifunctional phenolic antioxidant, lipid modulator, anti-inflammatory and antiatherosclerotic agent. J Pharmacol Exp Ther 2003,305:1116–1123. [DOI] [PubMed] [Google Scholar]
- 46. Kunsch C, Luchoomun J, Grey JY, Olliff LK, Saint LB, Arrendale RF, et al. Selective inhibition of endothelial and monocyte redox-sensitive genes by AGI-1067: a novel antioxidant and anti-inflammatory agent. J Pharmacol Exp Ther 2004,308:820–829. [DOI] [PubMed] [Google Scholar]
- 47. Tardif JC, McMurray JJ, Klug E, Small R, Schumi J, Choi J, et al. Effects of succinobucol (AGI-1067) after an acute coronary syndrome: a randomised, double-blind, placebo-controlled trial. Lancet 2008,371:1761–1768. 10.1016/S0140-6736(08)60763-1 [DOI] [PubMed] [Google Scholar]
- 48. Byrne RA, Mehilli J, Iijima R, Schulz S, Pache J, Seyfarth M, et al. A polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial vs. polymer-based drug-eluting stents. Eur Heart J 2009,30:923–931. 10.1093/eurheartj/ehp044 [DOI] [PubMed] [Google Scholar]
- 49. Massberg S, Byrne RA, Kastrati A, Schulz S, Pache J, Hausleiter J, et al. Polymer-free sirolimus- and probucol-eluting versus new generation zotarolimus-eluting stents in coronary artery disease: the Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting versus Zotarolimus-eluting Stents (ISAR-TEST 5) trial. Circulation 2011,124:624–632. 10.1161/CIRCULATIONAHA.111.026732 [DOI] [PubMed] [Google Scholar]
- 50. Mak KH, Belli G, Ellis SG, Moliterno DJ. Subacute stent thrombosis: evolving issues and current concepts. J Am Coll Cardiol 1996,27:494–503. [DOI] [PubMed] [Google Scholar]
- 51. Rodes J, Cote G, Lesperance J, Bourassa MG, Doucet S, Bilodeau L, et al. Prevention of restenosis after angioplasty in small coronary arteries with probucol. Circulation 1998,97:429–436. [DOI] [PubMed] [Google Scholar]
- 52. Daida H, Kuwabara Y, Yokoi H, Nishikawa H, Takatsu F, Nakata Y, et al. Effect of probucol on repeat revascularization rate after percutaneous transluminal coronary angioplasty (from the Probucol Angioplasty Restenosis Trial [PART]). Am J Cardiol 2000,86:550–552, A559. [DOI] [PubMed] [Google Scholar]
- 53. Kasai T, Miyauchi K, Kubota N, Kajimoto K, Amano A, Daida H. Probucol therapy improves long-term (>10-year) survival after complete revascularization: a propensity analysis. Atherosclerosis 2012,220:463–469. 10.1016/j.atherosclerosis.2011.09.051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Note: M, men; P, probucol group (standard drug treatment plus only probucol); C, control group (usual drug treatment without any lipid-lowering drugs); Duration*: duration of drug-use before PC
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.