Abstract
Developmental stuttering is a speech disorder most likely due to a heritable form of developmental dysmyelination impairing the function of the speech-motor system. Speech-induced brain-activation patterns in persons who stutter (PWS) are anomalous in various ways; the consistency of these aberrant patterns is a matter of ongoing debate. Here, we present a hierarchical series of coordinate-based meta-analyses addressing this issue. Two tiers of meta-analyses were performed on a 17-paper dataset (202 PWS; 167 fluent controls). Four large-scale (top-tier) meta-analyses were performed, two for each subject group (PWS and controls). These analyses robustly confirmed the regional effects previously postulated as “neural signatures of stuttering” (Brown 2005) and extended this designation to additional regions. Two smaller-scale (lower-tier) meta-analyses refined the interpretation of the large-scale analyses: 1) a between-group contrast targeting differences between PWS and controls (stuttering trait); and 2) a within-group contrast (PWS only) of stuttering with induced fluency (stuttering state).
Keywords: Persistent developmental stuttering, Functional neuroimaging, Meta-analysis, Activation likelihood estimation, ALE
1. INTRODUCTION
Persistent developmental stuttering (PDS) is a speech disorder affecting 1% of adults. Approximately 5% of children exhibit developmental stuttering, with onset typically between two and five years of age (Bloodstein, 1995). Spontaneous remission during childhood is common, with recovery rates estimated at 40-80%, phenomena suggesting both a common etiology and common mechanisms of recovery (Kell et al., 2009). Early theories of stuttering adopted a wide range of conceptual frameworks including psychodynamics, neurochemical and hormonal imbalances, and peripheral nerve and musculoskeletal abnormalities. More recently, converging studies from multiple laboratories have assembled compelling evidence that PDS is a heritable (Dworzynski, Remington, Rijsdijk, Howell, & Plomin, 2007; Kang et al., 2010) neurodevelopmental disorder, certainly affecting white matter (Chang, Erickson, Ambrose, Hasegawa-Johnson, & Ludlow, 2008; Cykowski, Fox, Ingham, Ingham, & Robin, 2010; Kell et al., 2009; Sommer, Koch, Paulus, Weiller, & Büchel, 2002; Watkins, Smith, Davis, & Howell, 2007) and possibly affecting grey matter(Kell et al., 2009). Jointly the reports of Cykowski (et al., 2010) and Kang (et al., 2010) point strongly to a mild form of developmental dysmyelination (likely a lysosomal storage disorder), with predominate involvement of left frontal white-matter tracts, at least in symptomatic individuals. Developmental stuttering, then, is best conceptualized as a developmental disconnection syndrome in which various components of the speech-production system are aberrantly connected and have impaired inter-regional communication leading to the symptom complex termed “stuttering”.
The functional neuroimaging literature in persons who stutter (PWS) supports the above etiological formulation in that it has repeatedly reported abnormal task-induced activation patterns during speech tasks in adults who stutter as compared to normally fluent controls subjects. As is the norm for human neuroimaging research, the great majority of functional neuroimaging studies in PWS have applied inter-subject averaging methods and reported their findings as activation coordinates in a standardized space. The nearly universal adoption of this analysis and reporting standard has fostered the development and application of coordinate-based meta-analysis methods (Fox, Lancaster, Laird, & Eickhoff, 2014), which compute activation likelihood estimations (ALE;(Turkeltaub, Eden, Jones, & Zeffiro, 2002)) across conceptually related groups of publications. In PDS, these methods were applied by Brown (et al., 2005) to identify functional-activation abnormalities associated with stuttering. In this meta-analysis, Brown reported several “neural signatures of stuttering”, including over activation of right inferior premotor cortex (operculum and insula) and cerebellum and under activation of auditory cortex (Brown, Ingham, Ingham, Laird, & Fox, 2005). These were interpreted as endorsing an “efference copy” as an explanatory account. Brown's ‘neural signatures’ of stuttering are widely cited and have been replicated by subsequent papers (Chang, Kenney, Loucks, & Ludlow, 2009; Lu et al., 2009). Nevertheless, Ingham (et al., 2012) and Wymbs (et al., 2013) have specifically challenged the “neural signatures of stuttering” reported by Brown and colleagues and, more generally, have argued in favor of a case-study approach and against the traditional group-mean approach as the most appropriate strategy for future research (R. J. Ingham, Grafton, Bothe, & Ingham, 2012; Wymbs, Ingham, Ingham, Paolini, & Grafton, 2013). Applying their proposed strategy, Wymbs (et al, 2013) studied four adults with PDS, imaging each subject on four separate occasions and came to the conclusion that “individual PWS may use different neural regions during overt stuttering, perhaps in response to different neuroanatomical abnormalities.” In the nine years since Brown's initial meta-analysis of stuttered speech, the PDS functional neuroimaging literature has grown considerably both in size and diversity. Concurrently, methods for coordinate-based meta-analysis have evolved, achieving greater statistical rigor and power (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012). The emerging controversy regarding this hypothesis suggests the need to re-address the “neural signatures” hypothesis using additional data and more advanced methods and to address the role of group-mean methods for future stuttering research.
Evolution of statistical parametric mapping (SPM) software is ongoing, with progressive improvements both in spatial normalization methods and in statistical analyses. As regards neuroimaging in PDS, these methodological improvements have fostered a diversity of experimental design and analysis strategies. In particular, there has been a shift from purely within-group analyses (SPMs created for each subject pool independently; e.g., (Braun, 1997; Fox, 1996) to between-group analysis (SPMs directly contrasting PWS and controls; e.g., (De Nil, Kroll, Lafaille, & Houle, 2003; Neumann et al., 2003). Broadly speaking, within-group contrasts (e.g., stuttering vs. induced fluency) characterize stuttering as a state, while between-group contrasts (e.g., induced fluency vs. natural fluency) characterize stuttering as a trait. The additional scope of experimental designs now available offer meta-analysis opportunities not available to Brown and colleagues.
The present study was intended to accomplish two aims. First, we sought to re-test Brown's “neural signatures” analysis, using a larger data set and more advanced meta-analytic methods. We hypothesized that – contrary to the arguments of Ingham (et al., 2012) and Wymbs (et al., 2013) – we would replicate Brown's original findings and extend them to regions often reported in the PDS literature that were not previously identified as potential “neural signatures” of stuttering. The SMA and sub-cortical regions, for example, would be likely candidates for detection using more data and improved methods. Second, we sought to determine whether – taking advantage of the availability of both within-group and between group SPM analyses -- we could identify patterns that would that distinguish the stuttering state (contrasting induced fluency with dysfluency in PWS) from stuttering trait (contrasting PWS with controls), a distinction previous explored (Fox, 2000).
2. METHODS
The methods applied here all entail activation-likelihood estimation (ALE) meta-analysis of the coordinate-format, tabular results of articles published in peer-reviewed journals reporting functional activation neuroimaging studies in adults with PDS and fluent controls. The methods for retrieving, quality filtering, statistical analysis and the experimental design of a hierarchical series of meta-analysis are described below.
2.1. Internet Search and Inclusion Filters
A pubmed.gov search (26.Nov.2013) cuing “stuttering” AND [“fMRI” OR “PET”] returned 109 papers, including the Brown et al. (2005) meta-analysis and its 8-paper dataset. The original inclusion criteria (coordinates reported, whole brain imaged, and overt speech) (Brown et al., 2005) were applied to this list. An additional criterion, ‘results reported in terms of univariate contrasts,’ was added to address new types of analyses not currently amenable to ALE meta-analysis (e.g. ‘feature extraction’ in (Lu et al., 2009)). Collectively, these selection criteria eliminated 92 papers, leaving 17 papers eligible for analyses. Bibliographies of all papers meeting the inclusion criteria were reviewed, but no additional qualifying papers were found. The resulting dataset (Table 1) was large enough to support large-scale analyses addressing the neural signatures hypotheses as well as sub-analyses targeting stuttering trait and stuttering state (below). Limitations were present, however. Too few studies (fewer than 5) were available to perform sufficiently powered meta-analyses on studies addressing children, gender, handedness, effects of speech therapy, or tasks other than overt reading.
Table 1.
Paper Selection
| Reference | Modality | PWS | CTL | Gender | Vocal task | Control | Fluency | Group Contrasts |
|---|---|---|---|---|---|---|---|---|
| Fox 1996 | PET | 10 | 10 | M | Paragraph reading | Rest | Yes | - |
| Braun 1997 | PET | 18 | 20 | M/F | Spontaneous narrative + sentence construction Correlations w/ dysfluency |
Orolaryngeal control | Yes | - |
| Fox 2000 | PET | 10 | 10 | M | Correations w/ stutter rate | Yes | - | |
| De Nil 2000 | PET | 10 | 10 | M | Word reading | Silent reading | - | PWS>CTL, CTL>PWS |
| De Nil 2003 | PET | 13 | 10 | M | Word reading | Visual baseline | - | - |
| Neumann 2003 | fMRI | 16 | 16 | M | Sentence reading | Visual baseline | - | PWS>CTL, CTL>PWS |
| Preibisch 2003 | fMRI | 16 | 16 | M | Sentence reading | Visual baseline | - | PWS>CTL, CTL>PWS |
| Ingham 2004 | PET | 10 | 10 | F | Correlations w/ stutter rate | Yes | - | |
| Ingham 2000 | PET | 4 | 4 | M | Paragraph reading | Rest | Yes | - |
| Neumann 2005 | fMRI | 9 | 0 | M | Sentence reading | Visual baseline | - | - |
| De Nil 2008 | fMRI | 15 | 15 | M | Word reading | Listening | Yes | PWS>CTL, CTL>PWS |
| Giraud 2008 | fMRI | 16 | 0 | M | Sentence reading Correlations w/ stutter severity |
Visual baseline | - | - |
| Watkins 2008 | fMRI | 12 | 10 | M/F | Sentence reading | Visual baseline | - | PWS>CTL, CTL>PWS |
| Chang 2009 | fMRI | 20 | 20 | M/F | Word repetition | Listening | - | PWS>CTL, CTL>PWS |
| Kell 2009 | fMRI | 13 | 13 | M | Sentence reading Correlations w/ stutter severity |
Silent reading | - | PWS>CTL, CTL>PWS |
| Toyomura 2011 | fMRI | 12 | 12 | M/F | Sentence reading | Rest | Yes | PWS>CTL |
| Ingham 2012 | PET | 18 | 12 | M | Sentence reading, monologue Correlations w/ stutter rate |
Rest | - | PWS>CTL, CTL>PWS |
The first 8 papers were included in Brown et al. (2005); others were identified by our literature search. All papers were included in the large-scale meta-analyses. Papers reporting fluency induction (‘Fluency’) were included in the within-group meta-analysis (cf. Methods). Papers reporting group contrasts were included in the between-group meta-analysis. Abbreviations: PWS=persons who stutter; CTL=control.
2.2 Activation Likelihood Estimation (ALE) Analysis Methods
The ALE algorithm was introduced by Turkeltaub (et al., 2002) and has evolved over time to address specific limitations, many of which were identified and acknowledged at that time. Laird (Laird et al., 2005) provided a false discovery rate (FDR) correction for multiple comparisons and a method for ALE-to-ALE statistical contrasts. (This was the ALE version applied by Brown et al., 2005). Eickhoff (Eickhoff et al., 2009) introduced empirical estimates of between-subject and between-template spatial variability (a modification of the functional volumes modeling spatial probability construct; (Fox et al., 1999; 2001) in place of user-selected Gaussian filtering. This paper also modified the permutation test for above chance clustering between experiments in an anatomically constrained space (grey-matter only), a transition from fixed-effects to random-effects inference. Turkeltaub (Turkeltaub et al., 2011) added corrections for variable numbers of foci per experiment and experiments per paper, to prevent undue weighting of ALE maps by individual experiments (e.g. with large numbers of foci) or individual papers (e.g. with multiple similar experiments). Each of these improvements increased the statistical rigor and specificity of ALE, and therefore of the analyses reported here.
All analyses were performed in GingerALE 2.3 (brainmap.org/ale/index.html) with a cluster-level threshold of p < 0.05, and a FDR multiple-comparison correction of p < 0.05). The ALE images produced in the steps above were pooled and randomly divided into 2 groups 10,000 times to create a null-distribution, permitting subsequent statistical testing for difference in ALE scores between the two groups (ALE-to-ALE contrast analyses). Resultant ALE scores were tested against a null hypothesis at each voxel to produce a voxel-wise p-value image. This image was thresholded with an FDR of p <0.05 and a minimum cluster size of 100 mm3
2.3. Hierarchical Experimental Design Strategy
Hierarchical designs are often applied with ALE meta-analysis. Large-scale (upper tier) analyses are performed first, pooling the largest possible sets of papers that can be meaningfully grouped to demonstrate main effects. Small-scale (lower tier) are then performed to refine the results and guide interpretation of the larger data sets (Fox, Parsons, & Lancaster, 1998). A hierarchical design was applied here to demonstrate the main effects of stuttering (the large-scale analyses) and also to demonstrate differences in stuttering state and stuttering trait (the small-scale analyses). In the following, the term “experiment” refers to a unique statistical contrast that yielded a published list of coordinates. Publications typically report multiple experiments, often with similar (but not identical) conditional contrasts.
2.3.1 Large-scale (Top-tier) Analyses: Comprehensive and Restricted Data Sets
Four large-scale meta-analyses were performed, two for each subject group (PWS and controls). Large-scale meta-analyses are effectively free of selection bias and have the highest statistical power, although the results may be challenging to interpret. All four top-tier meta-analyses pooled all available papers (n = 17), in a manner similar to Brown (et al., 2005).
Two meta-analyses (one per group) used “comprehensive”’ data sets, in which all available experiments from all papers were included, even if there were multiple similar experiments included in individual publications. Note, some papers report multiple experiments from the same group of subjects within a single paper, which will unduly weight a single subject group. Recent refinements of the ALE algorithms (Turkeltaub et al., 2011) include corrections for multiple experiments per paper, making this analysis less “unselective” than it might seem. This meta-analysis included 845 foci from 202 PWS and 359 foci 167 fluent controls.
Two meta-analyses used “restricted” data sets, in which only one experiment per subject group per paper was included, a technique for balancing the contribution of individual papers. This type of selection filtering was applied by Brown et al., 2005. It is a “manual” form of the correction later developed by Turkeltaub (et al., 2011). In the restricted dataset meta-analyses, we used task-minus-rest contrasts whenever possible, as they typically provide the greatest signal-to-noise ratio. In papers reporting only group contrasts (e.g. (Preibisch et al., 2003)), we included the PDS > Controls experiment (data table) in the PWS group and the Controls > PWS experiment in the controls group. Whenever fluency induction was used (e.g. Fox et al., 2000 reported dysfluency and induced fluency in the same group of PWS), we included the experimental condition without fluency induction, considering those results to be more representative of native stuttering. And as described by Brown et al. (2005), we used positive correlates with stutter or syllable rate when those were the only contrasts available. This large-scale analysis included 209 foci from 202 PDS subjects and 103 foci from 167 control subjects.
The resulting ALE maps from these analyses (both “comprehensive” and “restricted”) were subsequently compared using between-group contrast analysis (below, Figure 1).
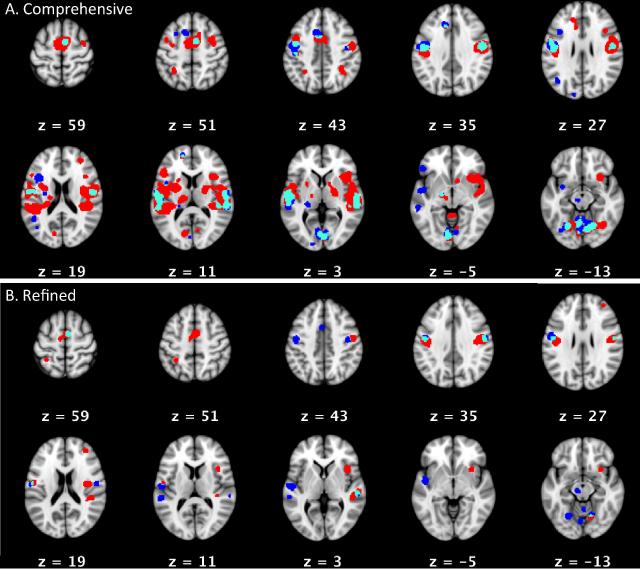
Figure 1. Large-Scale Meta-Analyses, (A) Comprehensive and (B) Refined.
The top section (A) shows areas of consistent activation for PDS (red) and controls (blue) in ‘comprehensive’ within-group meta-analyses, with areas of overlap in aqua (Cf. Supplementary Table 2 for xyz locations and anatomical labels). The bottom section (B) shows areas of consistent activation for PDS (red) and controls (blue) in the ‘refined’ “within-group meta-analyses, with areas of overlap in aqua (cf. Supplementary Table 2 for xyz locations and anatomical labels. All activations are overlaid on MNI152 atlas; slice numbers reference MNI152 space.
2.3.2 Stuttering Trait: Between-group contrast of PWS vs. Controls
Our group contrast analysis (i.e. PWS > Controls or Controls > PWS as reported by authors) explored group-wise activation differences between PWS and controls. The PWS > Controls analysis included 86 foci from 9 experiments from 9 papers and 113 subjects; the Controls>PDS analysis included 35 foci from 8 experiments from 8 papers and 101 subjects. Toyomura et al. (2011) only reported results for PDS>control and was only included in the first analysis. These analyses included both stuttered and induced fluency. The two resulting ALE maps from these analyses were subsequently compared using a contrast analysis (below, Figure 2A).
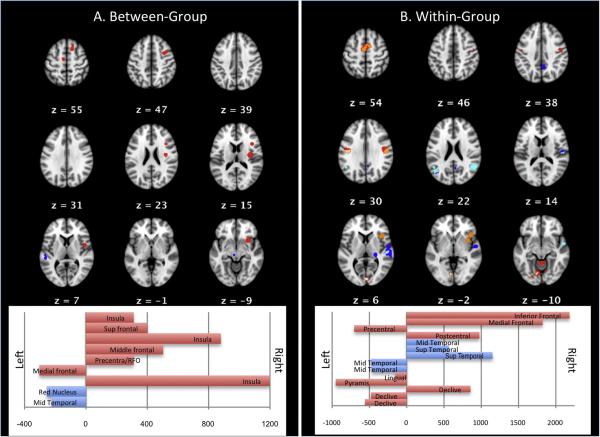
Figure 2. Targeted Sub-Analyses, (A) Between-Group and (B) Within-Group.
The left section (A) slices shows areas of consistent activation in the between-group (PDS vs. Controls) meta-analysis. PDS are shown in red, controls in blue. The laterality plot shows right-lateralized speech production in PDS compared to controls (cf. Supplementary Table 3 for xyz coordinates and anatomical labels). All activations are overlaid on MNI152 atlas; slice numbers reference MNI152 space. The right section (B) slices show areas of consistent activation during dysfluent (red) and fluent (blue) speech in the within-group meta-analysis (cf. Supplementary Table 4 for xyz locations and anatomical labels). Areas that retained significance in the contrast analysis are shown for PDS (orange) and controls (aqua). The laterality plot shows the contrast analysis, with frontal and cerebellar activations during dysfluency (red), and temporo-parietal activations during fluency (blue). Note: contrast analysis results (i.e. orange and aqua) are not shown on laterality plot. All activations are overlaid on MNI152 atlas; slice numbers reference MNI152 space. Abbreviations: Sup=superior; RFO=right frontal operculum; Mid=middle.
2.3.3 Stuttering State: Within-group contrast of Stuttering vs. Induced fluency
Our within-group analysis compared induced fluency with dysfluency in the PDS population. Activations during induced fluency numbered 142 foci from 8 papers and 89 subjects; activations during dysfluency (stuttering) numbered 119 foci from 7 papers and 77 subjects. The experiments were drawn from the same papers, except Toyomura et al. (2011), which only contributed to the ‘fluent’ results. The two resulting ALE maps from these analyses were subsequently compared using a contrast analysis (Below, Figure 2B)
3. RESULTS
ALE meta-analyses were performed hierarchically in two tiers: large-scale and small-scale. The large-scale (upper-tier) analyses pooled the largest possible sets of papers that could be meaningfully grouped. These were meant to re-test the previously postulated “neural signatures of stuttering” (Brown et al., 2005). The smaller-scale (lower tier) analyses were mean to guide interpretation of the effects observed in the more comprehensive analyses and, more specifically, to distinguish activation patterns characterizing stuttering as a neurophysiological trait from stuttering as a state.
3.1 Large-scale Analyses
Two types of large-scale analyses (“comprehensive” and “restricted”) were run on the 17-paper dataset, consisting of the Brown et al. (2005) dataset plus papers identified by our literature search. The ‘comprehensive analysis of PDS revealed 18 areas of statistically significant convergence across experiments, the largest in the right superior temporal gyrus, left red nucleus and midbrain, and bilateral frontal lobe, parietal lobe and cerebellum. The ‘comprehensive’ analysis of controls revealed 34 areas of statistically significant convergence across experiments, the largest in the right declive (vermis), left precentral gyrus, and in bilateral superior temporal gyri. (see Figure 1 and Supplementary Table 2). The ensuing PDS>Controls contrast analysis revealed 10 areas of statistically significant convergence in the right insula, left superior temporal gyrus, and bilateral frontal lobes. The Controls>PDS contrast analysis revealed 6 areas of statistically significant convergence in the left precentral and left superior temporal gyri. These areas of activation were much smaller in size than those detected in the PDS>Controls analysis (the largest were 408 and 3360 voxels, respectively). Fourteen areas of convergence between PDS and Controls were detected, the largest in the left precentral, and bilateral superior temporal gyri, and bilateral declive.
The restricted dataset analysis of PDS returned 14 areas of statistically significant convergence across experiments, the largest of which were in the right SMA, bilateral precentral gyri, and right insula. The restricted dataset analysis of controls identified 13 areas of statistically significant convergence across experiments, again including activations in bilateral precentral and superior temporal gyri and in the left red nucleus. A group contrast analysis of these two restricted analyses returned 8 areas of activation more consistently reported in PDS than in controls (right insula, SMA, and cerebellum, and bilateral precentral gyri) and 2 areas of activation more consistently reported in controls than in PDS: left auditory cortex and left precentral gyrus. No convergent areas were returned in the restricted analysis. Results are summarized in Figure 1 and Supplementary Table 2.
3.2 Stuttering Trait Analyses
Between-group differences were investigated with meta-analyses of author-reported contrasts (i.e. controls>PDS and PDS>controls). PDS>controls returned 7 areas of statistically significant convergence across experiments: one activation in the left cingulate motor area and multiple right-sided activations in the insula, SMA, prefrontal, and middle frontal gyri. Controls>PDS revealed 2 statistically significant areas of convergence across experiments: left middle temporal lobe and left red nucleus. No convergent areas were returned. Results are summarized in Figure 2A and Supplementary Table 3.
3.3 Stuttering State Analyses
A within-group (i.e within PDS) meta-analysis was used to investigate network differences between dysfluency speech and induced fluency. The dysfluency analysis revealed 10 areas of statistically significant convergence across experiments, in bilateral precentral gyri, right inferior and medial frontal gyri, and the left culmen, declive, tuber and right declive (all areas of the cerebellar vermis). The induced fluency analysis revealed 8 areas of statistically significant convergence across experiments , the largest in the right transverse temporal gyrus and bilateral superior temporal gyri. The contrast analysis of these results revealed 5 areas of statistically significant convergence across experiments during fluency, in bilateral superior and middle temporal gyri. Nine areas of statistically significant convergence across experiments were reported during dysfluent conditions, in the right inferior frontal and left medial frontal gyri, right postcentral and left precentral gyri, left lingual gyri, and bilateral cerebellar vermis (left pyramis and bilateral declive). No convergent areas were returned. Results are summarized in Figure 2B and Supplementary Table 4.
4. DISCUSSION
Through a hierarchical series of coordinate-based meta-analyses, the previously reported “neural signatures of stuttering” (Brown et al., 2005) were robustly confirmed, extended to previously unidentified regions (e.g., SMA) and refined by sub-analyses.
Two tiers of meta-analyses were performed. The top-tier of meta-analyses used all possible data sets (17 papers) to perform two meta-analyses per group (two in PWS; two in controls), which differed in the number of experiments allowed per paper (comprehensive and restricted). The top-tier meta-analyses replicated the “neural signature” regions and extended this designation to additional regions, most notably the SMA. The second tier of meta-analyses used sub-samples of this larger dataset to characterize stuttering trait effects (between-group contrasts of PWS with controls) and stuttering state effects (within-group contrasts of dysfluency with induced fluency in PWS). These sub-analyses provide interpretive guidance vis-à-vis the neural signature of stuttering reported by Brown (et al., 2005) and replicated here.
4.1 Cerebellar Findings
Cerebellar vermis over activation was one of Brown's ‘neural signatures of stuttering’ (Brown et al., 2005) Our large-scale (comprehensive and restricted) analyses reported cerebellar activations in both controls and PDS, with 5 areas of overlap between the 2 groups. No cerebellar activations were reported in the between-group meta-analysis, suggesting again that activations were similar in controls and PDS. The within-group (fluent-dysfluent) sub-analysis revealed left-lateralized (3/4 areas of activation) cerebellar activity during dysfluent conditions, without any corresponding activations during fluency. Since cerebellar activations did not vary significantly between PDS and controls across studies we can conclude that vermal activations are important for speech production, generally. We can further infer that unusually large, left-hemispheric cerebellar activations are related to dysfluent speech production in PDS. This lateralization is opposite the right-hemispheric cerebellar activations present during normal speech production (Stoodley & Schmahmann, 2009), extending lateralization abnormalities in PDS—-established in the cerebrum (Brown et al., 2005)—to the cerebellum. We will classify left cerebellar vermis overactivations, then, as related to a dysfluent state.
4.2 Cerebral Cortex Findings
Bilateral absence of auditory activation was also reported by Brown et al. (2005) as a ‘neural signature’ of stuttering (Brown et al., 2005). In our study, the large-scale analyses revealed extensive bilateral temporal activations in both groups; activations in PDS overlapped and extended beyond those reported in controls. Differences arose in the sub-analyses, however: left auditory (superior temporal gyrus) activations were greater in controls than in PDS, but bilateral auditory cortex activations were greater during fluency than dysfluency, especially on the right. No auditory cortex activations were present during dysfluency. Absence of left auditory cortex activation (compared to controls) indicated stuttering trait, and absence of right auditory cortex activation indicated dysfluent state. A bilateral absence of activation, as reported by Brown et al. (2005), would suggest dysfluency (right decreased activation) in someone with a stuttering trait (left decreased activation).
Activity in the right frontal operculum (RFO) / right anterior insula was the last ‘neural signature of stuttering’ reported by Brown et al (2005). In our large-scale analysis, RFO/insula activations were unique to PWS. The between-group analysis reported RFO/insula activations in PDS but not controls, and the within-group (induced-fluency-minus-dysfluency) analysis reported RFO/insula activations only during dysfluency. This finding is in seeming contrast to previous studies that have reported a negative correlation between RFO activity and stuttering severity (Kell et al., 2009; Preibisch et al., 2003) and concluded RFO activation was associated with fluency. Since the papers reporting a compensatory role for the RFO did not use fluency enhancing techniques, it is possible that this area is not mobilized by the external timing cues provided by chorus reading or paced speech, the methods used by most of the studies we analyzed. Moreover, activation patterns are inconsistent across different fluency enhancing techniques (Toyomura, Fujii, & Kuriki, 2011), suggesting that fluency can be achieved by various routes. One interpretation of these seemingly discordant results is that the insula is involved in natural (“endogenous”) fluency, but not the fluent speech produced by fluency induction. This could explain why it was active in our ‘Dysfluent’ (or absence-of-fluency-enhancing-techniques) analysis, but not our ‘Fluent’ analysis. The difference between natural fluency and ‘induced’ fluency has not, to our knowledge, been investigated with neuroimaging studies, but is an intriguing avenue for future research. Insula activations, then, indicate stuttering trait, but may or may not indicate dysfluent state.
Supplemental motor area (SMA) is reliably activated by speech production (Sörös et al., 2006), and our large-scale analysis showed activations in both controls and PWS. The between-group contrast returned PDS activations in the right pre-SMA, an area connected with prefrontal areas in primates (Picard & Strick, 2001) and involved in internally generated movements in fMRI task studies of humans(Nachev, Kennard, & Husain, 2008). The within-group (fluent-dysfluent) contrast showed bilateral SMA activations during dysfluency, an area shown to be connected with bilateral motor cortices in TMS studies (Narayana et al., 2012). We can conclude from the large-scale analysis that SMA activity is important to speech production in both PDS and controls. The pre-SMA, more active in PDS than controls, may be related to the internal movement generation defect described by Alm (2004). The SMA is likely related to the actual production of dysfluent speech, which is why it was active during dysfluency, not fluency. SMA activity indicates both stuttering trait and dysfluent state. The SMA is connected with the basal ganglia in nonhuman primates (Nachev et al., 2008), suggesting that network abnormalities involving these regions likely extend to subcortical regions.
4.3 Cerebral Sub-Cortex Findings
Subcortical activations in PDS are often reported (Giraud et al., 2008), but are poorly spatially concordant. Increased putamen activity has been correlated with stutter rate (Braun, 1997), and increased caudate activity has been associated with stuttering severity (Giraud et al., 2008). Bilateral increases in midbrain activity have been reported (Watkins et al., 2007), as well as increased left globus pallidus activity (R. J. Ingham et al., 2012). Our ‘comprehensive’ analysis detected subcortical activations in PDS in the left claustrum, globus pallidus, medial dorsal nucleus, substantia nigra/red nucleus, and right pulvinar. In controls, the only subcortical activations reported were in the left red nucleus and right caudate. The ‘refined’ analysis detected subcortical activations only in controls, in an area spanning the left red nucleus and substantia nigra. The between-group analysis reported greater activations in the left red nucleus of controls, and no subcortical activations were reported in the within-group (fluent-dysfluent) analysis. These results suggest an area of decreased red nucleus activity in PDS relative to controls, and diffuse, less concordant left midbrain overactivations in PDS. The ‘refined’ analysis results, which include the left substantia nigra, suggest that PDS abnormalities in substantia nigra function may be consistent across studies. We refer the reader to Alm (Alm, 2004) for an excellent review of the role of the basal ganglia in PDS pathology. We will focus our discussion on the red nucleus, an area not previously discussed (to our knowledge) with respect to PDS pathophysiology.
Although the red nucleus has not been discussed at length in previous reports, the results of this meta-analysis would not be possible if multiple papers had not reported red nucleus activations. These activations may have been overshadowed by larger cortical activations, reinforcing the importance of statistically rigorous methods for determining convergence between papers. The red nucleus has been implicated in normal speech production by fMRI studies (Sörös et al., 2006), stuttering has been reported after caudal midbrain infarction (Karakis, Ellenstein, Rosello, & Romero, 2009), and increased red nucleus activity is detectable two years after the start of long-term speech therapy (Neumann et al., 2005). Primate studies have identified the red nucleus as an important part of fronto-cerebellar networks, carrying (mostly motor) afferents from motor cortex, premotor cortex, and SMA to the cerebellum (Schmahmann, 1996). Functional studies have demonstrated connectivity between the red nucleus and cerebellum, thalamus, and (through primary motor cortex) to SMA, cingulate gyrus, and superior temporal gyrus (Sörös et al., 2006), all areas previously implicated in PDS (Brown et al., 2005; R. J. Ingham et al., 2012). Resting-state studies have reported red nucleus coactivations with temporal regions (Nioche, Cabanis, & Habas, 2008), areas implicated in fluency in our within-group contrast. It is not clear from these analyses whether decreased red nucleus activity compared to controls represents a primary defect or a consequence of lifelong compensation for a different primary defect (perhaps in the basal ganglia (Alm, 2004)). What is clear is that red nucleus activity is engaged by fluent speech production.
We propose a revised set of activation patterns characterizing ‘stuttering trait’ and ‘dysfluent state’: indicators of ‘stuttering trait’ (relative to controls) included (1) increased right insula/RFO activations, (2) increased SMA activations, and (3) decreased left auditory cortex activations, and (4) decreased left red nucleus activations. Indicators of ‘dysfluent state’ included (1) increased left cerebellar vermis activations, (2) increased SMA activations, (3) decreased right auditory cortex activations.
4.4 Strategic implications and future directions
The findings reported here strongly confirm and extend the “neural signatures of stuttering” first reported by Brown (et al., 2005) using a larger dataset and more statistically rigorous meta-analytic methods. This raises two questions. First, how are we to respond to the clear documentation of individual variability in PWS (Wymbs et al., 2013) and the strategic recommendations to shift our collective focus from group-mean mapping (upon which meta-analysis relies) to per-subject mapping (Ingham et al., 2012; Wymbs et al., 2013)? Second, what role should the meta-analyses reported here and, more generally, meta-analytic methods play in the future of research into developmental stuttering?
4.4.1 Individual variability: implications and practical applications
That individual variations in brain structure and functional organization exist is undeniable. Spatial normalization algorithms can partially (but never completely) correct for inter-individual structural variability; they correct for inter-individual functional variability only insofar as functional organization conforms to the topography of the neuroanatomical template. The popularity and impressive versatility of coordinate-based meta-analysis is a practical demonstration of the efficacy of spatial normalization (Fox et al., 2014). Yet, no reasonable person would anticipate that spatial normalization methods, however sophisticated, would remove all inter-individual (or inter-group) variability in brain functional organization. Thus, the observation that there remains significant variability is important, but in no way surprising. The more relevant question is what this implies about future neuroimaging research in developmental stuttering and, more generally, all brain disorders.
When making therapeutic choices, the therapist should strive to incorporate as much unique information regarding the individual patient as possible. In cancer therapy, genetic markers are being applied to guide choices of chemotherapeutic agents in a manner often characterized as “personalized medicine”. In neurosurgical interventions, the use of individual pre- and intra-operative maps of functional brain anatomy (e.g., to identify motor cortex, eloquent cortex, et cetera) is becoming widely used to improve patient outcome. So, the goal of using per-individual neuroimaging studies to inform choice of therapy and assess the responses to various types of therapy certainly has strong precedents. In the case of PDS, however, personalized therapy based on neuroimaging biomarkers – as proposed by Wymbs (et al., 2013) -- is a concept yet to be realized. To realize this vision, a scientifically rigorous rationale for selection among therapeutic options based on per-individual neuroimaging studies will need to be developed and defended.
4.4.2 Meta-analytic models: development and applications
To quantitatively characterize speech networks and their alterations in disorders, such as PDS, models are needed. In model construction, being data driven and explicit (i.e. testable) are cardinal virtues. Meta-analysis -- more than any other approach to model development – meets these requirements. In neuroimaging research, node-and-edge models (N&E) are widely employed. N&E models are applicable to many types of neuroimaging data (both structural and functional), accommodate numerous modeling conventions, and can represent virtually any brain network and any brain disorder. In N&E models, brain regions are represented as nodes. Co-variances between nodes comprise the edges (“paths”), that is, the inter-regional relationships. An important use of formal models is to direct analyses of primary (i.e., per patient) neuroimaging data sets. Meta-analytic models have be employed to improve diagnostic accuracy (Barron, Tandon, Lancaster, & Fox, 2014) and to direct therapies (Johansen-Berg et al., 2008). The meta-analytically determined regions from the various results reported here would be appropriately employed as nodes in N&E models of stuttering, fluent speech and induced fluency. The optimal modeling strategy applied would be determined by the dataset being modeled, but could include structural equation modeling, dynamic causal modeling, Granger causal modeling, partial least squares modeling and graph analytic (graph theory-based) modeling. The authors would argue that the most reasonable route to development of rigorous models of PDS and other speech disorders is through meta-analysis-informed modeling.
Gene discovery is another important application of neuroimaging methods, in general, and of meta-analysis, in particular. Neuroimaging genomics is a large and rapidly expanding field, applying structural and functional neuroimaging studies to identify genes associated with brain disorders (Glahn, Thompson, & Blangero, 2007). PDS is a heritable disorder (Andrews, Morris-Yates, Howie and Martin, 1991; Dwoszynski et al., 2007; Wittke-Thompson et al, 2007). Kang (et al., 2010) identified abnormalities in three genes associated with mucolipidoses, lysosomal storage disorders associated with neurologic symptoms. Although these genes were strongly associated in with stuttering, they could explain, at best 2-4% of the instance of stuttering in the populations studied. That is, there is much more work to be done to identify the full gamut of genes underlying developmental stuttering. The high rate of spontaneous recovery, particularly in females (Yairi & Ambrose, 1999), poses a significant hurdle for gene discovery using symptom status. Consequently, there is a pressing need for quantitative biomarkers, preferably biomarkers that could be readily applied in children, at the time of symptom onset. Neuroimaging is being applied in developmental disorders in other pediatric patients (e.g., autism spectrum disorders and attention-deficit/hyperactivity disorder). The regions identified here as “neural signatures of stuttering” are highly defensible starting points for the development of quantitative biomarkers assessing network status. For example, neuronal pathways connecting the various regions (nodes) could be assayed using diffusion tensor tractography in symptomatic children. Alternatively, network connectivity could be assayed using T2* images acquired during sleep (Manning et al., 2013). In both cases, such quantitative biomarkers promise to provide information about group and individual variability.
4.5 Conclusions
Despite arguments to the contrary, the “neural signatures of stuttering” proposed by Brown (et al., 2005) were robustly confirmed, extended and refined by applying ALE meta-analysis to a dataset more than double that tested by Brown and colleagues. The most notable addition to the regions identified as reliable neural signatures was the SMA. In addition, signatures of stuttering as a trait and stuttering as a state were identified. In response to arguments regarding the need for studies reporting groups of individual-subject analyses, we respond that the most appropriate potential role for “case report” style studies is in guiding therapeutic choices, although a rational basis for such choices remains to be developed. In defense of group-mean studies and meta-analysis thereof, we offer strategies for construction of data-driven models and biomarkers for gene discovery.
Supplementary Material
Highlights.
Activation likelihood estimation (ALE) meta-analyses of stuttering, induced fluency and fluency.
Findings replicate, extend, and refine previously postulated ‘neural signatures of stuttering’
Stuttering trait effects are identified by contrasting induced fluency (in persons who stutter) with fluency in controls.
Stuttering state effects identified by contrasting stuttering with induced fluency.
ACKNOWLEDGMENTS
We thank Juan Saenz, Michaela Robertson and P. Mickle Fox for technical assistance. This study was supported by funds from the National Institute of Health (NIMH: RO1 MH074457), P. Fox PI.
References
- Alm PA. Stuttering and the basal ganglia circuits: a critical review of possible relations. Journal of Communication Disorders. 2004;37(4):325–369. doi: 10.1016/j.jcomdis.2004.03.001. doi:10.1016/j.jcomdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Barron DS, Fox PT, Pardoe H, Lancaster J, Price LR, Blackmon K, et al. UNCORRECTED PROOF. Ynicl. 2014:1–8. doi:10.1016/j.nicl.2014.08.002. [Google Scholar]
- Bloodstein O. A Handbook on Stuttering. Singular Group; San Diego: 1995. [Google Scholar]
- Braun A. Altered patterns of cerebral activity during speech and language production in developmental stuttering. 1997:1–24. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: An ALE meta-analysis of functional neuroimaging studies. Human Brain Mapping. 2005;25(1):105–117. doi: 10.1002/hbm.20140. doi:10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. NeuroImage. 2008;39(3):1333–1344. doi: 10.1016/j.neuroimage.2007.09.067. doi:10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Kenney MK, Loucks TMJ, Ludlow CL. Brain activation abnormalities during speech and non-speech in stuttering speakers. NeuroImage. 2009;46(1):201–212. doi: 10.1016/j.neuroimage.2009.01.066. doi:10.1016/j.neuroimage.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski MD, Fox PT, Ingham RJ, Ingham JC, Robin DA. A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: A potential role for impaired myelination. NeuroImage. 2010;52(4):1495–1504. doi: 10.1016/j.neuroimage.2010.05.011. doi:10.1016/j.neuroimage.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nil L, Kroll RM, Kapur S, Houle S. A positron emission tomography study of silent and oral single word reading in stuttering and nonstuttering adults. 2000:1–17. doi: 10.1044/jslhr.4304.1038. [DOI] [PubMed] [Google Scholar]
- De Nil LF, Kroll RM, Lafaille SJ, Houle S. A positron emission tomography study of short- and long-term treatment effects on functional brain activation in adults who stutter. Journal of Fluency Disorders. 2003;28(4):357–380. doi: 10.1016/j.jfludis.2003.07.002. doi:10.1016/j.jfludis.2003.07.002. [DOI] [PubMed] [Google Scholar]
- De Nil LF, Beal DS, Lafaille SJ, Kroll RM, Crawley AP, Gracco VL. The effects of simulated stuttering and prolonged speech on the neural activation patterns of stuttering and nonstuttering adults. Brain and Language. 2008;107(2):114–123. doi: 10.1016/j.bandl.2008.07.003. doi:10.1016/j.bandl.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Dworzynski K, Remington A, Rijsdijk FH, Howell P, Plomin R. Genetic Etiology in Cases of Recovered and Persistent Stuttering in an Unselected, Longitudinal Sample of Young Twins. American Journal of Speech-Language Pathology. 2007;16(2):169. doi: 10.1044/1058-0360(2007/021). doi:10.1044/1058-0360(2007/021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59(3):2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. doi:10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. doi:10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C, et al. A PET study of the neural systems of stuttering. Nature. 1996;382:158–161. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong J-H, Lancaster JL. Brain correlates of stuttering and syllable production: a PET performance -correlation analysis. Brain. 2000;123:1985–2004. doi: 10.1093/brain/123.10.1985. [DOI] [PubMed] [Google Scholar]
- Fox PT, Parsons LM, Lancaster JL. Beyond the single study: function/location metanalysis in cognitive neuroimaging. Cognitive Neuroscience. 1998;1998(8):178–187. doi: 10.1016/s0959-4388(98)80138-4. [DOI] [PubMed] [Google Scholar]
- Fox PT, Huang A, Parsons LM, Xiong JH, Rainey L, Lancaster JL. Location-probability profiles for the mouth region of human primary motor-sensory cortex: model and validation. NeuroImage. 1999;13(1):196–209. doi: 10.1006/nimg.2000.0659. [DOI] [PubMed] [Google Scholar]
- Fox PT, Huang A, Parsons LM, Xiong JH, Rainey L, Lancaster JL. Functional volumes modeling: scaling for group size in averaged images. Human Brain Mapp. 2001;8(2):143–150. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<143::AID-HBM12>3.0.CO;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL, Laird AR, Eickhoff SB. Meta-analysis in human neuroimaging computational modeling of large-scale databases. Ann Rev Neurosci. 2014 doi: 10.1146/annurev-neuro-062012-170320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Neumann K, Bachoud-Levi A-C, Gudenberg, von AW, Euler H, Lanfermann H, Preibisch C. Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain and Language. 2008;104(2):190–199. doi: 10.1016/j.bandl.2007.04.005. doi:10.1016/j.bandl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Thompson PM, Blangero J. Neuroimaging endophenotypes: Strategies for finding genes influencing brain structure and function. Human Brain Mapping. 2007;28(6):488–501. doi: 10.1002/hbm.20401. doi:10.1002/hbm.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham RJ, Fox PT, Costello Ingham J, Zamarripa F. Is Overt Stuttered Speech a Prerequisite for the Neural Activations Associated with Chronic Developmental Stuttering? Brain and Language. 2000;75(2):163–194. doi: 10.1006/brln.2000.2351. doi:10.1006/brln.2000.2351. [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Fox PT, Ingham JC, Xiong J, zamarippa F, Hardies LJ. [Google Scholar]
- Lancaster J. Brain Correlates of Stuttering and Syllable Production: Gender Comparison and Replication. Journal of Speech, Language, and Hearing Research. 2004:1–22. doi: 10.1044/1092-4388(2004/026). [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Grafton ST, Bothe AK, Ingham JC. Brain activity in adults who stutter: Similarities across speaking tasks and correlations with stuttering frequency and speaking rate. Brain and Language. 2012;122(1):11–24. doi: 10.1016/j.bandl.2012.04.002. doi:10.1016/j.bandl.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex. 2008;18(6):1374–1383. doi: 10.1093/cercor/bhm167. doi:10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Riazuddin S, Mundorff J, Krasnewich D, Friedman P, Mullikin JC, Drayna D. Mutations in the Lysosomal Enzyme–Targeting Pathway and Persistent Stuttering. New England Journal of Medicine. 2010;362(8):677–685. doi: 10.1056/NEJMoa0902630. doi:10.1056/NEJMoa0902630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakis I, Ellenstein A, Rosello GR, Romero JR. Somnolence and Stuttering as the Primary Manifestations of a Midbrain Stroke. 2009:1–2. [PMC free article] [PubMed] [Google Scholar]
- Kell CA, Neumann K, Kriegstein, von K, Posenenske C, Gudenberg, von AW, Euler H, Giraud AL. How the brain repairs stuttering. Brain. 2009;132(10):2747–2760. doi: 10.1093/brain/awp185. doi:10.1093/brain/awp185. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping. 2005;25(1):155–164. doi: 10.1002/hbm.20136. doi:10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. Journal of Neuroscience. 2009;29(46):14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. doi:10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Ning N, Peng D, Ding G, Li K, Yang Y, Lin C. The role of large-scale neural interactions for developmental stuttering. Nsc. 2009;161(4):1008–1026. doi: 10.1016/j.neuroscience.2009.04.020. doi:10.1016/j.neuroscience.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9(11):856–869. doi: 10.1038/nrn2478. doi:10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Narayana S, Laird AR, Tandon N, Franklin C, Lancaster JL, Fox PT. Electrophysiological and functional connectivity of the human supplementary motor area. NeuroImage. 2012;62(1):250–265. doi: 10.1016/j.neuroimage.2012.04.060. doi:10.1016/j.neuroimage.2012.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann K, Euler HA, Gudenberg AWV, Giraud A-L, Lanfermann H, Gall V, Preibisch C. The nature and treatment of stuttering as revealed by fMRI. Journal of Fluency Disorders. 2003;28(4):381–410. doi: 10.1016/j.jfludis.2003.07.003. doi:10.1016/j.jfludis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Neumann K, Preibisch C, Euler HA, Gudenberg AWV, Lanfermann H, Gall V, Giraud A-L. Cortical plasticity associated with stuttering therapy. Journal of Fluency Disorders. 2005;30(1):23–39. doi: 10.1016/j.jfludis.2004.12.002. doi:10.1016/j.jfludis.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Nioche C, Cabanis EA, Habas C. Functional Connectivity of the Human Red Nucleus in the Brain Resting State at 3T. American Journal of Neuroradiology. 2008;30(2):396–403. doi: 10.3174/ajnr.A1375. doi:10.3174/ajnr.A1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. 2001:1–10. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Preibisch C, Neumann K, Raab P, Euler HA, Gudenberg, von AW, Lanfermann H, Giraud A-L. Evidence for compensation for stuttering by the right frontal operculum. NeuroImage. 2003;20(2):1356–1364. doi: 10.1016/S1053-8119(03)00376-8. doi:10.1016/S1053-8119(03)00376-8. [DOI] [PubMed] [Google Scholar]
- Ramage AE, Laird AR, Eickhoff SB, Acheson A, Peterson AL, Williamson DE, et al. A coordinate-based meta-analytic model of trauma processing in posttraumatic stress disorder. Human Brain Mapping. 2013;34(12):3392–3399. doi: 10.1002/hbm.22155. doi:10.1002/hbm.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. From Movement to Thought: Anatomic Substrates of the Cerebellar Contributionto Cognitive Processing. Human Brain Mapping. 1996:1–25. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Büchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. The Lancet. 2002;360(9330):380–383. doi: 10.1016/S0140-6736(02)09610-1. doi:10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Sörös P, Sokoloff LG, Bose A, McIntosh AR, Graham SJ, Stuss DT. Clustered functional MRI of overt speech production. NeuroImage. 2006;32(1):376–387. doi: 10.1016/j.neuroimage.2006.02.046. doi:10.1016/j.neuroimage.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Toyomura A, Fujii T, Kuriki S. Effect of external auditory pacing on the neural activity of stuttering speakers. NeuroImage. 2011;57(4):1507–1516. doi: 10.1016/j.neuroimage.2011.05.039. doi:10.1016/j.neuroimage.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-Analysis of the Functional Neuroanatomy of Single-Word Reading: Method and Validation. NeuroImage. 2002;16(3):765–780. doi: 10.1006/nimg.2002.1131. doi:10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping. 2011;33(1):1–13. doi: 10.1002/hbm.21186. doi:10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2007;131(1):50–59. doi: 10.1093/brain/awm241. doi:10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymbs NF, Ingham RJ, Ingham JC, Paolini KE, Grafton ST. Individual differences in neural regions functionally related to real and imagined stuttering. Brain and Language. 2013;124(2):153–164. doi: 10.1016/j.bandl.2012.11.013. doi:10.1016/j.bandl.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yairi E, Ambrose NG, Early childhood stuttering I. Persistent and recovery rates. J Speech Lang Hear Res. 1999;42:1097–1112. doi: 10.1044/jslhr.4205.1097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.