Summary
Cryptococcus neoformans is a facultative intracellular pathogen. The most distinctive feature of C. neoformans is a polysaccharide capsule that enlarges depending on environmental stimuli. The mechanism by which C. neoformans avoids killing during phagocytosis is unknown. We hypothesized that capsule growth conferred resistance to microbicidal molecules produced by the host during infection, particularly during phagocytosis. We observed that capsule enlargement conferred resistance to reactive oxygen species produced by H2O2 that was not associated with a higher catalase activity, suggesting a new function for the capsule as a scavenger of reactive oxidative intermediates. Soluble capsular polysaccharide protected C. neoformans and Saccharomyces cerevisiae from killing by H2O2. Acapsular mutants had higher susceptibility to free radicals. Capsular polysaccharide acted as an antioxidant in the nitroblue tetrazolium (NBT) reduction coupled to β-nicotinamide adenine dinucleotide (NADH)/phenazine methosulfate (PMS) assay. Capsule enlargement conferred resistance to antimicrobial peptides and the antifungal drug Amphotericin B. Interestingly, the capsule had no effect on susceptibility to azoles and increased susceptibility to fluconazole. Capsule enlargement reduced phagocytosis by environmental predators, although we also noticed that in this system, starvation of C. neoformans cells produced resistance to phagocytosis. Our results suggest that capsular enlargement is a mechanism that enhances C. neoformans survival when ingested by phagocytic cells.
Introduction
Cryptococcus neoformans is unique among pathogenic fungi because its cell body is surrounded by a polysaccharide capsule. The incidence of cryptococcosis has risen steadily during the past century because of the increasing number of immunocompromised patients. In addition to its clinical importance, C. neoformans has become a major model system for studying fungal infections. C. neoformans is both a pathogenic and an environmental fungus, and has the ability to infect many different hosts, including amoebas, slime molds, worms, insects and mammals (Casadevall and Perfect, 1998; Steenbergen et al., 2001; Mylonakis et al., 2002; Apidianakis et al., 2004). C. neoformans is a facultative intracellular pathogen that can survive and divide inside phagocytic cells, indicating that this yeast has developed mechanisms to evade intracellular killing in the phagolysosome (Tucker and Casadevall, 2002; Alvarez and Casadevall, 2006). The capacity for intracellular pathogenesis is an important feature, because phagocytic cells such as macrophages and neutrophils constitute the first line of defence against many fungal pathogens. In this sense, differences in macrophage activity have been related to the differential susceptibility to C. neoformans of different species (Shao et al., 2005). However, some recent findings suggest that the ability of C. neoformans to exploit macrophages is a mechanism that allows fungal dissemination in the host (Kechichian et al., 2007; Zaragoza et al., 2007).
One of the peculiarities of C. neoformans is that, in contrast to many fungal pathogens, it possesses well-defined virulence factors. Among them, the polysaccharide capsule contributes most to the overall virulence phenotype (McClelland et al., 2006). The cryptococcal polysaccharide capsule is believed to protect against environmental stresses, such as dehydration and other stress conditions (Aksenov et al., 1973; Casadevall and Perfect, 1998). In addition to surrounding the cell, the capsular polysaccharide is also released and secreted to the extracellular environment and this material is referred as exopolysaccharide. During infection, the exopolysaccharide has numerous deleterious effects on the host immune response (see review in Vecchiarelli, 2000).
An important feature of the capsule is that it can dramatically change in size in response to environmental conditions. In regular laboratory conditions that often involve growth in rich media, the capsule is usually small. However, a variety of media that induce capsule growth have been described (Granger et al., 1985; Vartivarian et al., 1993; Zaragoza et al., 2003a; Zaragoza and Casadevall, 2004). In vivo, C. neoformans infection is associated with massive capsule enlargement and the phenomenon seems to be important for virulence. In fact, capsule enlargement is an early morphological change that occurs during the first hours of infection in mice. The capsule also enlarges in size in vitro during the interaction with phagocytic cells (Feldmesser et al., 2001). Granger et al. (1985) reported that a mutant that could not enlarge the capsule in the presence of CO2 showed reduced virulence, and this has been also confirmed with other type of mutants (D’Souza et al., 2001). Although this morphological change is characteristic during the interaction with the host, its function remains unclear. So far, capsule enlargement has been involved in the inhibition of complement-mediated phagocytosis (Zaragoza et al., 2003b). Capsule enlargement is achieved by the accumulation of newly synthesized polysaccharide in the capsule in a short period of time (Maxson et al., 2007a), suggesting that it is a high-energy cost process. Consequently, we hypothesized that capsule enlargement is a process that confers advantage to the fungus during the interaction with the host. The aim of this work was to examine whether capsule growth was associated with a higher resistance to some of the stress factors that mimic the conditions that surrounds the fungal cells during its interaction with the host and phagocytic cells. Our results show that both capsular polysaccharide and capsule enlargement protects against a variety of stress conditions, implying a new mechanism that allows C. neoformans to survive and replicate inside macrophages.
Results
Killing by reactive oxygen species of cells with enlarged capsule in vivo and in vitro
We investigated whether capsular enlargement by C. neoformans conferred resistance to killing produced by reactive oxygen species (ROS). Cells with larger capsules were less susceptible to killing by ROS than cells with smaller capsules (Fig. 1, P-value at 3 h time point, 0.01). To investigate whether H2O2 carry-over to the agar plates had any influence in the results, we plated the sample (with and without hydrogen peroxide) at time zero and compared the viability and found no difference, indicating that H2O2 is diluted when the cells are plated. To exclude the possibility that H2O2 was affecting capsule size during the experimental conditions, we treated C. neoformans cells with large capsule with different H2O2 concentrations for 3 h, and measured for changes in the packed volume of the cells as described (Maxson et al., 2007a). The change in the packed volume of cells is an indirect measurement of the size of the capsule. We observed that even high H2O2 concentrations, such as 1 M H2O2, did not affect the packed volume of the cells during the 3 h incubation time (result not shown), indicating that in our conditions, H2O2 did not affect capsule size. This finding was confirmed by India Ink staining and microcopy observation. When we examined the effect at longer time periods, we observed that H2O2 killed cells with small and large capsule (result not shown).
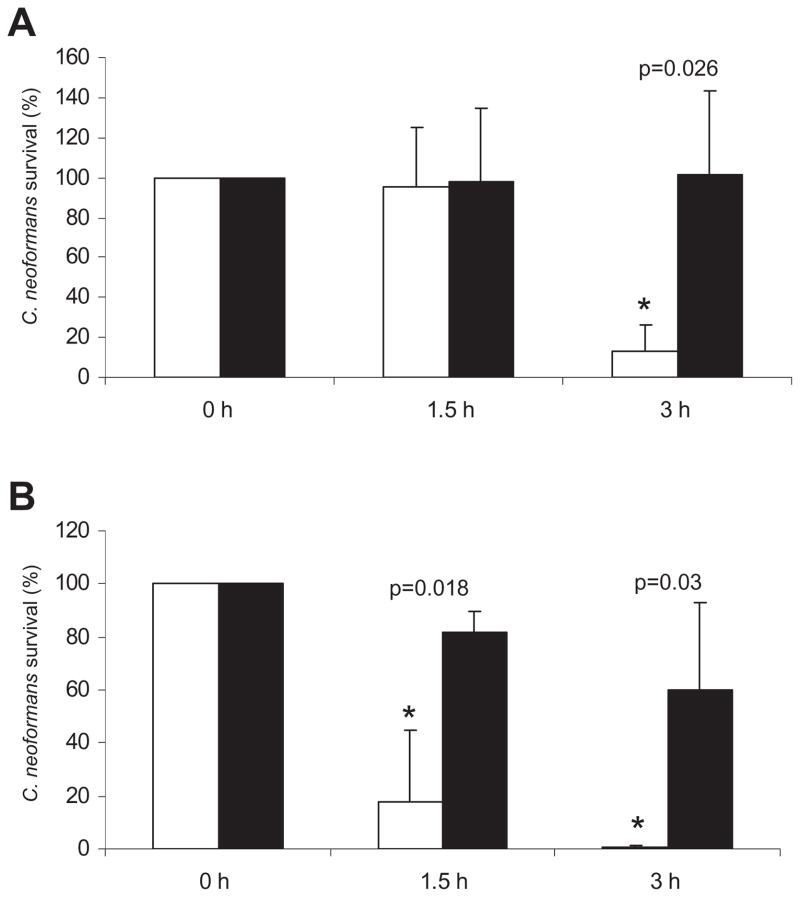
Fig. 1.
C. neoformans cells differing in capsule size manifest different susceptibility to H2O2. C. neoformans cells with small and large capsule (induced in diluted Sabouraud in MOPS buffer) were incubated with 0.5 mM H2O2 at 37°C. Parallel samples not exposed to H2O2 were carried out in parallel. At the indicated times, 100 μl aliquots were plated in Sabouraud, and viability was calculated at each time point as described in Experimental procedures. Solid line, small capsule; dotted line, cells with large capsule. At each time, the mean value and standard deviation of the viabilities from four different experiments are plotted. Asterisk indicates statistical differences at the time point indicated (P < 0.05).
Cells isolated directly from the lungs of infected mice during 18–24 h manifested enlarged capsules. After this short period of time, there is a significant increase in capsule size (result not shown). When we compared the susceptibility to H2O2 of cells from infected lungs and cells grown in regular Sabouraud medium, we observed that the cells recovered from mice were significantly more resistant to H2O2 than the cells grown in vitro (Fig. 2A) and this occurred even at a higher H2O2 concentration (2 mM, Fig. 2B). In fact, capsular enlargement that occurred in vivo was associated with higher resistance to killing by H2O2 than capsule enlargement induced in vitro.
Fig. 2.
Susceptibility of C. neoformans cells isolated from mice to H2O2. Cells with enlarged capsule were obtained from the lungs of infected mice as described in Experimental procedures. The cells were exposed to 0.5 mM (A) or 2 mM (B) H2O2, and survival was monitored as in (A) and as described in Experimental procedures. Open bars, cells grown in Sabouraud; closed bars, cells obtained from mice. Asterisks indicate statistical differences between cells grown in Sabouraud or isolated from infected mice, and the corresponding P-value is shown.
To confirm that the higher resistance observed in cells with enlarged capsule was directly due to a larger capsule size and not to higher catalase activity, we measured this enzymatic activity in our both types of cells, but found no difference (8.5 ± 2.6 mU per milligram of protein of enzymatic activity in cells with small capsule versus 10.2 ± 2 mU per milligram of protein in cells with enlarged capsule, P-value 0.4). However, in C. neoformans, it has been reported that the major enzymatic activity involved in ROS detoxification is gluthatione peroxidase (Brown et al., 2007). However, we could not detect this enzymatic activity (result not shown).
Influence of capsular polysaccharide in killing of C. neoformans and Saccharomyces cerevisiae by H2O2
We hypothesized that cells with large capsule were less susceptible to free radicals than cells with smaller capsules through a ‘buffering mechanism’, whereby oxygen-derived oxidants attacked preferentially the polysaccharide fibres. Consequently we investigated whether purified capsular polysaccharide protected cells with small capsule from killing by H2O2. Cells with small capsules suspended in capsular polysaccharide had reduced susceptibility to killing by H2O2 compared with cells suspended in distilled water (Fig. 3A).
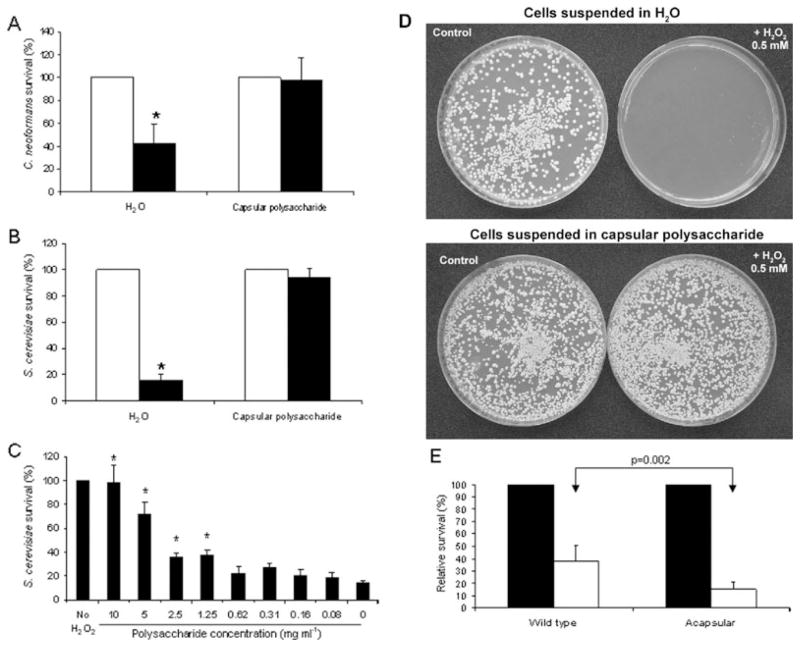
Fig. 3.
Purified capsular polysaccharide protects C. neoformans and S. cerevisiae. Purified capsular polysaccharide was obtained from cells with enlarged capsule by γ-irradiation as described in Experimental procedures. This polysaccharide was lyophilized and dissolved at 10 mg ml−1 (A and B) in distilled water. Cells from C. neoformans (A) or S. cerevisiae (B–D) were grown in Sabouraud, washed, and suspended in distilled water or capsular polysaccharide.
A and B. H2O2 0.5 mM was added to both samples (closed bars), and parallel samples to which no H2O2 was added were carried out in parallel (open bars). Viability was measured after 2 h of incubation at 37°C. Asterisk denotes statistical differences (P < 0.01).
C. Dose–response protection, using S. cerevisiae. Serial dilutions of the capsular polysaccharide were prepared (from 10 to 0.08 mg ml−1), and S. cerevisiae cells were added to each suspension. H2O2 (0.5 mM) was added to these samples. As controls, we included H2O2-treated and non-treated samples suspended in distilled water (left and right bars). Viability was measured after 2 h of incubation at 37°C. The experiment was performed in triplicates. In all the panels, the survival mean value and the standard deviation are plotted. Asterisk denotes statistical differences with the control where the cells were treated with H2O2 suspended in water (right bar).
D. Cells were treated as in (B), but plated after 24 h of incubation and pictures of the plates of the different samples were taken.
E. Cells from the wild-type strain (B3501) and the acapsular mutant (C536, cap59 disruptant) were placed in triplicates in wells from microdilution plates (106 cells per well, approximately) in the presence or absence of 0.5 mM H2O2. After 3 h incubation, the wells were washed, and XTT/menadione mixture was added to each well. Viability was calculated as Experimental procedures. Closed bars, controls without H2O2; open bars, 2 mM H2O2.
In a parallel approach, we investigated whether exogenous polysaccharide protected other fungal species, such as Saccharomyces cerevisiae, against ROS-induced killing. As shown in Fig. 3B, when H2O2 was added to cells in the presence of capsular polysaccharide, there was lower mortality than for cells suspended in water (Fig. 3B). To ascertain if there is a relationship between polysaccharide concentration and reduced killing, we performed a dose–response experiment. There was a decrease in the protection achieved below a polysaccharide concentration of 5 mg ml−1, and there was no statistical significant protection below 0.62 mg ml−1 (Fig. 3C).
Finally we investigated whether the protection observed would last during a longer incubation period. To address this issue, we incubated S. cerevisiae cells overnight in the presence of 0.5 mM H2O2 in a solution of capsular polysaccharide or in water as control. As shown in Fig. 3D, the cells remained viable after overnight exposure to H2O2 when capsular polysaccharide was present, and the survival rate was very similar to the one observed after 2 h of incubation (see Fig. 3B).
To demonstrate that the capsule played a protective role against free radicals, we compared the survival of a wild-type encapsulated strain (B3501) and an acapsular mutant (cap59) when exposed to free radicals. As acapsular mutants aggregate, we could not perform the survival assay by counting the number of colony-forming units (cfu), so we decided to use the XTT reduction assay. Using this approach, the acapsular mutant had a higher susceptibility to H2O2 exposure than the wild-type strain (Fig. 3E). We confirmed this result by adding exogenous capsular polysaccharide to the acapsular strain. When the acapsular strain was exposed to H2O2 in the presence of capsular polysaccharide (10 mg ml−1), there was a significant increase in the survival of the strain (survival of cells suspended in water in the presence of H2O2, 15 ±2%; survival of the same cells treated the same way, but suspended in capsular polysaccharide 10 mg ml−1, 75 ± 2%), which is consistent with the data presented in Fig. 3A and B).
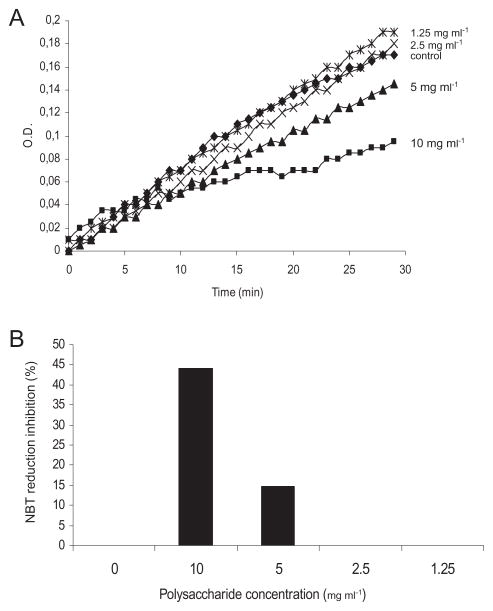
As soluble capsular polysaccharide protected C. neoformans and S. cerevisiae cells from killing by H2O2, we studied if it could behave as an antioxidant. Hence, we measured polysaccharide antioxidant ability using an assay in which electron transfer could be easily spectrophotometrically measured. The assay employed used the reduction of nitroblue tetrazolium (NBT) in the presence of β-nicotinamide adenine dinucleotide (NADH) and phenazine methosulfate (PMS) (Valentao et al., 2001; 2002; Ramallo et al., 2006). The addition of capsular polysaccharide affected the kinetics of NBT reduction in a dose-dependent manner (Fig. 4A). This inhibition was calculated to be around 50% at 10 mg ml−1 and there was no inhibition below 2.5 mg ml−1 (Fig. 4B). This result con-firmed that the capsular polysaccharide could act as an antioxidant and suggests a mechanism by which capsule enlargement protects against ROS.
Fig. 4.
Capsular polysaccharide affects NBT reduction in the NADH/PMS electron transfer system. NBT reduction induced by NADH and PMS was monitored by measuring absorbance at 540 nm in the absence or presence of different concentrations of capsular polysaccharide.
A. Optical density (O.D.) increase during 30 min.
B. Inhibition percentage of NBT reduction in the presence of different polysaccharide concentrations.
The experiment was performed in duplicates, obtaining almost identical results (P < 0.05).
Effect of H2O2 on physical properties of the capsular polysaccharide
Zeta potential and mobility determinations of native capsular polysaccharide preparations (Table 1) revealed negative values. When the polysaccharide samples were exposed to higher H2O2 concentrations and for longer periods of time, we found an increase in both zeta potential and mobility values (Table 1).
Table 1.
Effective diameter, polydispersity and diffusion coefficient of GXM-treated samples.
| Samples | Effective diameter (nm) | Polydispersity | Diffusion coefficient (cm2 s−1) × 10−9 | Zeta potential (mV) | Mobility (μs−1)/(V cm−1) |
|---|---|---|---|---|---|
| Control | 3620 ± 750 | 0.40 ± 0.016 | 1.76 | −37.99 ± 0.24 | −3.08 |
| 0.5 mM 2 h | 1500 ± 90 | 0.285 ± 0.040 | 3.27 | −37.99 ± 0.39 | −2.97 |
| 0.5 mM ON | 1930 ± 435 | 0.371 ± 0.034 | 3.36 | −32.05 ± 0.55 | −2.50 |
| 1 M 2 h | 1195 ± 300 | 0.389 ± 0.012 | 4.33 | −28.42 ± 0.77 | −2.22 |
| 1 M ON | 885 ± 53 | 0.354 ± 0.019 | 5.47 | −23.39 ± 0.76 | −1.83 |
ON, overnight.
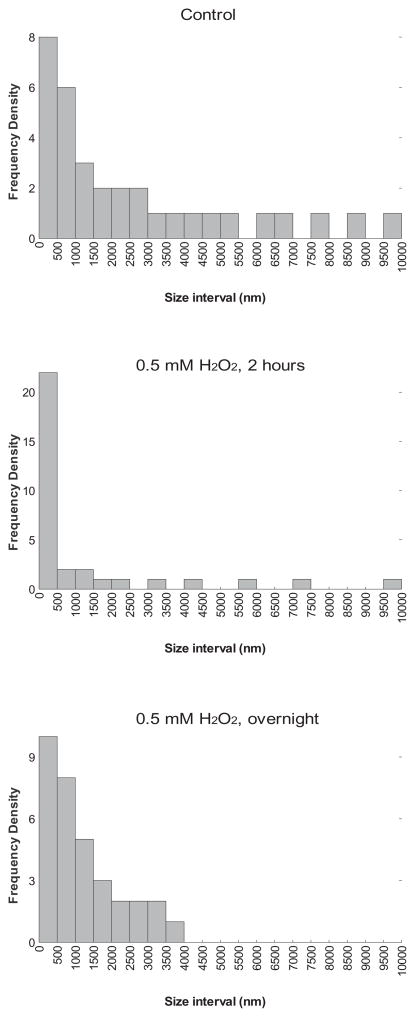
The average effective diameter and size distribution of capsular polysaccharide samples treated with H2O2 were determined. Treatment with H2O2 reduced the effective diameter in the samples in a concentration-dependent manner (Table 1). Polydispersity and diffusion coefficients were also calculated (Table 1). We also analysed the size distribution of the different polysaccharide samples treated with different H2O2 concentrations, by using dynamic light scattering (see Experimental procedures). We found that there was a variation in the size distribution of the different polysaccharide preparations after treatment with H2O2 (Fig. 5). The polysaccharide not treated with H2O2 consisted of three major size distributions (321–465 nm, 1584–2291 nm and 7822–10 000 nm). When the polysaccharide was treated with 0.5 mM H2O2 there was a decrease in the proportion of larger molecules, with the two major distributions centred at 0–2311 nm and 5623–10 000 nm. Interestingly, when the polysaccharide was incubated overnight with the same H2O2 concentration, there was an intermediate distribution pattern (Fig. 5) showing two major size classes, at 275–293 nm and 1788–2553 nm. In this condition, there were no fragments with a size bigger than 4500 nm. We observed similar results treating the polysaccharide samples with a high H2O2 concentration, such as 1 M (result not shown).
Fig. 5.
Reactive oxygen species affect the size of the polysaccharide molecules. The distribution of the molecular size of polysaccharide samples was measured after treatment with 0.5 mM H2O2 during 2 h or overnight at 37°C as described in Experimental procedures.
We also investigated whether ROS affected the binding of polysaccharide fibres to the cells. As stated above, 0.5 mM H2O2 did not affect capsule size. We tested if higher concentrations, such as 1 M, had any effect. We observed that 2 mM behaved in the same way as 0.5 mM. However, when cells with large capsule were exposed to high H2O2 concentrations (1 M) for a longer time period (overnight) there was a significant reduction in the packed cellular volume (result not shown). This phenomenon was not observed at lower concentrations (0.5 and 2 mM), a finding confirmed by India Ink staining (result not shown). This effect was not observed in acapsular mutants, indicating that the decrease in cellular volume occurred at the capsule level.
Effect of capsule size in the activity of antimicrobial peptides and antifungal drugs
Cells with small capsule manifested a significantly higher susceptibility to defensins relative to cells with larger capsules (Fig. 6A). The effect was dependent on the defensin concentration. At higher defensin concentrations, the peptide killed both types of cells equally. The same result was obtained when viability was measured using the XTT assay. This result suggests that capsule enlargement confers resistance to microbicidal peptides of the type produced by phagocytes.
Fig. 6. C. neoformans cells manifest different susceptibility to antimicrobial agents depending on their capsule size.
A. Cells with small and large capsule were exposed to different defensin concentrations, and after 30 min, viability was estimated by plating 100 μl on Sabouraud plates. The mean value and standard deviation from three different experiments are plotted. Solid line, cells with small capsule; dotted line, cells with large capsule. Asterisk denotes statistical differences between cells with small and large capsule at the defensin concentration indicated.
B. Cells with small and large capsule were exposed to different Amphotericin B concentrations during 3 h at 37°C, and viability was estimated by cfu enumeration as described in Experimental procedures. The average and the standard deviation of four different experiments are plotted. Solid line, cells with small capsule; dotted line, cells with large capsule. Asterisk denotes statistical differences between cells with small and large capsule at the Amphotericin B concentration indicated (P = 0.05).
We studied whether capsular enlargement protected against antifungal drugs. Although the minimum inhibitory concentration required to inhibit the growth of 90% of organisms (MIC90) was the same for both type of cells, we found that cells with enlarged capsule manifested reduced susceptibility to Amphotericin B compared with cells with smaller capsules at concentrations between 0.06 and 0.25 μg ml−1 (Fig. 6B). To ensure that once plated the cells were not affected by Amphotericin B, we plated cell samples at time zero, immediately after addition of the drug to the cell suspension. In these controls, no killing by Amphotericin B was observed, indicating that once plated, Amphotericin B is diluted on the plate and does not induce fungal death.
To test the role of the capsule in the susceptibility to other antifungal compounds which have a fungistatic (and not fungicidal) effect on C. neoformans, we measured the susceptibility of wild type (B3501) and isogenic acapsular mutant (cap59) to several antifungal drugs. As acapsular mutants clump in suspension, we could not use a microdilution reference method to measure the susceptibility pattern. So we decided to use E-test assay, which is performed in agar plates. Acapsular mutants were more susceptible to Amphotericin B, which is consistent with the findings obtained in Fig. 6B (Table 2). Concerning azole compounds, we did not find any significant difference between the strains, except for fluconazole. In this case, the MICs were higher in the wild-type strain than for the other azole derivatives, and we found that the acapsular strain was less susceptible to this compound. Both strains were resistant to caspofungin.
Table 2.
Susceptibility profile of wild-type and acapsular strains to antifungal compounds.
| MICs (mg l−1)
|
||
|---|---|---|
| Wild type | cap59 | |
| Amphotericin B | 0.5/0.5 | 0.125/0.125 |
| Itraconazole | 0.032/0.047 | 0.023/0.016 |
| Voriconazole | 0.064/0.094 | 0.094/0.125 |
| Posaconazole | 0.19/0.125 | 0.19/0.19 |
| Fluconazole | 4/3 | 64/> 256 |
| Caspofungin | > 32/> 32 | > 32/> 32 |
The minimal inhibitory concentration (MIC, mg l−1) for a variety of antifungal compounds was calculated for the wild-type (B3501) and acapsular strain (C536, cap59 disruptant) using E-test assay as described in Experimental procedures. The experiment was performed in duplicate in different days, and the MIC values obtained in both experiments are presented.
Capsule enlargement protects against engulfment by environmental predators
We investigated whether capsule enlargement protected against phagocytosis during its interaction with different hosts such as amoebas that efficiently phagocytose C. neoformans cells, without any requirement for opsonins. In these experiments, we incubated C. neoformans cells with Acathamoeba castellanii, and measured phagocytic index. In the initial experiments, we did not observe any phagocytosis, even using C. neoformans cells with small capsules. We considered whether the absence of phagocytosis in our control sample was due to the experimental conditions, in which the cells with small capsule were incubated in phosphate-buffered saline (PBS) as a control for the cells with large capsule, which were incubated in PBS + 10% fetal calf serum (FCS). To confirm that the incubation conditions affected the phagocytosis by amoeba, we grew cells in Sabouraud, placed them in PBS overnight, and then incubated them in Sabouraud for a short time (1 and 2 h). When the fungal cells were incubated back in Sabouraud, phagocytosis by amoeba increased, confirming that the pre-incubation conditions affected the phagocytosis percentage (Fig. 7A). When the cells were incubated overnight in PBS + serum to induce capsule enlargement, and then placed again in Sabouraud for a short time period, capsule size was not affected (result not shown). With this experimental approach, we established that the cells with small capsule were efficiently phagocytosed as described previously (Steenbergen et al., 2001). However, cells with large capsule remained resistant to phagocytosis (Fig. 7B). Hence, capsule enlargement confers protection to phagocytosis by environmental hosts.
Fig. 7. C. neoformans cells with different capsule size manifest differences in susceptibility of phagocytosis by A. castellanii.
A. C. neoformans cells were incubated overnight in PBS, and then transferred to Sabouraud or PBS for 1 or 2 h. Afterwards the cells were washed and used in phagocytosis experiments in the presence of A. castellanii as described in Experimental procedures. The mean value and standard deviation of three different experiments are shown. Statistical differences between samples are highlighted with arrows, and the P-value of the comparison is shown.
B. C. neoformans cells were incubated overnight in PBS or in PBS + 10% FCS or in Sabouraud, and then transferred to Sabouraud, PBS or PBS + 10% FCS for 2 h. Each bar is labelled first with the condition in which the cells were placed overnight and then with the condition in which they were placed for 2 h. The experiment was performed in triplicate, and the mean value and standard deviation are shown. Statistical differences between samples are highlighted with arrows, and the P-value of the comparison is shown.
Discussion
Capsule enlargement is a distinctive feature of C. neoformans that is associated with virulence for mammalian hosts. This process is highly dynamic and is strictly controlled by the cell (Zaragoza et al., 2006). Nevertheless, the mechanism by which capsular enlargement contributes in virulence is not well understood. Although capsule growth is associated with a slower yeast growth rate (Zaragoza and Casadevall, 2004), this process requires a large accumulation of polysaccharide on the capsule (Maxson et al., 2007a), implying a high-energy-consuming process. One intriguing question about capsule enlargement is why, in conditions of a slow growth rate, the yeast invests a high amount of energy in enlarging the capsule. Consequently, we hypothesized that capsule growth must have positive consequences for the yeast during the interaction with the host. It is known that capsule enlargement interferes with complement-mediated phagocytosis (Zaragoza et al., 2003b). By enlarging the capsule, C. neoformans locates complement proteins in an internal position, where they cannot interact with the phagocytic complement receptors. However, interference with phagocytosis is unlikely to be the only role for capsule enlargement for several reasons. First, phagocytosis of C. neoformans cells with enlarged capsule can occur in the presence of opsonins, and even glucuronoxylomannan (GXM)–CD18 interactions have been described, which promote phagocytosis in certain circumstances (Dong and Murphy, 1997; Netski and Kozel, 2002; Taborda and Casadevall, 2002). Second, capsule enlargement occurs after several hours (6–9 h) (Zaragoza et al., 2003a). As phagocytosis by alveolar macrophages can occur during the first 2 h of infection, it is reasonable to argue that capsule enlargement has other functions in virulence than simply interfering with engulfment by phagocytic cells.
Cryptococcus neoformans is an intracellular pathogen for mammalian phagocytic cells (Diamond and Bennett, 1973; Tucker and Casadevall, 2002) and environmental predators, such as amoebas (Steenbergen et al., 2001; 2003), which indicates that this pathogen has mechanisms that avoid killing by microbicidal factors found in the phagolysosome, which include oxygen- and nitrogen-derived free radicals, antimicrobial peptides, lytic enzymes and acidic pH. As a consequence of intra-cellular survival, C. neoformans can undergo intracellular replication and phagosome extrusion (Alvarez and Casadevall, 2006). Recently, it has been shown that cell-to-cell spread can also occur (Alvarez and Casadevall, 2007; Ma et al., 2007), reinforcing the idea that the intra-cellular environment is adequate for the survival of the yeast. We argued that capsule enlargement might play a role during this process, avoiding killing.
In agreement with our hypothesis, cells with enlarged capsules were more resistant to several stress factors that are likely to play a significant role in the phagolysosome, such as oxidative killing. In this regard, polysaccharides are known to be efficient free radical scavengers (Chan et al., 1991; Bylund et al., 2006) and protection against phagolysosomal oxidative burst would be a new function for the C. neoformans capsule in virulence. We found similar results in cells in which capsule size had been increased by passaging the cells through mice. It is reasonable to think that during the interaction with the host, in addition to capsule growth, other stress response pathways are activated that could explain in part the increased resistance of these cells. Although we cannot discard this possibility, our results are suggestive of and consistent with the notion that capsule growth is directly involved in resistance to host cell microbicidal mechanisms. We are also aware that in our in vitro experimental approach, cells with different capsule size have been incubated in different media, which in turn could affect the metabolic and stress response pathways activated in the cells. However, we could not detect any difference in the activities of enzymes directly involved in free radicals detoxification. For this reason, and to confirm the direct role of the capsule as a detoxifying structure, we performed a set of experiments using purified capsular polysaccharide. We have shown that purified capsular polysaccharide produces the same effect as capsule enlargement, and moreover, it acts as an antioxidant in our in vitro assays. In most of the experiments of this study, we used a polysaccharide concentration between 5 and 10 mg ml−1, and we observed that below these concentrations, there was a significant reduction in the protective effect. Previous findings report that the polysaccharide concentration in the capsule is approximately 6 mg ml−1 (Bryan et al., 2005), which indicates that the phenomena described in this article are potentially relevant to physiological conditions found during the interaction of the pathogen with the host. The molecular mechanism by which the capsular polysaccharide confers resistance to killing by H2O2 is unknown, but it is likely to reflect polysaccharide-mediated scavenging of oxygen-related oxidants. A similar situation has been described for some polysaccharides isolated from plants and algae, which present antioxidant activity, and no relationship between structure and antioxidant mechanism has been identified (Ruperez et al., 2002; Kardosova and Machova, 2006; Wang et al., 2007; Kodali and Sen, 2008)
The notion that the C. neoformans capsular polysaccharide acts as an antioxidant by reacting and scavenging H2O2-derived products is supported by the fact that exposure to ROS altered several physical properties of the capsular polysaccharide, such as the zeta potential. The change in zeta potential values could be explained by a neutralization of the negative charges contributed by glucuronic acids, possibly through chemical modification of the polysaccharide. Hydrogen peroxide also affected the distribution of polysaccharide size in the samples and tended to enrich the population of low-size molecules, although the highest effect was observed only when the polysaccharide was exposed for a short time to the peroxide. Longer incubations could allow structural rearrangements that promote self-association of the fibres. The fact that H2O2 changed the physical properties of the polysaccharide provides evidence for a reaction between these two entities. By increasing capsule size and the amount of polysaccharide in the capsule, the cell is presumably less susceptible to ROS through a buffering mechanism, so that free radicals would preferentially affect the capsule, leaving other cellular structures intact. This notion is consistent with the observation that encapsulated, but not acapsular cells replicate inside macrophages (Feldmesser et al., 2000a). In this sense, the capsule is not required for viability of the yeast (Chang and Kwon-Chung, 1994), which supports the idea that the effect of ROS on the polysaccharide fibres does not affect the survival of the C. neoformans cells. An interesting aspect of capsule and oxidant interactions is that free radicals can release the capsular polysaccharide, but only at high non-physiological concentrations, such as 1 M. This result suggests that the amount of polysaccharide accumulated in the cell could be sufficient to buffer physiological stress conditions. However, we noticed that some modifications found in the polysaccharide (Table 1) did not correlate with microscopic changes in capsule size. Although this might appear at first glance as a discrepancy in the results, we believe that there are several explanations for these results. First, it is possible that fibres of a smaller size generated as a consequence of ROS action still retain the ability to self-associate and produce a capsule of similar size. The structural factors that result in the binding of the polysaccharide fibres between them and to the cell wall are unknown, so it is possible that some of the changes observed in soluble samples do not directly involve capsular decrease. However, a decrease in capsule size is not, in principle, a requirement to obtain a protective effect in the presence of free radicals. In addition, polysaccharide samples used in Table 1 had been previously release from the capsule after γ-radiation treatment, which is an important experimental variation which makes the results not directly comparable. We believe that our results clearly demonstrate that free radicals can directly interact with capsular polysaccharide, which is a requirement for any antioxidant molecule.
In addition to free radicals, capsule growth decreased susceptibility to antimicrobial peptides and some antifungal drugs. Our results are in agreement with previous findings that suggested a role of the capsule in the defence against defensins (Alcouloumre et al., 1993). In the case of Amphotericin B, capsule enlargement produced an increase in the MIC50 measured for cells with small capsule. Amphotericin B exerts its action through binding to ergosterol and pore formation in the cell membrane. Many factors, including charge repulsion and/or steric hindrance produced by the high cross-linking of the polysaccharide fibres, might play a role in protection against this large antifungal drug. We cannot discard that capsule size is associated with other cellular changes that decrease susceptibility to these factors, such as a different cell wall or membrane composition. In this context, changes in membrane structure in response to nutrient deprivation have been related to increased susceptibility to antimicrobial peptides in C. albicans (Veerman et al., 2007). Other recent reports indicate that in Aspergillus terreus, resistance to Amphotericin B is associated with higher catalase activity, which suggests that part of the killing effect of the antifungal drug is mediated through the production of free radicals that are detoxified by this enzyme (Blum et al., 2008). In this sense, and if Amphotericin B induces an oxidative burst in the cells that contributes to killing, cells with larger capsule could be more protected due to the increase in the synthesis of polysaccharide molecules that occurs in these conditions. The fact that C. neoformans cells with enlarged capsule show increased resistance to Amphotericin B suggests that the susceptibility pattern observed in vitro might not correlate with the Amphotericin B therapeutical effect during infection due to capsular morphological changes. In addition, capsule size during infection depends on the organ where the yeast is located (Rivera et al., 1998), suggesting that the efficacy of antifungal treatments against C. neoformans could vary depending on the organ where the yeast is preferentially infecting. Although the capsule conferred resistance to Amphotericin B, it did not have any effect in the cases of voriconazole, posaconazole and itraconazole, and moreover, the absence of the capsule conferred resistance to fluconazole. Interestingly, fluconazole is the only hydrophilic azole compound. As the capsule is also highly hydrophilic, it may facilitate the uptake of this compound and in turn increase the susceptibility to fluconazole. This result could have clinical significance because fluconazole is the maintenance treatment for HIV+ patients affected with cryptococcal meningitis, and infections produced by acapsular strains have been described (Lacaz et al., 1993; Laurenson et al., 1998). This suggests that in these cases, a lack of response to fluconazole could be found. This finding could also explain the in vivo fluconazole resistance described in the literature (Assing et al., 2003; Sar et al., 2004; Bicanic et al., 2006). The capsule did not play a role in the susceptibility to caspofungin, a drug to which C. neoformans has intrinsic resistance (Abruzzo et al., 1995; Feldmesser et al., 2000b).
Efficient phagocytosis and killing by mammalian cells depend on the binding of opsonins, as the capsule has antiphagocytic properties (Kozel and Gotschlich, 1982). Binding of monoclonal antibodies and/or serum to the capsule produces a partial blocking (Zaragoza and Casadevall, 2006), and we argued that in this case, opsonin binding might interfere with the killing produced by the antimicrobial factors. Consequently, we selected a phagocytosis model where no opsonins were required for ingestion. Amoebas efficiently engulf C. neoformans in the absence of opsonins (Steenbergen et al., 2001), and this interaction is considered highly relevant, because it suggests an explanation for the origin of virulence in this fungal species (Steenbergen and Casadevall, 2003; Steenbergen et al., 2003). The fact that capsule growth inhibited phagocytosis in this model highlights the importance that this process has in avoiding phagocytosis and killing by different types of hosts. Moreover, our results suggest that during the interaction with amoebas, capsule enlargement might allow the yeast to evade the immune response of metazoal hosts, such as mammals.
We also made the intriguing observation that when the cells were starved in PBS, they became resistant to phagocytosis. This result suggests that phagocytosis is affected not only by capsule size, but also by the environment of the fungal cell prior to interaction with amoeba. In this regard, there are reports in the literature that the incubation medium can affect some important features of the capsule, such as size and packing (Dykstra et al., 1977; Jacobson et al., 1989; Nimrichter et al., 2007). We believe that this observation is highly relevant in the context of the environmental relationships. It is reasonable to argue that in the areas where amoebas and fungal cells can interact, the situation resembles starvation, which would induce a capsule structure resistant to phagocytosis, independently of the size of the structure. So we hypothesize that this would be a persistent mechanism to avoid fungal phagocytosis in the environment.
In summary, we demonstrate that capsule enlargement is associated with reduced susceptibility to both oxidative and antifungal killing. As capsule enlargement occurs following infection and ingestion by macrophages, the results suggest an explanation for the inability of macrophages to kill this organism. We believe that some of the findings described in this study provide insights into the role of other type of microbial capsules, which is a common feature in bacterial pathogens, and in consequence, highlight its importance during the interaction with the host.
Experimental procedures
Strains, growth media and India Ink staining
Cryptococcus neoformans H99 strain (serotype A; Perfect et al., 1980) was used in most experiments, because previous studies have demonstrated that this strain efficiently modulates its capsule size depending on environmental conditions (Zaragoza et al., 2003a). The wild-type strain B3501 (serotype D; Kwon-Chung, 1976) and the acapsular mutant C536 (Chang et al., 1995), and S. cerevisiae (ATCC 9763) were used in some experiments. The acapsular mutant was created by disruption of the CAP59 gene using B3501 as parental strain. The strains were routinely grown in liquid Sabouraud medium (Difco) at 30°C with moderate agitation. To induce capsule growth, the cells were transferred to PBS buffer with 10% fetal bovine serum (Zaragoza et al., 2003a) or in diluted Sabouraud (10%) in MOPS buffer (50 mM, pH 7.3) at 37°C, with shaking (Zaragoza and Casadevall, 2004). In these media, C. neoformans produces a significant increase in capsule size, from 2–3 μm to 10–15 μm. To visualize the size of the capsule, the cells were suspended in India Ink, and observed by regular light microscopy. A. castellanii (ATCC Strain 30324) was kindly provided by Dr Steinman (AECOM, Bronx, New York). The organisms were cultured in peptone-yeast extract-glucose broth, PYG (ATCC medium 354) in Falcon tissue culture flasks at 28°C. Amoeba cells were used when confluent on the bottom of the flask and were passaged every 7–10 days.
Oxidative stress induced by H2O2
Cryptococcus neoformans cells with small and large capsules were washed and suspended in water at a density of 2 × 103 cells ml−1. Hydrogen peroxide was added at different concentrations and the cells were incubated at 37°C for different times. Controls included tubes without hydrogen peroxide incubated in parallel. Each sample was done by triplicate. Then, 50 and 100 μl were plated in Sabouraud plates, and incubated at 30°C for 2 days. The number of cfu was counted, and viability was expressed as the percentage of colonies obtained in the treated samples compared with the controls to which no H2O2 was added. The experiment was performed at least three times in different days. In some experiments, to test the role of the capsule as antioxidant, the cells were suspended in a solution of purified capsular polysaccharide (10 mg ml−1), and viability after H2O2 exposure was compared with samples suspended in distilled water.
XTT viability assay
In some experiments, XTT assay was used instead of cfu counting. C. neoformans cells (106) were placed in wells from microdilution plates (96-well plates), and H2O2 was added to different final concentrations. In some experiments, capsular polysaccharide to a final concentration of 10 mg ml−1 was also added. The total volume of the mixture was 100 μl. In all experiments, the same amount of dead cells (inactivated by incubating 30 min at 60°C) was included as negative controls. After incubating for 3 h, the plate was washed, and 100 μl of XTT 0.5 mg ml−1 in PBS containing menadione 25 μM was added in each well. The optical density at 492 nm was measured after 5–7 h of incubation at 37°C. Then, the optical density of the wells containing dead cells was subtracted from the optical density of each well, and the relative survival was compared in each sample compared with the well to which no H2O2 was added. Each treatment was performed in three different wells.
Capsule size changes measurement
Changes in capsule size were estimated by measuring the packed volume of the cells in the absence or presence or free radicals (see method in Maxson et al., 2007a). C. neoformans cells with large capsule (around 108 ml−1) were suspended in distilled water and treated with different concentrations of H2O2 (0.5 and 2 mM and 1 M). A tube without any treatment was carried out in parallel as control. The samples were incubated for 3 h or overnight at 37°C, and washed twice with distilled water and suspended in the same volume, having each sample the same cell density. Sixty microlitres from each suspension was placed inside a haematocrit tube. After overnight packing of the cells by gravity, the volume occupied by the cells was calculated (Maxson et al., 2007a).
γ-Radiation and capsule isolation
Capsular polysaccharide was obtained as in Maxson et al. (2007b) with some modifications. Briefly, capsule enlargement was induced at a large scale (around 109−1010 cells in at least 1 l of 10% Sabouraud medium in MOPS 50 mM, pH 7.3), and cells were extensively washed and suspended in distilled water. The cells were heat-inactivated by incubating them at 55°C for 30 min, then placed in a 137Cs γ-irradiator (MARK 1–30 irradiator, Shepherd and Associates), and irradiated. A dose of 200 greys effectively released more than 90% of the capsule from the cells, and this dose was routinely used in our experiments. The γ-irradiator belonged to the National Center for Biotechnology (CSIC, Madrid, Spain), and its use was preceded by the approval from the Biosafety Commission from this institution. After irradiation, the cells were centrifuged at 3500 g, and the supernatant was transferred to a new tube. The polysaccharide was lyophilized, weighted and suspended in sterile water at 10 mg ml−1. The soluble capsular polysaccharide obtained through this protocol was heated again at 55°C for 30 min to eliminate remaining C. neoformans cells.
NBT reduction assay in the presence of NADH and PMS. Antioxidant role for capsular polysaccharide
To test the antioxidant role of the capsular polysaccharide we used the NBT reduction assay coupled to NADH and PMS (Valentao et al., 2001; 2002; Ramallo et al., 2006). In this system, NADH is oxidized by transferring electrons to PMS, which acts like an electron transfer intermediate, able to reduce NBT molecules. NBT reduction can be easily monitored by absorbance measurement at 540 nm. Briefly, the reaction was performed in 96-well plates using 250 μl per assay. Each sample contained phosphate buffer (40 mM, pH 7.6), NADH (166 μM) and NBT (43 μM). The mix was incubated for 2 min at room temperature and NBT reduction was started by the addition of 2.7 μM PMS. One parallel well to which no PMS was added was left as a control. All the reagents were freshly prepared at a 10× concentrated stock, except the buffer, which was prepared at 5×. The plate was immediately placed in an iEMS Reader MF (Lab-Systems, Finland) and incubated at 37°C. Optical density was monitored every 30 s during 30 min. Each condition was performed in two independent replicate wells. In some assays, capsular polysaccharide at 10, 5, 2.5 and 1.25 mg ml−1 was added to the wells. The antioxidant efficacy of the polysaccharide was estimated by calculating the relative decrease in the NBT reduction compared with the controls to which no polysaccharide was added.
Zeta potential and dynamic light scattering analysis of H2O2-treated polysaccharide samples
Capsular polysaccharide was obtained from C. neoformans after γ-irradiation treatment as described above. Parallel samples containing 4 mg in 1 ml of distilled water were treated with H2O2 (0.5 mM or 1 M) for 2 h or overnight at 37°C. After the treatment, the samples were dialysed in water overnight and then lyophilized. The samples were suspended at 1 mg ml−1 in ultrapurified water. Effective diameter and polydispersity of all the capsular polysaccharide samples were measured by Quasi elastic light scattering in a 90Plus/BI-MAS Multi Angle Particle Sizing analyser (Brookhaven Instruments, Holtsville, NY) following the manufacturer’s recommendations. We used non-H2O2-treated polysaccharide as control. Size distribution of the particles was calculated based on Non-Negatively constrained Least Squares (NNLS) algorithm. The results show average of 20 runs (Frases et al., 2008). Zeta potential and mobility were measured using a Zeta potential analyser (Zeta-Plus, Brookhaven Instruments, Holtsville, NY) following the manufacturer’s recommendations (Nosanchuk and Casadevall, 1997; Maxson et al., 2007b). The results show the average of 20 runs. Zeta potential is defined as the potential gradient (mV) that develops across the interface between a boundary liquid in contact with a solid and the mobile diffuse layer in the body of the liquid. The mobility is the velocity of the particles moving to an electrode of the opposite charge when an electric field is applied.
Killing by antimicrobial peptides
Defensin PG-1 (Lehrer and Ganz, 1996) was kindly provided by Dr Nosanchuk. This short peptide has anticryptococcal activity (Doering et al., 1999; Martinez and Casadevall, 2006). Cells with small and large capsule were washed with PBS and suspended in the same buffer at 103 cells ml−1 with different concentrations of Defensin PG-1 and incubated at 37°C for 30 min. Controls included parallel samples without defensins. After incubation with and without defensin, the number of cfu was determined by plating 100 μl on Sabouraud’s agar. The experiment was performed three times on three different days.
Mouse infection and isolation of fungal cells from infected mice
To obtain cells with large capsule induced under physiological conditions, we infected CD1 mice (male, around 25 g weight, Charles River Laboratories) with 5 × 107C. neoformans cells. Briefly, C. neoformans (H99 strain) was grown in liquid Sabouraud medium (20 ml), washed with PBS and suspended at 109 cells ml−1. Fifty microlitres from this suspension was injected intratracheally in the mice. After 18–24 h, the mice were sacrificed by cervical dislocation, and the lungs were isolated and homogenized in 10 ml of PBS containing collagenase A (1 mg ml−1). The homogenate was incubated at 37°C for 90 min with occasional shaking (vortex), and after this time, the cells were extensively washed (at least five times) with 30 ml of distilled water. The cells were finally suspended in 2–5 ml of water, and counted with a haemocytometer. This protocol gave a clean C. neoformans preparation, as ascertained by microscopic examination of the suspension. The cells density was adjusted to 103 cells ml−1, and H2O2 was added as described above. As control, cells incubated in Sabouraud were exposed to the same H2O2 concentration in parallel.
Cell-free protein extract preparation and catalase and gluthatione peroxidase activity assay
Catalase activity was measured according to Aebi (1984) with some modifications. Briefly, cells with small and large capsule were obtained from 25 ml of the corresponding medium (Sabouraud or diluted Sabouraud in MOPS buffer). The cells were collected by centrifugation, and kept at −20°C until the activity was measured. To prepare protein cell-free extracts, the cells were thawed in 0.5 ml of imidazole buffer (20 mM, pH 7) at 4°C, and the cells were broken by vigorous vortexing after addition of 1 g of glass beads (0.5 mm diameter). The homogenates were centrifuged and supernatants were transferred to clean micro-centrifuge tubes. The catalase reaction mixture (500 μl final volume) contained phosphate buffer (50 mM KH2PO4, pH 7) and 20 mM H2O2. After stabilization of the absorbance at 260 nm, the reaction involved the measurement of catalase activity by the addition of 25 or 50 μl of the protein cell-free extract to the reaction mixture. The enzymatic activity was expressed in units, defined as the amount of enzyme necessary to hydrolyse 1 μmol of H2O2 in 1 min. Protein concentration was measured with the biocinchoninic acid (BCA) protein assay kit (Pierce), using bovine serum albumin (BSA) as standard and enzymatic activity was normalized by the protein concentration in each sample. Gluthatione peroxidase was measured as described in Veal et al. (2002).
Amphotericin B-induced killing assay
Cells with small and large capsules (obtained as described above) were washed and suspended in distilled water at a cell density of 2 × 103 ml−1. One hundred microlitres from this suspension were added to 96-well plates containing 100 μl of 2 × RPMI medium buffered with MOPS at pH 7 containing different concentrations of Amphotericin B, ranging from 16 to 0.032 μg ml−1. The last two wells in each row did not contain antifungal drug, and they were used as control. After addition of the cells, the plate was incubated at 30°C for 3 h with shaking 5 min every hour at 1320 r.p.m. Then 100 μl from each well was directly plated in Sabouraud plates and incubated at 30°C for 2 days. Viability was calculated as described above. The experiments were performed in triplicate and they were performed several times in different days.
Antifungal susceptibility testing using E-test assay
E-test strips (amphotericine B, voriconazole, fluconazole, itraconazole, posaconazole and caspofungin) were supplied by AB Biodisk (Stockholm, Sweden). The yeast inoculum was adjusted with a spectrophotometer by adding sterile 0.85% NaCl to match the transmittance that was produced by a 0.5 McFarland standard at 530 nm. A volume of 240 μl was spread on Sabouraud plates and these plates were let to dry before the strips were applied. The MICs were read after 48 h of incubation at 30°C. The MIC was read as the lowest concentration at which the border of the elliptical inhibition zone intersected the scale on the strip. Microcolonies inside the inhibition zone were ignored.
Phagocytosis of C. neoformans by amoeba
Confluent A. castellanii cells, grown as described above, were removed from culture flasks and counted. The cells were suspended in PBS, and after plating 106 cells per well in 96-well plates, they were allowed to adhere at 28°C for 1 h. During this time, C. neoformans cells with different capsule size were also washed, counted and suspended in PBS. Once amoebas adhered to the plate, C. neoformans cells were added and co-incubated with A. castellanii cells at 28°C (incubation time dependent on experiment). Plates were then removed from the incubator, centrifuged to sediment A. castellanii to the bottom of the plate and the supernatant was removed by aspiration. One hundred microlitres of ice-cold methanol were added to each well, and plates were incubated at 4°C for 30 min. Methanol was then removed and PBS was added twice to wash the wells. For staining of A. castellanii cells, a 1:20 dilution of Giemsa was added for 30 min. After rinsing twice with PBS, the plates were viewed at 40× and counted to determine the phagocytic index. The phagocytic index is defined as the number of A. castellanii with internalized yeast per total amoeba cells counted as a percentage. For the determination of phagocytic index approximately 100 cells were counted in 6–10 wells in each condition.
Statistics
Differences in fungal survival were analysed using t-test, after assaying normality using the Kolgomorov–Smirnoff test using the Unistast software for Excel (Unistat, London, UK).
Acknowledgments
We thank Dr J.D. Nosanchuk for the use of defensins and Dr Steinman for the kind gift of A. castellanii strains. We thank Dr J.C. Arguelles and Pilar González (Universidad de Murcia, Spain) for providing protocols to measure catalase activity, and Drs Carlos and Juana Maria Gancedo (CSIC, Spain) for the permission to use their technical resources and for their helpful discussions. We are indebted to Dr F. Usera and Rosa Hidalgo for their collaboration, help and technical support in the use of the γ-irradiator from the animal facility from the National Center for Biotechnology (CSIC, Spain). We warmly thank Josefa Casas for her technical support, and all the members from the Mycology Service from the National Center for Microbiology (Instituto de Salud Carlos III) for their helpful discussions. M.V.C. is funded by a research contract from the Agencia Española de Cooperación Internacional (AECI). O.Z. is a ‘Ramón y Cajal’ fellow from the Ministerio Español de Educación y Ciencia (MEC) and is funded by Grants MPY1025/06 from the MEC and 1181/06 from el Instituto de Salud Carlos III.
References
- Abruzzo GK, Flattery AM, Gill CJ, Kong L, Smith JG, Krupa D, et al. Evaluation of water-soluble pneumocandin analogs L-733560, L-705589, and L-731373 with mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1995;39:1077–1081. doi: 10.1128/aac.39.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Aksenov SI, Babyeva IP, Golubev VI. On the mechanism of adaptation of micro-organisms to conditions of extreme low humidity. Life Sci Space Res. 1973;11:55–61. [PubMed] [Google Scholar]
- Alcouloumre MS, Ghannoum MA, Ibrahim AS, Selsted ME, Edwards JE., Jr Fungicidal properties of defensin NP-1 and activity against Cryptococcus neoformans in vitro. Antimicrob Agents Chemother. 1993;37:2628–2632. doi: 10.1128/aac.37.12.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16:2161–2165. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Casadevall A. Cell-to-cell spread and massive vacuole formation after Cryptococcus neoformans infection of murine macrophages. BMC Immunol. 2007;8:16. doi: 10.1186/1471-2172-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y, Rahme LG, Heitman J, Ausubel FM, Calderwood SB, Mylonakis E. Challenge of Drosophila melanogaster with Cryptococcus neoformans and role of the innate immune response. Eukaryot Cell. 2004;3:413–419. doi: 10.1128/EC.3.2.413-419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assing K, Birgens H, Arendrup M. Cryptococcus neoformans var neoformans resistant to fluconazole in an HIV-negative patient with chronic lymphocytic leukemia. Clin Microbiol Infect. 2003;9:441–444. doi: 10.1046/j.1469-0691.2003.00571.x. [DOI] [PubMed] [Google Scholar]
- Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43:1069–1073. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- Blum G, Perkhofer S, Haas H, Schrettl M, Wurzner R, Dierich MP, Lass-Florl C. Potential basis for amphotericin B resistance in Aspergillus terreus. Antimicrob Agents Chemother. 2008;52:1553–1555. doi: 10.1128/AAC.01280-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Campbell LT, Lodge JK. Cryptococcus neoformans, a fungus under stress. Curr Opin Microbiol. 2007;10:320–325. doi: 10.1016/j.mib.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan RA, Zaragoza O, Zhang T, Ortiz G, Casadevall A, Dadachova E. Radiological studies reveal radial differences in the architecture of the polysaccharide capsule of Cryptococcus neoformans. Eukaryot Cell. 2005;4:465–475. doi: 10.1128/EC.4.2.465-475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund J, Burgess LA, Cescutti P, Ernst RK, Speert DP. Exopolysaccharides from Burkholderia cenocepacia inhibit neutrophil chemotaxis and scavenge reactive oxygen species. J Biol Chem. 2006;281:2526–2532. doi: 10.1074/jbc.M510692200. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Perfect JR. Cryptococcus neoformans. Washington, DC: American Society for Microbiology Press; 1998. [Google Scholar]
- Chan J, Fan XD, Hunter SW, Brennan PJ, Bloom BR. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Kwon-Chung KJ. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Wickes BL, Kwon-Chung KJ. Further analysis of the CAP59 locus of Cryptococcus neoformans: structure defined by forced expression and description of a new ribosomal protein-encoding gene. Gene. 1995;167:179–183. doi: 10.1016/0378-1119(95)00640-0. [DOI] [PubMed] [Google Scholar]
- D’Souza CA, Alspaugh JA, Yue C, Harashima T, Cox GM, Perfect JR, Heitman J. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21:3179–3191. doi: 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond RD, Bennett JE. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973;7:231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TL, Nosanchuk JD, Roberts WK, Casadevall A. Melanin as a potential cryptococcal defence against microbicidal proteins. Med Mycol. 1999;37:175–181. [PubMed] [Google Scholar]
- Dong ZM, Murphy JW. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect Immun. 1997;65:557–563. doi: 10.1128/iai.65.2.557-563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra MA, Friedman L, Murphy JW. Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect Immun. 1977;16:129–135. doi: 10.1128/iai.16.1.129-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000a;68:4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmesser M, Kress Y, Mednick A, Casadevall A. The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J Infect Dis. 2000b;182:1791–1795. doi: 10.1086/317614. [DOI] [PubMed] [Google Scholar]
- Feldmesser M, Kress Y, Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology. 2001;147:2355–2365. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- Frases S, Nimrichter L, Viana NB, Nakouzi A, Casadevall A. Cryptococcus neoformans capsular polysaccharide and exopolysaccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot Cell. 2008;7:319–327. doi: 10.1128/EC.00378-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DL, Perfect JR, Durack DT. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985;76:508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson ES, Tingler MJ, Quynn PL. Effect of hypertonic solutes upon the polysaccharide capsule in Cryptococcus neoformans. Mycoses. 1989;32:14–23. doi: 10.1111/j.1439-0507.1989.tb02163.x. [DOI] [PubMed] [Google Scholar]
- Kardosova A, Machova E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia. 2006;77:367–373. doi: 10.1016/j.fitote.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75:4792–4798. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali VP, Sen R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a pro-biotic bacterium. Biotechnol J. 2008;3:245–251. doi: 10.1002/biot.200700208. [DOI] [PubMed] [Google Scholar]
- Kozel TR, Gotschlich EC. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- Kwon-Chung KJ. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia. 1976;68:821–833. [PubMed] [Google Scholar]
- Lacaz CS, Heins-Vaccari EM, Melo NT, Moreno-Carvalho OA, Sampaio ML, Nogueira LS, et al. Neurocryptococcosis caused by nonencapsulated Cryptococcus neoformans. Arq Neuropsiquiatr. 1993;51:395–398. doi: 10.1590/s0004-282x1993000300019. [DOI] [PubMed] [Google Scholar]
- Laurenson IF, Ross JD, Milne LJ. Microscopy and latex antigen negative cryptococcal meningitis. J Infect. 1998;36:329–331. doi: 10.1016/s0163-4453(98)94567-4. [DOI] [PubMed] [Google Scholar]
- Lehrer RI, Ganz T. Endogenous vertebrate antibiotics. Defensins, protegrins, and other cysteine-rich antimicrobial peptides. Ann N Y Acad Sci. 1996;797:228–239. doi: 10.1111/j.1749-6632.1996.tb52963.x. [DOI] [PubMed] [Google Scholar]
- Ma H, Croudace JE, Lammas DA, May RC. Direct cell-to-cell spread of a pathogenic yeast. BMC Immunol. 2007;8:15. doi: 10.1186/1471-2172-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland EE, Bernhardt P, Casadevall A. Estimating the relative contributions of virulence factors for pathogenic microbes. Infect Immun. 2006;74:1500–1504. doi: 10.1128/IAI.74.3.1500-1504.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Casadevall A. Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect Immun. 2006;74:6118–6123. doi: 10.1128/IAI.00995-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson ME, Cook E, Casadevall A, Zaragoza O. The volume and hydration of the Cryptococcus neoformans polysaccharide capsule. Fungal Genet Biol. 2007a;44:180–186. doi: 10.1016/j.fgb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Maxson ME, Dadachova E, Casadevall A, Zaragoza O. Radial mass density, charge, and epitope distribution in the Cryptococcus neoformans capsule. Eukaryot Cell. 2007b;6:95–109. doi: 10.1128/EC.00306-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc Natl Acad Sci USA. 2002;99:15675–15680. doi: 10.1073/pnas.232568599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netski D, Kozel TR. Fc-dependent and Fc-independent opsonization of Cryptococcus neoformans by anticapsular monoclonal antibodies: importance of epitope specificity. Infect Immun. 2002;70:2812–2819. doi: 10.1128/IAI.70.6.2812-2819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimrichter L, Frases S, Cinelli LP, Viana NB, Nakouzi A, Travassos LR, et al. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot Cell. 2007;6:1400–1410. doi: 10.1128/EC.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosanchuk JD, Casadevall A. Cellular charge of Cryptococcus neoformans: contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect Immun. 1997;65:1836–1841. doi: 10.1128/iai.65.5.1836-1841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect JR, Lang SDR, Durack DT. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol. 1980;101:177–194. [PMC free article] [PubMed] [Google Scholar]
- Ramallo IA, Zacchino SA, Furlan RL. A rapid TLC autographic method for the detection of xanthine oxidase inhibitors and superoxide scavengers. Phytochem Anal. 2006;17:15–19. doi: 10.1002/pca.874. [DOI] [PubMed] [Google Scholar]
- Rivera J, Feldmesser M, Cammer M, Casadevall A. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect Immun. 1998;66:5027–5030. doi: 10.1128/iai.66.10.5027-5030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruperez P, Ahrazem O, Leal JA. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J Agric Food Chem. 2002;50:840–845. doi: 10.1021/jf010908o. [DOI] [PubMed] [Google Scholar]
- Sar B, Monchy D, Vann M, Keo C, Sarthou JL, Buisson Y. Increasing in vitro resistance to fluconazole in Cryptococcus neoformans Cambodian isolates: April 2000 to March 2002. J Antimicrob Chemother. 2004;54:563–565. doi: 10.1093/jac/dkh361. [DOI] [PubMed] [Google Scholar]
- Shao X, Mednick A, Alvarez M, van Rooijen N, Casadevall A, Goldman DL. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J Immunol. 2005;175:3244–3251. doi: 10.4049/jimmunol.175.5.3244. [DOI] [PubMed] [Google Scholar]
- Steenbergen JN, Casadevall A. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microbes Infect. 2003;5:667–675. doi: 10.1016/s1286-4579(03)00092-3. [DOI] [PubMed] [Google Scholar]
- Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci USA. 2001;98:15245–15250. doi: 10.1073/pnas.261418798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen JN, Nosanchuk JD, Malliaris SD, Casadevall A. Cryptococcus neoformans virulence is enhanced after growth in the genetically malleable host Dictyostelium discoideum. Infect Immun. 2003;71:4862–4872. doi: 10.1128/IAI.71.9.4862-4872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborda CP, Casadevall A. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity. 2002;16:791–802. doi: 10.1016/s1074-7613(02)00328-x. [DOI] [PubMed] [Google Scholar]
- Tucker SC, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci USA. 2002;99:3165–3170. doi: 10.1073/pnas.052702799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentao P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML. Antioxidant activity of Centaurium erythraea infusion evidenced by its super-oxide radical scavenging and xanthine oxidase inhibitory activity. J Agric Food Chem. 2001;49:3476–3479. doi: 10.1021/jf001145s. [DOI] [PubMed] [Google Scholar]
- Valentao P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, de Lourdes Basto M. Studies on the antioxidant activity of Lippia citriodora infusion: scavenging effect on superoxide radical, hydroxyl radical and hypochlorous acid. Biol Pharm Bull. 2002;25:1324–1327. doi: 10.1248/bpb.25.1324. [DOI] [PubMed] [Google Scholar]
- Vartivarian SE, Anaissie EJ, Cowart RE, Sprigg HA, Tingler MJ, Jacobson ES. Regulation of cryptococcal capsular polysaccharide by iron. J Infect Dis. 1993;167:186–190. doi: 10.1093/infdis/167.1.186. [DOI] [PubMed] [Google Scholar]
- Veal EA, Toone WM, Jones N, Morgan BA. Distinct roles for glutathione S-transferases in the oxidative stress response in Schizosaccharomyces pombe. J Biol Chem. 2002;277:35526–35531. doi: 10.1074/jbc.M111548200. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol. 2000;38:407–417. doi: 10.1080/mmy.38.6.407.417. [DOI] [PubMed] [Google Scholar]
- Veerman EC, Valentijn-Benz M, Nazmi K, Ruissen AL, Walgreen-Weterings E, van Marle J, et al. Energy depletion protects Candida albicans against antimicrobial peptides by rigidifying its cell membrane. J Biol Chem. 2007;282:18831–18841. doi: 10.1074/jbc.M610555200. [DOI] [PubMed] [Google Scholar]
- Wang P, Jiang X, Jiang Y, Hu X, Mou H, Li M, Guan H. In vitro antioxidative activities of three marine oligosaccharides. Nat Prod Res. 2007;21:646–654. doi: 10.1080/14786410701371215. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, Casadevall A. Experimental modulation of capsule size in Cryptococcus neoformans. Biol Proced Online. 2004;6:10–15. doi: 10.1251/bpo68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Casadevall A. Monoclonal antibodies can affect complement deposition on the capsule of the pathogenic fungus Cryptococcus neoformans by both classical pathway activation and steric hindrance. Cell Microbiol. 2006;8:1862–1876. doi: 10.1111/j.1462-5822.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, Fries BC, Casadevall A. Induction of capsule growth in Cryptococcus neoformans by mammalian serum and CO(2) Infect Immun. 2003a;71:6155–6164. doi: 10.1128/IAI.71.11.6155-6164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Taborda CP, Casadevall A. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur J Immunol. 2003b;33:1957–1967. doi: 10.1002/eji.200323848. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, Telzak A, Bryan RA, Dadachova E, Casadevall A. The polysaccharide capsule of the pathogenic fungus Cryptococcus neoformans enlarges by distal growth and is rearranged during budding. Mol Microbiol. 2006;59:67–83. doi: 10.1111/j.1365-2958.2005.04928.x. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, Alvarez M, Telzak A, Rivera J, Casadevall A. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect Immun. 2007;75:2729–2739. doi: 10.1128/IAI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]