Abstract
Background
Patient-level risk factors for delayed graft function (DGF) have been well-described. However, the OPTN definition of DGF is based on dialysis in the first week, which is subject to center-level practice patterns. It remains unclear if there are center-level differences in DGF, and if measurable center characteristics can explain these differences.
Methods
Using 2003-2012 SRTR data, we developed a hierarchical (multilevel) model to determine the association between center characteristics and DGF incidence after adjusting for known patient risk factors, and to quantify residual variability across centers after adjustment for these factors.
Results
Of 82,143 deceased donor kidney transplant recipients, 27.0% developed DGF, with a range across centers of 3.2-63.3%. A center’s proportion of preemptive transplants (OR 0.83, per 5% increment; 95%CI:0.74-0.93;P=0.001) and kidneys with >30 hours of cold ischemia time (OR 0.95, per 5% increment; 95%CI:0.92-0.98;P=0.001) were associated with less DGF. A center’s proportion of donation after cardiac death donors (OR 1.12, per 5% increment; 95%CI:1.03-1.17; P<0.001) and imported kidneys (OR 1.06, per 5% increment; 95%CI:1.03-1.10; P<0.001) were associated with more DGF. After patient- and center-level adjustment, only 41.8% of centers had DGF incidences consistent with the national median and 28.2% had incidences above the national median.
Conclusions
Significant heterogeneity in DGF incidences across centers, even after adjusting for patient and center-level characteristics, calls into question the generalizability and validity of the current DGF definition. Enhanced understanding of center-level variability and improving the definition of DGF accordingly may improve DGF’s utility in clinical care and as a surrogate endpoint in clinical trials.
Keywords: delayed graft function, kidney transplantation, Scientific Registry of Transplant Recipients
INTRODUCTION
Delayed graft function (DGF), as defined by the need for dialysis in the first seven days post-transplant, is collected on every kidney transplant recipient in the United States through the Organ Procurement and Transplantation Network (OPTN). Many studies have explored the role of DGF in directing clinical care and predicting post-transplant outcomes [1-6]. In fact, the U.S. Food & Drug Administration (FDA) has explored DGF as a potential surrogate endpoint in trials to test agents in a more rapid, cost-effective manner than using long-term graft outcomes as an endpoint [7]; FDA approval of DGF as a surrogate endpoint would certainly have profound effects on drug development in transplantation.
However, there is substantial heterogeneity in the reported associations between DGF and transplant outcomes [8-17]. For example, some have reported that DGF is an independent predictor of graft loss [18-20], while others have suggested that its effects are neutral except when associated with acute rejection [12, 21, 22]. Butala and colleagues reported a 5-fold increase in the relative risk of 1-year graft loss in DGF patients, and Shoske and colleagues reported a reduction in 1-year graft survival from 91% to 75% in DGF patients who did not have a rejection episode during their index transplant hospitalization [8, 23]. In a study with longer follow-up for rejection, Troppmann and colleagues reported 1- and 5-year actuarial graft survival of 99% and 89% for transplant recipients with neither DGF nor rejection, compared to 100% and 88% for DGF patients without rejection [22]. However, those patients that developed rejection and DGF had graft survival of 84% and 63%. Others have found that DGF is associated with poorer graft function, but not lower graft survival [24]. This heterogeneity renders the use of DGF as a surrogate endpoint challenging, yet the sources of this heterogeneity remain unclear.
Since DGF involves a subjective decision to treat a patient with dialysis in the first week following a transplant, one explanation for the heterogeneity of DGF’s effects between single-center reports could be heterogeneity in center-level post-transplant dialysis practice patterns. Those centers that have a low threshold for dialysis, such as for minor perturbations of fluid status or minor elevations of potassium, will necessarily have a higher rate of DGF, independent of patient factors. The goal of this study was to explore and quantify the center-level heterogeneity of DGF following kidney transplantation, to determine whether or not center-level factors that can be ascertained from OPTN data are associated with DGF beyond patient factors, and to examine the residual variability in DGF incidences across centers after accounting for patient and center level factors.
RESULTS
DGF Incidence
Of 82,143 patients undergoing deceased donor kidney transplants (DDKT), 22,185 (27.0%) developed DGF. The incidence of DGF varied widely across 177 centers, from 3.2%-63.3% (median 27.3%, IQR:18.7-33.8%).
Patient-Level Factors
Males were more likely to experience DGF (29.5% vs. 23.0%; P<0.001), as were African American patients (32.1% vs. 23.6% in Caucasians vs. 26.3% in Hispanic/Latinos; P<0.001) (Table 1). Recipients of grafts from donors with elevated serum creatinine (>1.5 mg/dL) (38.1% vs. 24.8%; P<0.001), diabetes (32.7% vs. 26.6%; P<0.001), and hypertension (34.0% vs. 24.3%; P<0.001) were more likely to experience DGF, as were recipients of imported grafts (30.5% vs. 25.8%; P<0.001) and recipients of grafts from donation after cardiac death (DCD) donors (43.0% vs. 24.5%; P<0.001) and expanded criteria donors (ECD) (33.2% vs. 25.6%; P<0.001).
Table 1.
Patient-level characteristics, by the development of DGF.
| DGF (N=22,185) | No DGF (N=59,958) | P-value | |
|---|---|---|---|
| Donor Characteristics
| |||
| Mean Age (SD) | 41.9 (15.8) | 37.7 (16.8) | <0.001 |
| African-American | 3,007 (26.3%) | 8,440 (73.7%) | 0.055 |
| Hypertension | 7,694 (34.0%) | 14,961 (66.0%) | <0.001 |
| Diabetes Mellitus | 1,859 (32.7%) | 3,826 (67.3%) | <0.001 |
| Creatinine > 1.5mg/dL | 5,111 (38.1%) | 8,307 (61.9%) | <0.001 |
| Donation After Cardiac Death | 4,202 (43.8%) | 5,389 (56.2%) | <0.001 |
| Expanded Criteria Donor | 5,002 (33.2%) | 10,042 (66.7%) | <0.001 |
| Imported Kidney | 6,577 (30.5%) | 15,002 (69.5%) | <0.001 |
| Median CIT (IQR) | 19 (13.3-24.6) | 16.4 (11.2-22.0) | <0.001 |
|
| |||
| Recipient Characteristics
| |||
| Mean Age (SD) | 52.7 (12.7) | 51.7 (13.2) | <0.001 |
| Male | 14,854 (29.5%) | 35,444 (70.5%) | <0.001 |
| African-American | 8,888 (32.1%) | 18,775 (67.9%) | <0.001 |
| Median Peak PRA (IQR) | 4 (0-36) | 3 (0-37) | 0.7 |
| Zero-HLA-Mismatch | 1,738 (20.8%) | 6,597 (79.1%) | <0.001 |
| Prior Transplant | 3,113 (27.3%) | 8,298 (72.7%) | 0.5 |
DGF=delayed graft function, CIT=cold ischemia time, IQR=interquartile range, SD=standard deviation, PRA=panel reactive antibody
Transplant Center Factors
Center-level kidney transplant volume during the 10-year study period ranged from 151-1,797 (median 421, IQR: 275-633) (Table 2). Of total transplant volume at a given center, the median proportion from deceased donors was 64.6% (IQR: 57.0-74.1%). Of total DDKT volume at a given center, the median proportion from DCD donors was 11.5% (IQR: 6.1%-16.0%), hypertensive donors was 26.7% (IQR: 22.2-31.8%), diabetic donors was 6.4% (IQR: 4.6-8.3%), African-American donors was 11.9% (IQR: 6.8-17.6%), African-American recipients was 29.9% (IQR: 14.0-43.5%), donors over the age of 65 was 2.4% (IQR: 1.2-4.6%), and recipients over the age of 65 was 15.3% (IQR: 12.1-19.1%). Kidneys with over 30 hours of cold ischemia time (CIT) represented over 50% of transplants in a handful of centers, but a much smaller proportion in most (median 6.5%, IQR:3.2-17.6%).
Table 2.
Characteristics of the 177 studied transplant centers. The percentages represent the proportion of transplants at a center that fit the corresponding characteristic.
| Minimum | 25th Percentile | Median | 75th Percentile | Maximum | |
|---|---|---|---|---|---|
| DGF Incidence | 3.2% | 18.7% | 27.3% | 33.8% | 63.3% |
| Center Volume (over 10 years) | 151 | 275 | 421 | 633 | 1797 |
| Proportion of Transplants that Are DDKT | 18.1% | 57.0% | 64.6% | 74.1% | 97.5% |
| Proportion of Donation After Cardiac Death KT | 0.0% | 6.1% | 11.5% | 16.0% | 40.4% |
| Proportion of Expanded Criteria Donors | 1.7% | 11.6% | 17.0% | 22.9% | 47.8% |
| Proportion of Imported Kidneys | 8.7% | 16.4% | 21.0% | 29.5% | 77.5% |
| Proportion of KT with CIT > 30 Hours | 0.0% | 3.2% | 6.5% | 17.6% | 91.3% |
| Proportion of KT with Donor Creatinine >1.5 mg/dL | 2.3% | 10.6% | 13.6% | 18.7% | 41.2% |
| Proportion of Preemptive KT | 0.4% | 4.8% | 6.7% | 10.6% | 24.2% |
| Proportion of Diabetic Donors | 1.7% | 4.6% | 6.4% | 8.3% | 14.5% |
| Proportion of Hypertensive Donors | 11.4% | 22.2% | 26.7% | 31.8% | 52.6% |
| Proportion of KT with Donor Age > 65 Years | 0.0% | 1.2% | 2.4% | 4.6% | 14.6% |
| Proportion of KT with Recipient Age > 65 Years | 4.2% | 12.1% | 15.3% | 19.1% | 34.4% |
| Proportion of KT with African-American Donors | 1.1% | 6.8% | 11.9% | 17.6% | 36.4% |
| Proportion of KT with African-American Recipients | 0.4% | 14.0% | 29.9% | 43.5% | 91.6% |
DGF = delayed graft function, DDKT = deceased donor kidney transplant, KT = kidney transplants, CIT = cold ischemia time
Patient-Level Model
After adjusting for patient-level factors, only 38.4% of centers had predicted incidences of DGF that would have put them in a category consistent with the national median (Figure 1A). 28.8% of centers had predicted incidences of DGF above the national median. The remaining 32.7% of centers had predicted incidences of DGF below the national median. The adjusted relative odds of DGF across centers ranged from 0.11 to 3.02 (IQR: 0.64-1.37).
Figure 1.
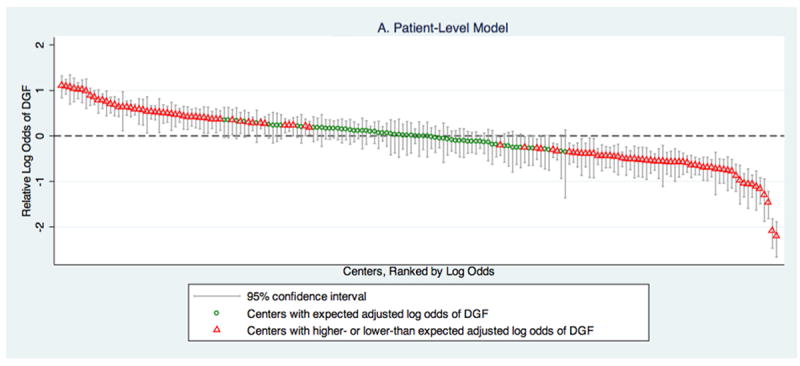
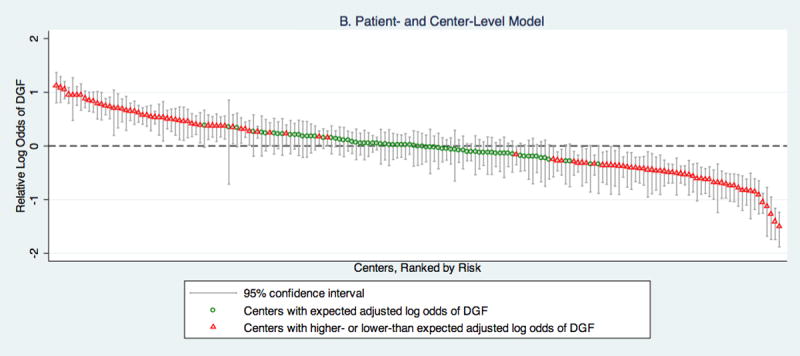
Relative likelihood of DGF, based on (A) a model that only includes patient-level characteristics and (B) a model that combines patient and center-level characteristics, by center.
A displays the log odds of DGF at a center relative to the national median based on adjustment for patient-level characteristics. Based on this model, 38.4% (68/177) of centers have 95% confidence intervals that encompass the national median log odds of DGF. The relative log odds ranged from -2.20-1.11 (IQR:-0.44-0.31), which corresponds to a relative odds of DGF range across centers of 0.11-3.02 (IQR: 0.64-1.37).
B displays the log odds of DGF at a center relative to the national median based on adjustment for patient and center-level characteristics. Based on this model, 41.8% (74/177) of centers have 95% confidence intervals that encompass the national median log odds of DGF. The relative log odds ranged from -1.50-1.12 (IQR: -0.34-0.34), which corresponds to a relative odds of DGF range across centers of 0.22-3.08 (IQR: 0.71-1.41).
DGF=delayed graft function, IQR=interquartile range
Multilevel (Combined Patient and Center-Level) Logistic Model
After adjusting for patient-level factors, there were a number of factors at the center-level that were statistically significantly associated with DGF (Table 3). For every 5% increase in a center’s use of preemptive transplants, there was a 17.0% decrease in the odds of DGF (OR 0.83; 95% CI: 0.74-0.93; P=0.001). Holding all other factors constant, the proportion of preemptive transplants would be associated with a 19.7% absolute decrease in the odds of DGF between centers at the 25th percentile of preemptive transplant use (4.8%) and those at the 75th percentile (10.6%). For every 5% increase in a center’s use of DCD donors, there was an 11.9% increase in the odds of DGF (OR 1.12; 95% CI: 1.03-1.17; P<0.001). Holding all other factors constant, the proportion of DCD donors would be associated with a 23.6% absolute increase in the odds of DGF between centers at the 25th percentile of DCD donor use (6.1%) and those at the 75th percentile (16.0%). For every 5% increase in a center’s use of imported kidneys, there was a 6.1% increase in the odds of DGF (OR 1.06; 95% CI: 1.03-1.10; P<0.001). Holding all other factors constant, the proportion of imported kidneys would be associated with a 16.1% absolute increase in the odds of DGF between centers at the 25th percentile of imported kidney use (16.4%) and those at the 75th percentile (29.5%). For every 5% increase in a center’s use of kidneys with cold ischemia time >30 hours, there was a 5.0% decrease in the odds of DGF (OR 0.95; 95% CI: 0.92-0.98; P=0.001). Holding all other factors constant, the proportion of kidneys with cold ischemia time >30 hours would be associated with a 14.4% absolute decrease in the odds of DGF between centers at the 25th percentile of use of kidneys with cold ischemia time >30 hours (3.2%) and those at the 75th percentile (17.6%). In other words, independent of an individual’s kidney length of CIT, being transplanted at a center with increased experience transplanting kidneys with CIT>30 is associated with a lower likelihood of DGF.
Table 3.
Adjusted odds ratio for the development of DGF in a model combining patient- and center-level characteristics
| Adjusted Odds Ratio (95% Confidence Interval) | P-value | |
|---|---|---|
|
Donor Factors
| ||
| Age (5-year increments) | 1.07 (1.07-1.08) | <0.001 |
| Hypertension | 1.40 (1.34-1.46) | <0.001 |
| Donation After Cardiac Death | 2.73 (2.57-2.91) | <0.001 |
| Serum Creatinine >1.5 mg/dL | 1.94 (1.85-2.02) | <0.001 |
| Cold Ischemia Time (5 hour Increments) | 1.18 (1.16-1.19) | <0.001 |
|
| ||
|
Recipient Factors
| ||
| Age (5-year increments) | 1.00 (0.99-1.00) | 0.2 |
| Male | 1.49 (1.43-1.55) | <0.001 |
| Peak PRA (5% increments) | 1.00 (1.00-1.00) | <0.001 |
| Zero-HLA-Mismatch | 0.73 (0.68-0.78) | <0.001 |
| Prior Transplant | 0.99 (0.93-1.05) | 0.7 |
|
| ||
|
Center-Level Factors
| ||
| Proportion of Preemptive Transplants (5% increments) | 0.83 (0.74-0.93) | 0.001 |
| Proportion of Donation After Cardiac Death Donors (5% increments) | 1.12 (1.03-1.17) | <0.001 |
| Proportion of Transplants with Cold Ischemia Time >30 Hours (5% increments) | 0.95 (0.92-0.98) | 0.001 |
| Proportion of Imported Kidneys (5% increments) | 1.06 (1.03-1.10) | <0.001 |
DGF = delayed graft function, BMI=body mass index, PRA = panel reactive antibody
Year of transplant, donor blood type, donor cause of death, donor creatinine, donor cardiac arrest after event leading to death, donor race, donor BMI, use of pulsatile perfusion, and recipient race were also included in the model.
Center volume and the proportions of deceased donor transplants, ECD transplants, donors with elevated serum creatinine, diabetic donors, hypertensive donors, donors >65 years of age, recipients >65 years of age, African-American donors, and African-American recipients were not statistically significantly associated with DGF and were therefore excluded from the final model.
After adjusting for patient and center-level factors, 41.8%of centers had predicted incidences of DGF that would have put them in a category consistent with the national median (Figure 1B). 28.2% had predicted incidences of DGF above the national median. The remaining 29.9% of centers had predicted incidences of DGF below the national median. The adjusted relative odds of DGF across centers ranged from 0.22 to 3.08 (IQR: 0.71-1.41).
DISCUSSION
In this national study of center-level factors and DGF, we found significant heterogeneity in a patient’s likelihood of developing DGF based on the center at which the transplant is performed. While the median incidence of DGF was 27.3%, the range was 3.2-63.3%. Even after adjusting for patient and center-level characteristics, there remained significant heterogeneity in the predicted DGF incidences across centers. After patient-level adjustment, only 38.4% of centers had DGF incidences consistent with the national median. Adjusting for patient and center characteristics increased that, but only to 41.8%. Center-level characteristics associated with decreased DGF included a center’s proportion of preemptive transplants (OR 0.83, per 5% increment; 95% CI: 0.74-0.93; P=0.001) and its proportion of kidneys with cold ischemia time >30 hours (OR 0.95 per 5% increment; 95% CI: 0.92-0.98; P=0.001). The increased use of DCD donors (OR 1.12, per 5% increment; 95% CI: 1.03-1.17; P<0.001) and imported kidneys (OR 1.06, per 5% increment; 95% CI: 1.03-1.10; P<0.001) were associated with increased DGF.
Our patient level-factors were consistent with the findings of Irish and colleagues [1, 2]. For example, they reported that donation after cardiac death was the factor most strongly associated with DGF. We also found that to be the case. Like them, we found similar associations and point estimates for male recipients, African-American recipients, and donors with hypertension. Donor age, but not recipient age, was associated with DGF in their studies as well as ours. These comparable findings lend face validity to the patient-level component of our model.
The etiology of the significant residual variability across centers is likely multifactorial, reflecting the complexity of perioperative care and practices across centers. For example, donor management [25, 26], anesthetic and perioperative fluid administration practices [28, 29], immunosuppression practices[3, 6, 30], which likely vary across centers, influence an individual transplant recipient’s likelihood of developing DGF. Factors such as these may explain the significant residual variability of predicted DGF incidence after adjusting for patient- and center-level factors.
To our knowledge, this is the first study to report on center-level effects in the development of DGF. Notable strengths of this study include its large sample size and its inclusion of nearly all centers in the United States. Limitations of this study include its retrospective, observational nature and the difficulty in drawing causal inferences from studies using large, administrative databases. The center-level factors that we tested were ones that were simultaneously mechanistically plausible and measurable. However, it is quite possible that there are other patient- or center-level effects that are not captured by this database that might influence a patient’s likelihood of developing DGF.
Many definitions of DGF are currently in use, though significant logistic and methodological challenges exist in utilizing a DGF definition based on serum creatinine levels, glomerular filtration rate, or urine output. The need for dialysis within the first seven days post-transplant is the most frequently used definition in the transplant literature [31]. However, we have demonstrated that DGF using this definition is subject to marked heterogeneity across transplant centers, even after accounting for patient- and center-level characteristics. For DGF to have clinical and research utility, a tenable definition of DGF would be resistant to such heterogeneity.
In conclusion, DGF, as defined by the need for dialysis in the first week after a kidney transplant, might not be biologically comparable from one center to another. While there are many patient-level factors associated with DGF, there are center-level factors, notably the proportion of preemptive transplants, DCD donors, imported transplants, and kidneys with cold ischemia time >30 hours, that are also associated with DGF. And even after adjustment for those patient and center-level factors, significant variability in the likelihood of DGF remains across centers, perhaps reflecting the subjective nature of the decision to dialyze a patient in the first week post-transplant. Ideally, a new definition should be designed and developed that is independent of these center-level treatment patterns. Care must be exercised in the use of the current DGF definition as an outcome in multi-center studies where post-transplant management is not standardized.
MATERIALS AND METHODS
Study Population
Patients 18 years of age and older undergoing non-preemptive, kidney-only DDKT between January 1, 2003 and December 31, 2012, as reported to the OPTN and distributed by the Scientific Registry of Transplant Recipients (SRTR), were selected for patient-level analysis. The SRTR includes information on all donors, wait-listed transplant candidates, and transplant recipients in the U.S. provided by members of the OPTN, and has been well-described elsewhere [32]. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. Patients with missing DGF information were excluded from analysis (n=3). In addition, we excluded transplant centers that performed exceptionally few kidney transplants, defined as an average of 10 or fewer transplants per year, in order to maintain regression model stability.
Patient-Level Logistic Model
Multivariable logistic regression exploring donor and recipient-level associations with the development of DGF was performed to ensure that our patient-level variables were consistent with previous DGF prediction models. Donor and graft variables included age, race, blood type, hypertension, diabetes mellitus, elevated serum creatinine, cause of death, DCD, ECD, import from another region, use of pulsatile perfusion, and CIT. Recipient variables included age, sex, race, peak panel reactive antibody (PRA), zero-HLA-mismatch, hypertension, and prior transplantation. These variables were included based on biological plausibility and published studies [1, 2]. Using a hierarchical (multi-level) model with a center-level random intercept, we calculated the expected and observed incidence of DGF across transplant centers in the United States, based on these identified patient-level predictors.
Multilevel (Combined Patient- and Center-Level) Logistic Model
To explore whether center-level characteristics were associated with DGF, above and beyond just patient-level characteristics, we fit a hierarchical model that incorporated plausible center-level characteristics that were measured in or could be calculated from SRTR data. Total DDKT volume was included in the model and was defined as the total number of transplants performed at the center that fit inclusion criteria for this study. Experience managing the patient-level risk factors known to be associated with DGF was also incorporated into the center-level model, as the proportion of total DDKT comprised of the following: DCD, ECD, imported kidneys, transplants with CIT>30 hours, donors with creatinine>1.5 mg/dL, diabetic donors, hypertensive donors, donors over age 65, recipients over age 65, African-American donors, and African-American recipients. Proportion of DDKT at a center (versus live donor KT) and the proportion of a center’s transplants that were preemptive transplants were also included as center-level variables, as we hypothesized that a center’s experience with these patients might inform their management of post-transplant dialysis. Center level variables that were not statistically significant in the multivariate model were not included in the final model for parsimony. The hierarchical model also included the following patient-level donor variables: age, race, blood type, diabetes, hypertension, BMI, serum creatinine, cause of death, donation after cardiac death, CIT, need for inotropic support, cardiac arrest after the event leading to death, use of pulsatile perfusion, and transplant year. The following patient-level recipient variables were included as well: age, history of prior transplant, zero-HLA-mismatch, peak PRA, sex, and race.
Statistical Analysis
All analyses were performed using Stata 12.1 (StataCorp, College Station, TX), with a two-tailed alpha level of 0.05. Hierarchical modeling was performed with the xtmelogit command.
Acknowledgments
Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number F32DK093218 (to BJO). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Role of the Sponsor: none
Abbreviations
- BMI
body-mass index
- CIT
cold ischemia time
- DCD
donation after cardiac death
- DDKT
deceased donor kidney transplantation
- DGF
delayed graft function
- ECD
expanded criteria donor
- IQR
interquartile range
- OPTN
Organ Procurement and Transplantation Network
- PRA
panel reactive antibody
- SD
standard deviation
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Author Contributions:
BJO, NTJ, and DLS participated in research design. BJO, NTJ, ECH, JGW, KJVA, NG, DLS contributed to data analysis. BJO, NTJ, ECH, JGW, KJVA, NG, RAM, NMD, and DLS wrote the paper. BJO, NTJ, ECH, JGW, KJVA, NG, RAM, NMD, and DLS participated in the performance of the research.
Conflict of Interest Disclosures: The authors have no relevant disclosures or conflicts of interest.
References
- 1.Irish WD, et al. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10(10):2279–86. doi: 10.1111/j.1600-6143.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 2.Irish WD, et al. Nomogram for predicting the likelihood of delayed graft function in adult cadaveric renal transplant recipients. J Am Soc Nephrol. 2003;14(11):2967–74. doi: 10.1097/01.asn.0000093254.31868.85. [DOI] [PubMed] [Google Scholar]
- 3.McTaggart RA, et al. Sirolimus prolongs recovery from delayed graft function after cadaveric renal transplantation. Am J Transplant. 2003;3(4):416–23. doi: 10.1034/j.1600-6143.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith KD, et al. Delayed graft function and cast nephropathy associated with tacrolimus plus rapamycin use. J Am Soc Nephrol. 2003;14(4):1037–45. doi: 10.1097/01.asn.0000057542.86377.5a. [DOI] [PubMed] [Google Scholar]
- 5.Shaffer D, et al. A pilot protocol of a calcineurin-inhibitor free regimen for kidney transplant recipients of marginal donor kidneys or with delayed graft function. Clin Transplant. 2003;17(Suppl 9):31–4. doi: 10.1034/j.1399-0012.17.s9.5.x. [DOI] [PubMed] [Google Scholar]
- 6.Gonwa TA, et al. Immunosuppression for delayed or slow graft function in primary cadaveric renal transplantation: use of low dose tacrolimus therapy with post-operative administration of anti-CD25 monoclonal antibody. Clin Transplant. 2002;16(2):144–9. doi: 10.1034/j.1399-0012.2002.1o078.x. [DOI] [PubMed] [Google Scholar]
- 7.Cavaille-Coll M, et al. Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant. 2013;13(5):1134–48. doi: 10.1111/ajt.12210. [DOI] [PubMed] [Google Scholar]
- 8.Butala NM, et al. Is delayed graft function causally associated with long-term outcomes after kidney transplantation? Instrumental variable analysis. Transplantation. 2013;95(8):1008–14. doi: 10.1097/TP.0b013e3182855544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman HI, et al. Delayed function reduces renal allograft survival independent of acute rejection. Nephrol Dial Transplant. 1996;11(7):1306–13. [PubMed] [Google Scholar]
- 10.Kayler LK, Srinivas TR, Schold JD. Influence of CIT-induced DGF on kidney transplant outcomes Influence of CIT-induced DGF on kidney transplant outcomes. Am J Transplant. 2011;11(12):2657–64. doi: 10.1111/j.1600-6143.2011.03817.x. [DOI] [PubMed] [Google Scholar]
- 11.Mallon DH, et al. Defining delayed graft function after renal transplantation: simplest is best. Transplantation. 2013;96(10):885–9. doi: 10.1097/TP.0b013e3182a19348. [DOI] [PubMed] [Google Scholar]
- 12.Marcen R, et al. Delayed graft function does not reduce the survival of renal transplant allografts. Transplantation. 1998;66(4):461–6. doi: 10.1097/00007890-199808270-00008. [DOI] [PubMed] [Google Scholar]
- 13.Ojo AO, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–74. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 14.Prommool S, et al. Time dependency of factors affecting renal allograft survival. J Am Soc Nephrol. 2000;11(3):565–73. doi: 10.1681/ASN.V113565. [DOI] [PubMed] [Google Scholar]
- 15.Shoskes DA, Cecka JM. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66(12):1697–701. doi: 10.1097/00007890-199812270-00022. [DOI] [PubMed] [Google Scholar]
- 16.Tejani AH, et al. Predictive factors for delayed graft function (DGF) and its impact on renal graft survival in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Pediatr Transplant. 1999;3(4):293–300. doi: 10.1034/j.1399-3046.1999.00057.x. [DOI] [PubMed] [Google Scholar]
- 17.Woo YM, et al. Early graft function and patient survival following cadaveric renal transplantation. Kidney Int. 1999;55(2):692–9. doi: 10.1046/j.1523-1755.1999.00294.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnston O, et al. Reduced graft function (with or without dialysis) vs immediate graft function--a comparison of long-term renal allograft survival. Nephrol Dial Transplant. 2006;21(8):2270–4. doi: 10.1093/ndt/gfl103. [DOI] [PubMed] [Google Scholar]
- 19.Quiroga I, et al. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant. 2006;21(6):1689–96. doi: 10.1093/ndt/gfl042. [DOI] [PubMed] [Google Scholar]
- 20.Yarlagadda SG, et al. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24(3):1039–47. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 21.Troppmann C, et al. Delayed graft function, acute rejection, and outcome after cadaver renal transplantation. The multivariate analysis. Transplantation. 1995;59(7):962–8. doi: 10.1097/00007890-199504150-00007. [DOI] [PubMed] [Google Scholar]
- 22.Troppmann C, et al. Delayed graft function in the absence of rejection has no long-term impact. A study of cadaver kidney recipients with good graft function at 1 year after transplantation. Transplantation. 1996;61(9):1331–7. doi: 10.1097/00007890-199605150-00008. [DOI] [PubMed] [Google Scholar]
- 23.Shoskes DA, Halloran PF. Delayed graft function in renal transplantation: etiology, management and long-term significance. J Urol. 1996;155(6):1831–40. doi: 10.1016/s0022-5347(01)66023-3. [DOI] [PubMed] [Google Scholar]
- 24.Boom H, et al. Delayed graft function influences renal function, but not survival. Kidney Int. 2000;58(2):859–66. doi: 10.1046/j.1523-1755.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 25.Marshall R, et al. Adverse effect of donor vasopressor support on immediate and one-year kidney allograft function. Surgery. 1996;120(4):663–5. doi: 10.1016/s0039-6060(96)80014-6. discussion 666. [DOI] [PubMed] [Google Scholar]
- 26.Patel SJ, et al. Risk factors and consequences of delayed graft function in deceased donor renal transplant patients receiving antithymocyte globulin induction. Transplantation. 2008;86(2):313–20. doi: 10.1097/TP.0b013e31817ef190. [DOI] [PubMed] [Google Scholar]
- 27.Lodhi SA, et al. Pulsatile pump decreases risk of delayed graft function in kidneys donated after cardiac death. Am J Transplant. 2012;12(10):2774–80. doi: 10.1111/j.1600-6143.2012.04179.x. [DOI] [PubMed] [Google Scholar]
- 28.Dawidson IJ, et al. Intraoperative albumin administration affects the outcome of cadaver renal transplantation. Transplantation. 1992;53(4):774–82. doi: 10.1097/00007890-199204000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Sprung J, et al. Anesthesia for kidney transplant surgery. Anesthesiol Clin North America. 2000;18(4):919–51. doi: 10.1016/s0889-8537(05)70202-9. [DOI] [PubMed] [Google Scholar]
- 30.Stallone G, et al. Addition of sirolimus to cyclosporine delays the recovery from delayed graft function but does not affect 1-year graft function. J Am Soc Nephrol. 2004;15(1):228–33. doi: 10.1097/01.asn.0000102469.32182.8c. [DOI] [PubMed] [Google Scholar]
- 31.Yarlagadda SG, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23(9):2995–3003. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaubel DE, et al. Analytical approaches for transplant research, 2004. Am J Transplant. 2005;5(4 Pt 2):950–7. doi: 10.1111/j.1600-6135.2005.00837.x. [DOI] [PubMed] [Google Scholar]


