Abstract
The aim of this study was to investigate whether consumption of probiotic fermented milk containing Bifidobacterium bifidum YIT 10347 improves symptoms in patients with functional gastrointestinal disorders (FGID). Thirty-seven FGID patients (18 male, 19 female) aged 12–80 years (mean ± SD, 52.6 ± 17.5 years) whose condition had not improved despite being seen at several medical institutions consumed 100 mL/day of B. bifidum YIT 10347 fermented milk for 4 weeks. Symptoms were evaluated after the enrollment period (BL: baseline), sample consumption period (CP) and 4 weeks after the CP (FP: follow-up period). Gastrointestinal symptoms were evaluated using the Gastrointestinal Symptom Rating Scale (GSRS) and the Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease (FSSG); psychological symptoms were evaluated using the Profile of Mood States (POMS) short form. Concentrations of salivary stress markers and the oxidative stress marker urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG) were measured. GSRS subscale scores for abdominal pain, diarrhea, and constipation significantly improved relative to BL after consumption of the fermented milk, as did FSSG subscale scores for symptoms of acid-related dyspepsia. Some subjective psychological symptoms improved. POMS scores significantly improved, and “Anger-Hostility” subscale scores significantly decreased after the consumption period, while “Vigor” subscale scores marginally increased during the consumption period. The concentrations of urinary 8-OHdG and the stress marker salivary cortisol were significantly lower at CP but returned to baseline levels at FP. Continuous consumption of B. bifidum YIT 10347 fermented milk is expected to improve gastrointestinal symptoms and reduce psychological stress in FGID patients.
Keywords: B. bifidumYIT 10347, functional dyspepsia, psychological symptoms, gastrointestinal symptoms, functional gastrointestinal disorders, stress
INTRODUCTION
Functional gastrointestinal disorders (FGID) are conditions in which uncomfortable gastrointestinal symptoms appear in the absence of organic abnormalities such as inflammation [1]. In the esophagus, it manifests as nonerosive reflux disease with symptoms of heartburn; in the stomach, it manifests as functional dyspepsia (FD) with chronic indigestion and stomach pain; and in the bowels, it manifests as irritable bowel syndrome with chronic abnormal bowel movements such as diarrhea or constipation accompanied by abdominal pain and abdominal discomfort. These symptoms repeatedly overlap and progress, so although FGID is not fatal, it is problematic for patients because it can cause a considerable decrease in quality of life.
Factors such as accelerated or delayed gastric emptying, abnormal secretion of gastric acid, visceral hypersensitivity, mental and psychological stress and Helicobacter pylori infection are thought to contribute to the pathology of FD in a complex manner. Thus, it is fundamentally treated with guidance on lifestyle and diet, and pharmacotherapy with prokinetics, antacids and antianxiety drugs has been shown to be somewhat effective. However, the placebo effect can be strong, and thus it is difficult to say whether therapeutic drugs have an adequate additive effect. Patients with FGID have no organic disease and normal blood biochemical test results, so the primary objective of treatment is to improve symptoms for the patient’s satisfaction. Accordingly, food should be a preferred method for improving gastrointestinal symptoms, as it poses no risk of side effects, is less burdensome to patients and reduces medical costs.
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [2], and they have been shown to have various effects on the bowels, from regulation of intestinal function to immunomodulation [3,4,5,6]. Recent studies have also shown that they can be effective in not only the lower digestive tract but also the upper digestive tract including the stomach and esophagus. One notable probiotic that shows promising effects in the stomach is Bifidobacterium bifidum YIT 10347, which has previously been shown to inhibit H. pylori activity, prevent injury to the gastric mucosa and improve symptoms of general malaise in patients who test positive for H. pylori [7,8,9].
Therefore, the objective of this pilot study was to investigate the effects of continuous consumption of B. bifidum YIT 10347 fermented milk in FGID patients whose condition did not improve with several types of pharmacotherapy.
MATERIALS AND METHODS
Probiotics
B. bifidum YIT 10347 was obtained from the culture collection of the Yakult Central Institute for Microbiological Research (Tokyo, Japan).
Sample beverages
B. bifidum YIT 10347 fermented milk was prepared by anaerobic culture in pasteurized milk for 24 hrs at 37°C. Flavoring was added to the culture to adjust the taste, and the culture was packed into paper containers (100 mL per container) and shipped to participants every week as the sample beverage. Sample beverages were stored in a refrigerator (≤ 10°C) before they were shipped to study participants and were kept refrigerated during shipping. The sample beverages contained at least 1×107 cfu/ml of B. bifidum YIT 10347 and Streptococcus thermophilus YIT 2021.
Study design
This clinical trial was an open-label pilot study to validate the effects of consumption of B. bifidum YIT 10347 fermented milk on gastrointestinal symptoms. The study was approved in advance by the Institutional Review Board of Toho University and was conducted in accordance with the Declaration of Helsinki.
Participants
Among all FGID patients being examined at hospitals affiliated with Toho University (Tokyo, Japan) and at Urita Clinic (Aomori, Japan), 42 patients (21 male, 21 female) aged 12–80 years (mean ± SD, 53.0 ± 17.9 years) who were diagnosed as having functional gastrointestinal disorder by the principal investigator were selected to participate in this study. Participants were given a thorough written explanation of the objective and content of this study through an informed consent form, and all participants provided informed consent by signing the informed consent form.
Patients who did not give consent, were likely to ingest the beverage incorrectly, had a gastrointestinal tumor, were pregnant, were nursing, had cardiac failure, had renal failure, or were allergic to yogurt or dairy products were excluded.
The average number of medical institutions at which a participant had been seen was 3.5 (range: 1–8). Seventeen participants were taking prokinetics, 15 were taking antianxiety drugs, 15 were taking polycarbophil, 29 were taking probiotics, 19 were taking anticholinergics, and 8 were taking ramosetron. As the objective of this study was to validate the additive effects of B. bifidum YIT 10347 fermented milk, use of these drugs during the study period was not restricted.
Protocol
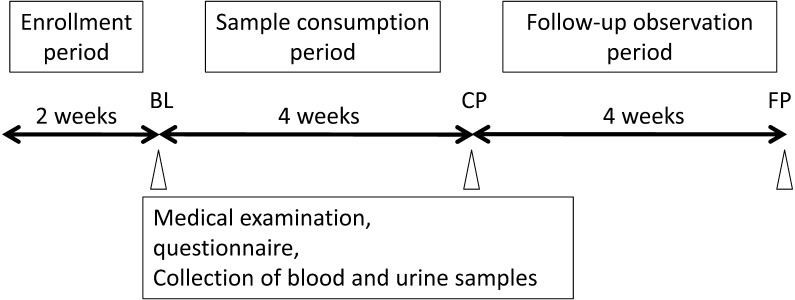
This open-label study to evaluate the effects of consumption of B. bifidum YIT 10347 fermented milk comprised a 2-week enrollment period, a 4-week sample consumption period during which 1 bottle of the sample beverage (100 mL) was consumed per day and a 4-week follow-up observation period (Fig. 1). Participants were instructed not to change their routine diet or other lifestyle habits, and no restrictions were placed on use of routinely taken drugs.
Fig. 1.
Study protocol.
At 3 points during the study, namely, at baseline after the 2-week enrollment period (BL), after 4 weeks of sample consumption (CP) and 4 weeks after the end of the sample consumption period (FP), medical examinations for digestive symptoms and general health were conducted by a doctor, and blood and urine samples were collected.
Gastrointestinal symptoms were evaluated using the Gastrointestinal Symptom Rating Scale (GSRS; Japanese version) and the Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease (FSSG) [10, 11]. The GSRS is a questionnaire that evaluates subjective severity of gastrointestinal symptoms over the week prior to evaluation. It comprises 15 items, and a higher score indicates more severe symptoms. This questionnaire is divided into subscales that allow for separate evaluation of reflux syndrome, abdominal pain syndrome, indigestion syndrome, diarrhea syndrome and constipation syndrome [12]. The FSSG is a questionnaire developed in Japan that evaluates symptoms of patients with gastroesophageal reflux disease [13]. It comprises 12 items and is divided into subscales that allow for separate evaluation of reflux symptoms and acid-related dyspepsia.
Subjective psychological symptoms were evaluated using the Profile of Mood States (POMS) short form [13]. The POMS was developed in the United States by McNair et al. [14,15,16] as a questionnaire for evaluating mood and is characterized by its ability to measure transient mood states that change depending on the conditions under which subjects are placed. It also has 6 mood subscales that allow for simultaneous evaluation of “Tension-Anxiety”, “Depression-Dejection”, “Anger-Hostility”, “Vigor”, “Fatigue” and “Confusion”. The short form of the Japanese version of the POMS produces similar results to the full-length version [17]. Furthermore, it does not take as much time and effort from subjects because it has only 30 items, and it can measure changes in mood over a short period of time that result from an intervention [18].
In addition, the stress markers cortisol, secretory immunoglobulin A (sIgA), α-amylase and chromogranin A were measured in saliva samples collected while participants were fasting in the morning at a predetermined time. Cortisol concentration was measured using a salivary cortisol enzyme assay kit (Salimetrics, State College, PA, USA), and sIgA was measured using a salivary secretory IgA indirect enzyme assay kit (Salimetrics). Alpha-amylase concentration was measured using a salivary α-amylase assay kit (Salimetrics). Chromogranin A was measured using a human chromogranin A EIA kit (Yanaihara Institute, Shizuoka, Japan).
Urine samples were collected in the morning while participants were fasting. The oxidative stress marker urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG) was measured using a DNA damage enzyme-linked immunosorbent assay kit (Cosmo Bio, Tokyo, Japan). Samples were sent to SRL, which performed measurements (Tokyo, Japan).
Five participants dropped out of the study after the sample consumption period ended due to personal reasons unrelated to the study. Ultimately, 37 participants (18 male, 19 female) aged 12–78 years (mean ± SD, 52.6 ± 17.5 years) completed the study. Furthermore, data from the 5 participants who dropped out of the study while it was ongoing could have influenced the results, so these data were excluded from compilation and analysis.
Statistical analysis
The effectiveness of the sample beverage was evaluated based on the following items: (1) abdominal symptoms (subjective symptom questionnaire), (2) psychological symptoms (subjective symptom questionnaire), (3) salivary stress markers and (4) oxidative stress markers. Data regarding effectiveness are shown as means ± SE, and they were statistically analyzed when required.
SAS version 8.02 for Windows (SAS Institute Japan, Tokyo, Japan) was used for statistical analysis. Baseline values (BL) were compared with values at each observation point (CP and FP). Two-sample paired Wilcoxon signed rank tests were used with a significance level of 5%, and descriptive statistics and 95% confidence intervals for mean values of continuous variables were calculated. A significance level of p<0.05 was considered to indicate a significant difference, and a significance level of 0.05 ≤ p≤0.1 was considered to indicate marginal significance.
RESULTS
Evaluation of participant safety based on results of medical examinations
The safety of the fermented milk was evaluated based on the results of medical examinations of 37 patients, excluding the 5 patients who withdrew their consent and dropped out, conducted by physical checkup every 4 weeks beginning at the start of the sample beverage consumption period. The primary objective of the medical examinations was to check for adverse events associated with gastrointestinal symptoms. The examinations revealed adverse events (intestinal gas and bloating) in 2 patients. These were either mild or moderate.
The possibility of a causal relationship between the sample beverage and symptoms of burping, rumbling in the lower abdomen, changes in bowel movements, and bloating, all of which are transient symptoms characteristic of consumption of fermented milk products, could not be ruled out. However, the possibility of a causal relationship was ruled out for all other symptoms, as they were present before the study began. The adverse events identified through medical examinations were transient and mild, which indicates that the studied food posed no safety risk to the participants.
Gastrointestinal symptoms
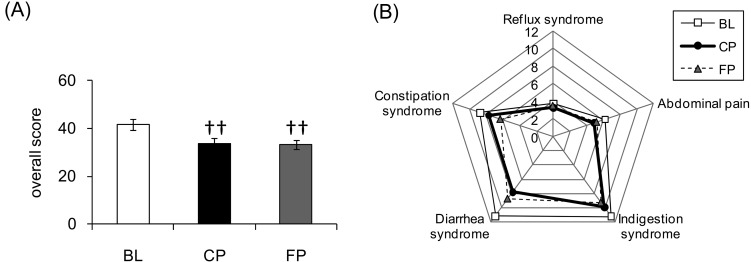
Figure 2 shows the results for the GSRS scores. Total scores were significantly lower at CP and FP relative to BL. Reflux syndrome subscale scores were lower at CP than at BL, but the difference was only marginally significant (p=0.06). Abdominal pain, diarrhea and constipation syndrome subscale scores were significantly lower at CP and FP relative to BL. Indigestion syndrome subscale scores were significantly lower at FP relative to BL.
Fig. 2.
Results of the Gastrointestinal Symptom Rating Scale (GSRS). Time-dependent changes in scores for the GSRS.
(A) GSRS total scores. Values are expressed as the mean ± standard error. ††p<0.01 vs. BL. (B) Average subscale scores (reflux syndrome, abdominal pain, indigestion syndrome, diarrhea syndrome and constipation syndrome).
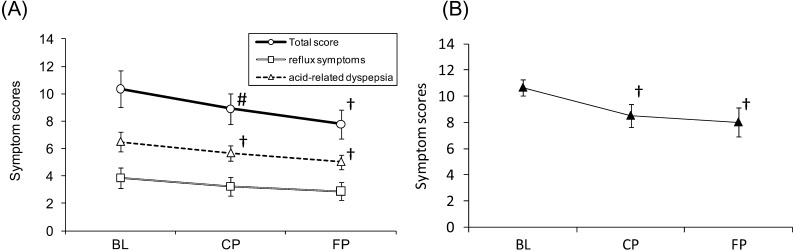
The results from the FSSG questionnaire are shown in Fig. 3. When the scores of the 35 participants who completed the questionnaire without error were analyzed, total scores were found to be marginally lower at CP and significantly lower at FP relative to BL. No significant difference in score was found for the reflux symptoms. Acid-related dyspepsia scores were significantly lower at CP and FP relative to BL. Furthermore, when a stratified analysis was conducted on the 14 participants determined to have FD (defined as an acid-related dyspepsia score of 8 or higher at BL), a more marked decrease in scores was observed after consumption of B. bifidum YIT 10347 fermented milk, with the scores at CP and FP being significantly lower relative to BL (p=0.01, p=0.02).
Fig. 3.
Results of the Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease (FSSG). Time-dependent changes in scores for the FSSG.
(A) Open circles indicate total scores, open squares show reflux symptoms, and open triangles indicate acid-related dyspepsia (n=35). (B) Closed triangles show acid-related dyspepsia scores of 8 or higher at BL (n=14). Values are expressed as the mean ± standard error. †p<0.05 vs. BL; #p<0.1 vs. BL.
Profile of mood states
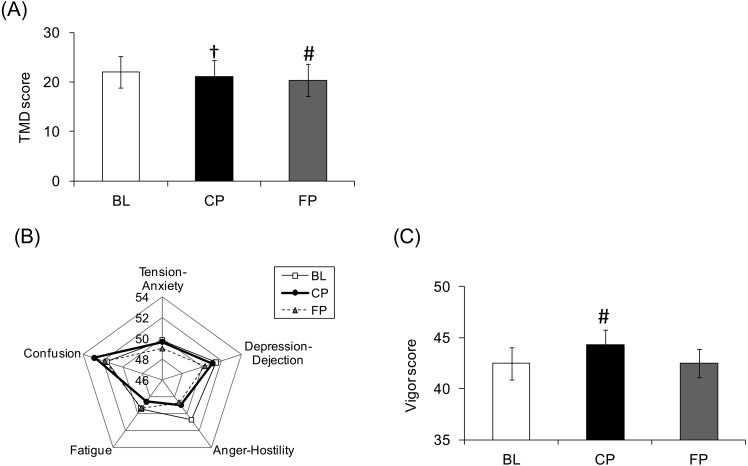
POMS data are shown in Fig. 4. Scores for “Total Mood Disturbance (TMD)” and “Anger-Hostility” significantly improved between BL and CP. Scores for “Vigor” marginally improved between BL and CP.
Fig. 4.
Results of the Profile of Mood States (POMS). Time-dependent changes in scores for the POMS.
(A) Total mood disturbance scores. Values are expressed as the mean ± standard error. †p<0.05 vs. BL; #p<0.1 vs. BL. (B) Average subscale scores of negative items (Tension-Anxiety, Depression-Dejection, Anger-Hostility, Fatigue, Confusion). (C) Vigor scores of subscale positive items. Values are expressed as the mean ± standard error. †p<0.05 vs. BL; #p<0.1 vs. BL.
Stress markers in saliva
Changes in stress markers are shown in Table 1. Salivary cortisol concentrations were significantly lower at CP but returned to BL levels at FP. At FP, a marginally significant increase in sIgA was seen along with a significant increase in chromogranin A. No significant difference was observed for any other marker.
Table 1. Stress markers in saliva.
| BL | CP | FP | ||
| Cortisol | (μg/dl) | 0.19 ± 0.02 | 0.16 ± 0.02† | 0.18 ± 0.02 |
| sIgA | (μg/ml) | 255.0 ± 33.4 | 337.3 ± 61.6 | 322.3 ± 39.7# |
| α-amylase | (U/ml) | 87.4 ± 12.9 | 93.6 ± 17.7 | 95.9 ± 15.4 |
| Chromogranin A | (p mol/mg protein) | 12.8 ± 2.7 | 10.8 ± 2.5 | 27.7 ± 5.2† |
Values are expressed as the mean ± standard error. †p<0.05 vs. BL; ♯p<0.1 vs. BL.
Oxidative stress marker 8-hydroxy-2’-deoxyguanosine
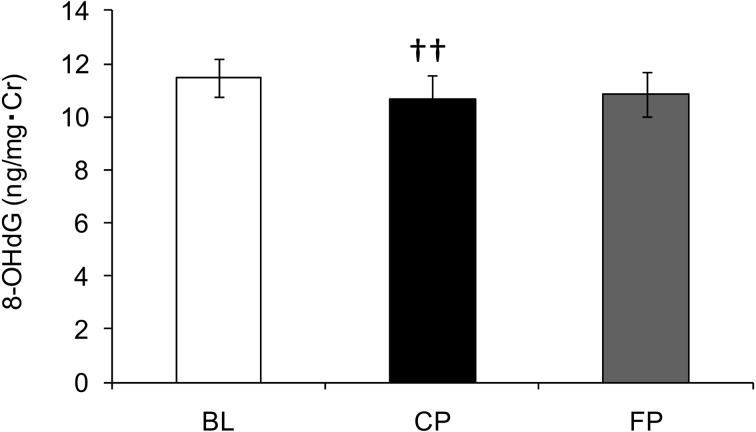
Measurements of the urinary oxidative stress marker 8-OHdG are shown in Fig. 5. 8-OHdG levels significantly decreased between BL and CP and marginally increased between CP and FP (p=0.06).
Fig. 5.
Urinary 8-hydroxy-2’-deoxyguanosine level. Time-dependent changes in the urinary 8-OHdG concentration.
Values are expressed as the mean ± standard error. ††p<0.01 vs. BL.
DISCUSSION
This study found that average measurements of gastrointestinal symptoms (GSRS, FSSG), psychological symptoms (“Anger-Hostility” and “TMD” on the POMS), salivary stress markers (cortisol) and oxidative stress markers (8-OHdG) of a group of FGID patients improved after 4 weeks of continuous consumption of B. bifidum YIT 10347 fermented milk.
Among gastrointestinal symptoms, significant improvements were seen in abdominal pain, diarrhea, constipation and dyspepsia after consumption of the fermented milk. All the participants in this study had seen no improvement in their condition despite being seen at several medical institutions. It is a very interesting finding that consumption of a very safe food yielded considerable improvement of FGID symptoms in spite of this characteristic of the participants.
B. bifidum YIT 10347 fermented milk has previously been shown to improve gastrointestinal symptoms in patients who tested positive for H. pylori [7]. It was once believed that this occurs because lactic acid and acetic acid produced by B. bifidum YIT 10347 inhibit H. pylori activity in the stomach, which prevents H. pylori from damaging the gastric mucosa and consequently improves gastrointestinal symptoms. It is certainly true that a meta-analysis has shown that H. pylori eradication is effective in improving dyspepsia symptoms in approximately 10% of FD patients and that another meta-analysis of Asian patients found H. pylori eradication to be more effective in that population than in Europeans and Americans [19, 20]. However, epidemiological studies have not shown a clear association between H. pylori infection and FD [21,22,23], and many patients still experience FD symptoms even after H. pylori eradication. This indicates that a mechanism other than H. pylori eradication induced the improvement in gastrointestinal symptoms observed after consumption of B. bifidum YIT 10347 fermented milk in this study.
An animal study has shown that B. bifidum alleviates acid/ethanol-induced gastric injury [9]. The explanation for this is that B. bifidum YIT 10347, which adheres well to cells in the stomach, promotes accelerated production of the gastroprotective factor mucin in host cells. Furthermore, experiments with cultured cells have also shown that B. bifidum YIT 10347 regulates NF-κB signaling pathways [8]. There are numerous mechanisms underlying the onset of FGID symptoms, which makes it difficult to explain causal relationships. However, a possible reason why gastrointestinal symptoms improved is that the abovementioned effects of B. bifidum YIT 10347 worked to protect the stomach, thereby alleviating visceral hypersensitivity, which is one symptom of FD.
In a previous trial, improvement of gastrointestinal symptoms was observed after 8 weeks of consumption of fermented milk [7]. In the present study, improvement of some symptoms was observed at CL, that is, after only 4 weeks of consumption of fermented milk. Because B. bifidum YIT 10347 is known to remain alive in the intestines [24], we speculate that consumption of B. bifidum YIT 10347 fermented milk ameliorates the intestinal environment, resulting in increased stress tolerance and improved gastrointestinal and psychological symptoms. Ongoing maintenance of this amelioration is a probable reason for the improvement seen after 4 weeks of consumption of fermented milk in this study.
In addition to the improvement in gastrointestinal symptoms observed after consumption of B. bifidum YIT 10347 fermented milk in this study, there was improvement in psychological symptoms and significant decreases in salivary stress markers and oxidative stress markers. Psychological stress is a major factor in FGID and is said to directly cause abnormal gastric motor function, visceral hypersensitivity and, by extension, gastrointestinal symptoms [25]. It has long been noted that stress alters intestinal flora, but recent studies have shown that differences in intestinal flora alter the responsiveness of the hypothalamic-pituitary-adrenal axis, which is a major pathway used during exposure to stress [26]. The brain and gut microbes interact by transmitting information to one another through the nervous system, the endocrine system, and the immune system. Similarly, consumption of B. bifidum YIT 10347 fermented milk is thought to possibly lead to improvement in psychological symptoms by improving the intestinal environment.
The sample beverage in this study contained B. bifidum YIT 10347 and S. thermophilus YIT 2021. As mentioned above, B. bifidum YIT 10347, but not S. thermophilus YIT 2021, has been reported to demonstrate gastroprotective effects, suppressing H. pylori-induced IL-8 secretion of human gastric cells [7] and acute gastric injury in an animal model [9]. Furthermore, unlike B. bifidum YIT 10347, S. thermophilus YIT 2021 has not been reported to survive transport to the intestines. This suggests that B. bifidum YIT 10347 is the active ingredient of the fermented milk. Further trials with an improved design are necessary to determine the precise role of B. bifidium YIT 10347 in improving the intestinal environment, gastrointestinal environment and psychological symptoms.
Conclusion
Continuous consumption of B. bifidum YIT 10347 fermented milk may be beneficial, as it improves gastrointestinal symptoms and reduces psychological stress in patients with refractory FGID.
Acknowledgments
The authors thank all the volunteers who enrolled in the clinical trial. We also thank H. Shibahara-Sone of Yakult Central Institute and Mr. D. Nozaki, O. Watanabe and C. Nonaka of Yakult Honsha Co. Ltd. for technical support and helpful advice.
REFERENCES
- 1.Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE. 2000. Rome II. The Functional Gastrointestinal Disorders – Diagnosis, Pathophysiology and Treatment: A Multinational Consensus, 2nd ed. Degnon Associates, [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations and World Health Organization2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a joint FAO/WHO expert consultation. FAO, Rome, Italy and WHO, Geneva, Switzerland. Available at: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf (accessed 2014-08-13).
- 3.Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. 2001. Probiotics: effects on immunity. Am J Clin Nutr 73Suppl: 444S–450S. [DOI] [PubMed] [Google Scholar]
- 4.Reid G, Hammond JA. 2005. Probiotics. Some evidence of their effectiveness. Can Fam Physician 51: 1487–1493. [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazaki K, Matshzaki T. 2008. Health properties of milk fermented with Lactobacillus casei strain Sirota (LcS). In Handbook of fermented functional foods, 2nd ed, Edward RF (ed), CRC Press, Boca Raton, pp. 165–172. [Google Scholar]
- 6.Cain AM, Karpa KD. 2011. Clinical utility of probiotics in inflammatory bowel disease. Altern Ther Health Med 17: 72–79. [PubMed] [Google Scholar]
- 7.Miki K, Urita Y, Ishikawa F, Iino T, Shibahara-Sone H, Akahoshi R, Mizusawa S, Nose A, Nozaki D, Hirano K, Nonaka C, Yokokura T. 2007. Effect of Bifidobacterium bifidum fermented milk on Helicobacter pylori and serum pepsinogen levels in humans. J Dairy Sci 90: 2630–2640. [DOI] [PubMed] [Google Scholar]
- 8.Shirasawa Y, Shibahara-Sone H, Iino T, Ishikawa F. 2010. Bifidobacterium bifidum BF-1 suppresses Helicobacter pylori-induced genes in human epithelial cells. J Dairy Sci 93: 4526–4534. [DOI] [PubMed] [Google Scholar]
- 9.Gomi A, Harima-Mizusawa N, Shibahara-Sone H, Kano M, Miyazaki K, Ishikawa F. 2013. Effect of Bifidobacterium bifidum BF-1 on gastric protection and mucin production in an acute gastric injury rat model. J Dairy Sci 96: 832–837. [DOI] [PubMed] [Google Scholar]
- 10.Svedlund J, Sjödin I, Dotevall G. 1988. GSRS—a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 33: 129–134. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita Y, Miki M, Tanimura T. 2007. [QOL of Japanese patients with GERD]. Nihon Rinsho 65: 1539–1544(in Japanese). [PubMed] [Google Scholar]
- 12.Hongo M, Fukuhara S, Green J. 1999. Evaluation of QOL in gastroenterology: evaluating QOL with the Japanese version of the GSRS. Diagnosis and Treatment. 87: 731–736[Japanese]. [Google Scholar]
- 13.Kusano M, Shimoyama Y, Sugimoto S, Kawamura O, Maeda M, Minashi K, Kuribayashi S, Higuchi T, Zai H, Ino K, Horikoshi T, Sugiyama T, Toki M, Ohwada T, Mori M. 2004. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol 39: 888–891. [DOI] [PubMed] [Google Scholar]
- 14.McNair DM, Lorr M, Droppleman LF. 1992. Profile of Mood States. Educational and Industrial Testing Service, San Diego. [Google Scholar]
- 15.McNair DM, Lorr M, Droppleman LF. 1992. POMS Manual. Multi-Health Systems Inc, Tront. [Google Scholar]
- 16.McNair DM, Heuchert JWP. 2003. Profile of Mood States Technical Update 2003. Multi-Health Systems Inc, Tront. [Google Scholar]
- 17.Yokoyama K, Araki S, Kawakami N, Tkakeshita T. 1990. [Production of the Japanese edition of profile of mood states (POMS): assessment of reliability and validity]. Nippon Koshu Eisei Zasshi 37: 913–918(in Japanese). [PubMed] [Google Scholar]
- 18.Yokoyama K. 2005. Guide to the POMS short form and case studies. Kaneko Shobo, Tokyo. [Google Scholar]
- 19.Moayyedi P, Soo S, Deeks JJ, Delaney B, Harris A, Innes M, Oakes R, Wilson S, Roalfe A, Bennett C, Forman D. 2011. WITHDRAWN: Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev CD002096. [DOI] [PubMed] [Google Scholar]
- 20.Jin X, Li YM. 2007. Systematic review and meta-analysis from Chinese literature: the association between Helicobacter pylori eradication and improvement of functional dyspepsia. Helicobacter 12: 541–546. [DOI] [PubMed] [Google Scholar]
- 21.Danesh J, Lawrence M, Murphy M, Roberts S, Collins R. 2000. Systematic review of the epidemiological evidence on Helicobacter pylori infection and nonulcer or uninvestigated dyspepsia. Arch Intern Med 160: 1192–1198. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura A, Adachi K, Takashima T, Murao M, Katsube T, Yuki M, Watanabe M, Kinoshita Y. 2001. Prevalence of functional dyspepsia and its relationship with Helicobacter pylori infection in a Japanese population. J Gastroenterol Hepatol 16: 384–388. [DOI] [PubMed] [Google Scholar]
- 23.Heikkinen M, Färkkilä M. 2002. Long-term outcome of functional dyspepsia: effect of Helicobacter pylori infection. A 6- to 7-year follow-up study. Scand J Gastroenterol 37: 905–910. [DOI] [PubMed] [Google Scholar]
- 24.Fujimoto J, Watanabe K. 2013. Quantitative detection of viable Bifidobacterium bifidum BF-1 cells in human feces by using propidium monoazide and strain-specific primers. Appl Environ Microbiol 79: 2182–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miwa H, Watari J, Fukui H, Oshima T, Tomita T, Sakurai J, Kondo T, Matsumoto T. 2011. Current understanding of pathogenesis of functional dyspepsia. J Gastroenterol Hepatol 26Suppl 3: 53–60. [DOI] [PubMed] [Google Scholar]
- 26.Sudou N. 2005. Stress and intestinal flora. J Intest Microbiol 19: 25–29(in Japanese). [Google Scholar]